- Submissions

Full Text
Gerontology & Geriatrics Studies
Aging Heart: Recent Research and Concepts
Vinod Nikhra*
Hindu Rao Hospital, India
*Corresponding author:Vinod Nikhra, Senior Chief Medical Officer and Teaching faculty, Hindu Rao Hospital, A public sector thousand-bed multi specialty Hospital, India
Submission: August 23, 2017; Published: September 18, 2017

ISSN : 2578-0093Volume1 Issue1
Abstract
Cardiovascular aging: Age is the most important determinant of cardiovascular (CV) health. There occurs an exponential rise in both the CV risk and the incidence of CV events in older adults parallel with the advancing age. The aging process and its impact, in turn, depend on many factors, from individual genetic constitution to his or her patterns of living which include the environment and lifestyle influencing the risk factors variously.
SPathophysiology of CV aging: Some individuals may age without much deterioration in body organs and systems, whereas others may be ridden with the extreme age-associated changes. Further, the aging process may affect some body systems more severely whereas others are spared from a serious disability. Thus, aging is associated with a progressive but varying decline in numerous physiological functions, and significantly affects the body organs and various systems including the heart and vasculature.
Aging and CV diseases: With advancing age, there is an increase in incidence and prevalence of atherosclerosis, hypertension, coronary artery disease and heart failure; cerebrovascular and peripheral vascular disease. The patho-physiological alterations at macro level include altered ventricular systolic and diastolic functions and diminished cardiac reserve, cardiac hypertrophy, increased arterial stiffness, and impaired endothelial function. Simultaneously, important changes are taking place at micro level; at cellular, sub cellular and molecular levels. The macro- and micro level changes have impact on quality of life, and affect the individual survival.
Retarding CV aging: The age-associated changes in CV structure and function pose the older adults to the increased risk for CV Diseases. The lifestyle interventions to prevent and delay the CV changes that accompany aging will reduce the prevalence of CVDs. But, retarding the rate of progression of CVDs at subclinical level need to be considered before the clinical disease becomes manifest. Some strategies aim to reduce the oxidative stress which exacerbates aging process and leads to debility to human body in general and CVS in particular. The most promising data comes from experimental biology high lightening calorie restriction as a tool for reduction in oxidative stress and increasing longevity.
Keywords: Aging; Aging process cardiac aging; Vascular aging; Cardiovascular disease; Heart failure; Oxidative stress; Lifestyle interventions; Retarding aging; Calorie restriction; CRAN
The Aging Process and Epidemiology of Cardio-Vascular Diseases
Aging, per se, is the most important risk factor for cardiovascular diseases (CVDs). At individual level, it is the most important determinant of cardiovascular (CV) health. There occurs an exponential rise in both the CV risk and the incidence of CV events in older adults parallel with the advancing age. Statically, the older adults (>60 years old) account for more than 80% of coronary heart disease, more than 75% of congestive heart failure, and more than 70% of a trial fibrillation patients [1]. It is estimated that by 2030, approximately 20% of the population will be aged 65 or older, and in this age group, CVDs will amount to over 40% of all deaths [2,3] and rank as the leading cause of disability and sub optimal quality of life during later years.
The increased age-dependent exposure time to various risk factors including hypertension, diabetes, hypercholesterolemia and smoking is accompanied by age associated interactions of the intrinsic aging of heart and vasculature with progressive structural changes and functional decline occurring with age. Further, the changes in CV structure and functions due to the intrinsic aging increase susceptibility to oxidative stress related injury and contribute to increased CV morbidity and mortality. These changes alter the substrate on which CVDs are superimposed in several ways, altering the occurrence, presentation, and manifestations of heart disease in older adults and lowering the threshold for clinically significant signs and symptoms. The aging, thus, has an impact on the risk and severity of the clinical manifestations for CVDs; which is true for clinically overt disease as well as subclinical or occult diseases, such as silent coronary atherosclerosis.
To prove this point, various studies including the Framingham Heart Study [4] and the Baltimore Longitudinal Study on Aging highlight the changes that occur with age in healthy humans, and their association and relevance to the increased incidence of LVH, chronic heart failure, and AF seen with advancing age [5]. With aging, the declining cardio protective systems and increasing disease processes predispose to the development of heart failure, which is largely a disease of the elderly. About 50% of all heart failure diagnoses and 90% of all heart failure deaths occur in this segment of the population [6].
The aging process and its impact, in turn, depend on many factors, from individual genetic constitution to his or her patterns of living which include the environment and lifestyle influencing the risk factors variously. Some individuals may age without much deterioration in body organs and systems, whereas others may be ridden with the extreme age-associated changes. The latter group having severe alterations in cardiovascular structure and functions display ‘unsuccessful cardiovascular aging’. What is more, these individuals may be otherwise healthy older adults. Thus, highlighting the fact that the aging process may affect some body systems more severely whereas others are spared from a serious disability.
Age-Associated Changes in Cardiac Morphology and Physiology
In general, aging is associated with a progressive but varying decline in numerous physiological functions, and significantly affects the body organs and various systems including the heart and vasculature. With advancing age, there is an increase in incidence and prevalence of atherosclerosis, hypertension, coronary artery disease and cardiac failure; cerebrovascular and peripheral vascular disease. The patho-physiological alterations at macro level include altered ventricular systolic and diastolic functions and diminished cardiac reserve, cardiac hypertrophy and failure, increased arterial stiffness, and impaired endothelial function. There are underlying changes are taking place at micro level; at cellular, sub cellular and molecular levels.
The macro-and micro level changes become apparent as various patterns of diseases of heart and vasculature; have impact on quality of life, and affect the individual survival. In apparently normal aging healthy individuals, from the age of 20 to 85 years, there is a gradual increase in LV wall thickness, alterations in the diastolic filling pattern; impaired LV ejection and HR reserve capacity, and altered heart rhythm [7,8]. The age-associated increase in arterial stiffness has long been considered to parallel or to cause the ageassociated increase in blood pressure [9].
These age-associated changes compromise the cardiac reserve capacity and affect the threshold for symptoms and signs. The progressive decline in LV compliance with age may go unnoticed (i.e., subclinical diastolic dysfunction), but with the occurrence of stress, it can manifest as overt heart failure. The development of heart failure with apparently preserved systolic function, as shown by a ‘normal’ ejection fraction, occurs in about one-third to one-half of older patients with heart failure [10]. Congestive heart failure, in the older adults, is often a consequence of age-related alterations in blood pressure and cardiac and vascular structure and function.
Increased arterial stiffening
There is an age-associated increase in intimal media (IM) thickening and a reduction in compliance signifying an increase in arterial stiffness [11]. It is believed that age-associated increase in IM thickness in humans represents an early stage of atherosclerosis. The excessive IM thickening predicts the co-existence of silent CAD, and the increased IM thickness an independent predictor of future clinical CVD events [12]. Pulse wave velocity (PWV), a marker of vascular stiffening, increases with age both sexes. Further, the ageassociated increase in PWV occurs independently of atherosclerosis. With arterial stiffening; there is increased central systolic arterial pressure, a decrease in diastolic arterial pressure, and an increase in the pulse pressure. The elevated pulse pressure is associated with progression of IM thickening and the IM thickening, in turn, is associated with widening of pulse pressure.
The arterial stiffness influences the development of atherosclerosis, which through endothelial cell dysfunction and other mechanisms, promotes vascular stiffness. Various risk factors, including hypertension, smoking, dyslipidemia, diabetes, diet, and so far undefined genetic factors, interact with vascular aging to activate an atherosclerotic plaque [13]. Thus, atherosclerosis, which increases with aging, can be called an interaction of vascular aging with various atherosclerotic risk factors [14]. The evidence to support this view has come from studies in older animals where atherogenic diets result in the similar elevations of plasma lipids causing markedly severe atherosclerotic lesions.
Altered arterial pressure
The elevated pulse pressure is an independent risk factor for future CV events [15] and studies have shown that individuals manifesting elevations in systolic and pulse pressures are more likely to develop clinical disease. Pulse pressure is a useful hemodynamic indicator of conduit artery vascular stiffness. The measurement of pulse wave velocity or carotid intima-media thickness is important indicator of vascular stiffness and vascular aging [16].
Framingham study reported an age-dependent rise in average systolic blood pressure across all adult age groups and inferred that pulse pressure is a better predictor of coronary disease risk than the systolic or diastolic pressure. The average diastolic pressure rises until 50 years of age, levels off from ages 50 to 60, and declines thereafter. Owing to the decline in diastolic pressure in older adults in whom systolic pressure is increasing, isolated systolic hypertension is the most common form of hypertension and associated with an increased cardiovascular disease risk. The age-dependent increase in blood pressure contributes to increasing left ventricular hypertrophy (LVH), which is an independent risk factor for CVD.
An emerging concept in the treatment of hypertension recognizes that progressive vascular damage can continue to occur even when arterial pressure is controlled. Thus, the drugs that retard or reverse age-associated vascular wall remodeling and increased stiffness will be preferable to those that lower pressure without affecting the vascular wall properties.
Cardiac LV after load and vascular after load
The cardiac component of after load during exercise increases slightly with age because of increase in heart size in older adults [17]. The components of vascular after load-conduit artery compliance characteristics, reflected as pulse waves, are altered with aging, and there is increased vascular load on the LV. The increase in LV wall thickness with aging balances the expected increase in cardiac after load because of increased LV volume in older adults during stress. Optimal ejection fraction occurs when ventricular and vascular loads are matched.
The precise cardiac and vascular load matching in younger persons is preserved in older adults at rest. During exercise, however, a mismatch in loading occurs due to altered LV and vascular elastic. Such LV arterial-ventricular load mismatch in older adults during exercise may be an important mechanism for the deficit in the LVEF reserve [18].
Heart rhythm (HR)
The HR variability, declines steadily with age and has been linked to increased risk for CV morbidity and mortility. There is an increase in the prevalence and complexity of both supraventricular and ventricular arrhythmias at rest, ambulatory, or during exercise in otherwise healthy older adults.
Isolated a trial premature beats (APBs) appear on resting ECG in 5% to 10% of subjects older adults and are generally not associated with heart disease. There occurs a steep increase in the prevalence of VE with advancing age in both those clinically free of heart disease and in unselected populations. A trial fibrillation (AF) is detected in approximately 3% to 4% of healthy volunteers over age 60 years, which is 10-fold higher than in the general adult population. The PSVT is an early clue for an increased risk for future AF. Another risk factor for AF is the increase in left a trial size that occurs with advancing age.
Left ventricular changes
According to Framingham Heart Study and Baltimore Longitudinal Study on Aging, based on the data from apparently healthy adults, there was an age-dependent increase in LV wall thickness measured by echocardiography in both men and women, indicating increased prevalence of LV hypertrophy (LVH) with age, even in the absence of clinical hypertension. The LVH has been shown to be associated with increased risk for coronary heart disease, sudden death, stroke, and overall cardiovascular disease.
Left Ventricular Diastolic Function is highly prevalent in older adults, and adversely affects the exercise capacity and predisposes to the development of diastolic heart failure, amounting to more than half of the heart failure cases in patients older than 75 years. The LV ejection fraction (EF) is preserved during aging. The value of EF<50% is indicative of impaired LV systolic function. Also, the maximum EF achievable during exercise decreases with age. There is age-associated failure to augment EF with exercise indicating age-associated loss of EF reserve. Healthy older adults can augment their cardiac index 2.5-fold during exercise over at rest, whereas those at the younger age can increase their cardiac index 3.5-fold.
Aortic and mitral valvular changes
There occurs myxomatous degeneration and collagen deposition leading to valvular sclerosis. Aortic valve sclerosis is present in 30%-80% of older adults, and often develops in parallel with progression of atherosclerosis in other vessels and accompanied by an increased occurrence of CV events and mortality. The aortic valve sclerosis can advance into aortic stenosis when severe thickening, stiffening, and calcification of the leaflet and valvular annulus occur, leading to valvular obstruction.
In addition, aortic regurgitation, also related to the calcification of the aortic cusps and annulus occurs in 13%-16% of the older adults. Mitral annular calcification (MAC) commonly found with aortic valve sclerosis and is associated with aging. Individuals with MAC have increased risks of mitral stenosis and regurgitation, heart failure, a trial fibrillation, conduction system diseases, stroke, coronary and vascular diseases. The valvular changes superimposed on ventricular changes in older adults result in a severe compromise in the cardiac functional reserve capacity, make the heart more susceptible to stress and lower the threshold for disease-related symptoms and signs.
Altered neurohormonal regulation
The detrimental effects of β-adrenergic signaling on CV health are mediated by demand for augmented cardiac output that results from sympathetic signaling and depending on the duration of the stress, the altered neurotransmitter release might be a basis for apparent impairment of sympathetic CV regulation that occurs with aging. The beta-blockers (β-adrenergic receptor inhibitors) have shown a survival benefit in patients with heart failure in clinical trials.
Age-Related CV Changes: Underlying Mechanisms at Sub-Cellular and Molecular Levels
Vascular aging and atherosclerosis
The aging is an independent risk factor for atherosclerosis and at micro level the vascular cellular senescence contributes to the pathogenesis of atherosclerosis. In various animal models, vascular aging is associated at micro level with enhanced expression of adhesion molecules in aorta and arteries [19]. There occurs an increased arterial thickening and stiffness, increased arterial permeability, and dysfunctional endothelium. The activity of the endothelial nitric oxide synthase (eNOS) isoform is markedly reduced [20] and there occurs reduction in NO, which is a critical vasodilator, helping in regulating vascular tone and inhibiting vascular inflammation, thrombosis, and aberrant cellular proliferation. The loss of NO promotes endothelial cell senescence.
There is a decreased activity of angiogenic growth factors such as vascular endothelial growth factor (VEGF) with aging and alterations in extracellular enzymes, matrix proteins, which affect endothelial cell proliferation and migration. With aging, the endothelial barriers become incompetent and vascular smooth muscle cells migrate into sub endothelial spaces and deposit extracellular matrix proteins that result in intimal thickening. There occurs senescence-associated expression of several regulators of the cell cycle, attrition of telomeres, and suppression of telomerase activity. The interaction of the risk factors with the age-altered vascular substrate leads to vascular inflammation, vascular remodeling, and oxidative stress; and provides a reduced threshold for genesis and promotion of atherosclerosis.
The transcription factors belonging to the peroxisome proliferator-activated receptor family (PPAR), modulate several gene expressions, are anti-inflammatory and significantly reduced during aging. With aging, there is a high level of circulating proinflammatory cytokines like TNFα, IL-6, IL-1β and IL-17, matrix metallo proteinases (MMPs) and other adhesion molecules. Agerelated changes in platelet activation result in hypercoagulability of the blood and increase risk of thrombus formation in older adults. Additionally, there are COX-mediated alterations in eicosanoids in an aging endothelium. The eicosanoids, PGE2, PGI2 and PGD2 are vasorelaxants, whereas TxA2 and the endoperoxide, PGH2, are vasoconstrictors. With aging, the ratio of the vasoconstrictive factors is increased, and this may play a significant role in the agerelated vascular resistance and atherosclerosis. There also, occurs alteration of Ca2+ handling, which is the signaling molecule of excitation-contraction coupling. Its reuptake into the SR is impaired in cardiac aging and heart failure (HF).
Cardiac aging, cardiomyopathy and HF
In a response to increased vascular resistance, there occurs remodelling of myocardium which includes myocyte hypertrophy and alterations in extracellular matrix (ECM). The cardiac hypertrophy results in increased myocardial oxygen demand and decreased coronary perfusion pressure due to compression of the coronary microcirculation. This causes mismatch in oxygen/ nutrient supply-demand and induces a relative myocardial ischemia. At the sub cellular level, mitochondrial oxidative stress and dysfunction playan important rolein cardiac aging (Figure 1).
Figure 1: The molecular mechanisms of cardiac aging.
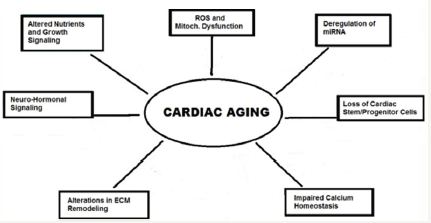
In the experiments, the mice over expressing catalase, mCAT mice, displayed greatly attenuated cardiac aging phenotypes, including reduced cardiac hypertrophy and improved diastolic function and myocardial performance. The attenuated cardiac aging phenotypes in mCAT mice were having significantly reduced mitochondrial protein oxidative stress and damage, further supporting the role of mitochondrial ROS in cardiac aging. Another factor, the PPAR-γ coactivator 1α (PCG-1α) is the key regulator of mitochondrial biogenesis, and enhances mitochondrial function in the heart. Increasing evidence suggests that micro RNAs (miRNAs) are important regulators of aging and cardiovascular diseases [21], and several recent studies have highlighted the role of miRNAs in cardiac aging. The evidence suggests that increased miR-34a expression in the aged heart contributes to cardiac aging [22].
Figure 2: The Concept of HF Iceberg.
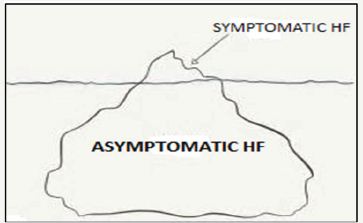
These changes reduce functional reserve and predispose the aged hearts to the development of heart failure. The HF adversely affects the cardiac pumping capacity, and in most cases is asymptomatic for a variable period. The symptomatic HF patients represent the visible part of an iceberg and best defined as the acute decomposition of chronic HF (Figure 2). There is a rising incidence of HF worldwide and it is a major health concern carrying an increased morbidity and potential loss of work days, hospitalization and substantial cost of treatment. Furthermore, its clinical course is gradually progressive, which affects QOL especially during later years of life.
At the cellular and sub cellular levels, the HF is associated with defects in mitochondrial function. The alterations in substrate metabolism contribute to contractile dysfunction and LV remodeling. At the advanced stages of HF, the myocardium has low ATP content due to a decreased ability to generate ATP by oxidative metabolism and loss of contractile strength. There is a progressively reduced myocardial performance, mainly due to the loss of myocardial tissue and dysfunctional viable myocytes, or a combination of both. Myocardial metabolism is altered and the substrate utilization switches from mostly fatty acids to glucose. In addition to metabolic derangements, there is increased adrenergic tone which adds further to metabolic dysregulation and the progression of myocardial dysfunction.
Reactive oxygen species and oxidative stress
The aging process brings forth both visible and tangible changes in the human body organs and systems, and the oxidative damage at cellular and molecular levels appears to be the primary cause. The oxidative stress tends to be pervasive among cellular organelles and macromolecules, and the cumulative oxidative damage consequent to oxidative stress leading to inflammatory response at cellular and sub cellular levels, brings forth various aging-associated changes [23]. The incidence of cardiovascular diseases correlates with age, which in turn appears to be the result of oxidative stress and cumulative damage at cellular and molecular levels. Further, the aging process does not affect all organs and systems equally. It appears that organ systems with greater cell turnover suffer the most accumulated DNA damage.
At a cellular level, the oxidative damage to mitochondria results in acidification of the cytoplasm and release of cytochrome c. At the sub cellular level there is damage to DNA and other sub cellular structures, and gradual loss of the telomere cap, due to several reactive molecular species that include reactive oxygen species (ROS) and reactive nitrogen species, reactive aldehyde species, transition metal intermediates and advanced glycation end (AGE) products. The oxidative stress leading to irreversible damage signals either cell cycle arrest or apoptosis heralding the aging process at the tissue level.
The ROS production increases with age and the cardiomyocytes become more susceptible to oxidative stress. As the result, the cardiomyocytes undergo apoptosis and necrosis. The necrosis promotes the proinflammatory and profibrotic environment. There results cardiomyocyte senescence, indicated by the increased expression of senescence markers, such factors as p53, p21, p16, and β-galactosidase activity, and decreased telomere length and nuclear and mitochondrial DNA damage. The rate of cardiomyocytes regeneration from cardiac stem cells pool in the aging heart is inadequate to maintain cardiomyocyte numbers in response to cardiomyocyte loss.
The mitochondral dyamics and energetics
But, ROS induced oxidative stress and oxidative damage is not a linear mechanism. The organisms, at cellular levels, are now known to efficiently sense and neutralize ROS under normal conditions through cellular antioxidant systems, which include the superoxide dismutases (SOD), catalase, and the glutathione peroxidases (GPxs), among others. Further, there appears to be a complexity of ROS and antioxidant systems. On one hand, the ROSinduced oxidative stress causes damage to sub cellular organelle; on the other hand, there appear to exist numerous biological roles for ROS in signaling and the stress response. Mitochondrial ROS are required for a proper response to hypoxia in the heart through activation of hypoxia inducible factor 1 (HIF-1), which is necessary for ischemic preconditioning [26].
There exists the phenomenon that oxidative stress might promote longevity and metabolic health.This has been validated by recent studies in Caenorabditis elegans, a nematode, demonstrating that some conditions which increase mitochondrial ROS production also significantly increase lifespan via mitochondrial ROS-mediated activation of HIF-1.
The calorie restriction, especially glucose restriction, also extends lifespan in various model organisms, including Saccharomyces cerevisae, Drosophila melanogaster, and C. elegans, by inducing mitochondrial respiration and increasing oxidative stress. Thus, oxidative stress appears to be crucial as the signal to induce stress response [27]. This concept of mitochondrial hormesis (mitohormesis) hypothesizes that a low dose of oxidative stress, induced by caloric restriction, exercise, or other stimuli, may trigger an adaptive response that improves overall stress response and endogenous antioxidant defense. Thus, may lead to a long-term check on oxidative stress and the extension of lifespan.
The evidence supports this theory. The damaged, high-ROS producing mitochondria have been demonstrated to accumulate in aging cardiac tissue, and play a major role in pathologic cardiac hypertrophy. Further, the CR as well as inhibition of mTOR by treatment with rapamycin in animal studies increase autophagy and prevent mitochondrial dysfunction, and extend lifespan. Mitochondrial homoplasmy is another phenomenon, where a pool of variant but functional form of mitochondria having mutant mtDNA exists without showing any defect in respiratory activity, and escapes auto phagosomal degradation or even expands through mitochondrial fission [29].
The mitochondrial-turnover/lysosomal theory can successfully explain this phenomenon of aberrant autophagy. Another important phenomenon is the mitochondrial fusion, which allows two mitochondria to mix and repair their contents and provides mtDNA stability as well as tolerance to mtDNA mutations. It separates dysfunctional mitochondria organelle which is subsequently targeted for mitophagy.
There occurs deterioration in mitochondrial energetic with heart failure in human and experimental animals. The studies have noted an age-dependent increase in mitochondrial protein-carbonylation in mice, which is indicative of increased oxidative damage to mitochondrial proteins. The accumulation of mitochondrial DNA mutations increases apoptosis and the mice develop cardiomyopathy with marked LV hypertophy, systolic and diastolic dysfunction, and impaired overall myocardial performance, and have a shortened lifespan.
The mitochondrial dysfunction and aberrant ROS production may contribute to a direct damaging effect to cellular macromolecules, apoptosis and cellular aging through interference with normal signaling and energetic [30]. The age-dependent decline also favors mitochondrial ROS production, as well as mitochondrial DNA mutation and protein damage amplifying ROS, which may be a critical factor in cardiac aging as well as in other organs aging [31].
Cardiac neurohormonal regulation and oxidative stress
IGF-1 signaling modulates other factors in the longevity network by inducing mTOR activity through regulation of Akt activity, which in turn inactivates the Fork head box O (FOXO) by phosphorylation. The mechanism involves activation of endogenous antioxidants and sirtuin-1 (SIRT1) [34]. In human, the IGF1 levels significantly decrease with age. The low serum IGF1 correlates with an increased risk of heart failure among older adults. The role of IGF1 in cardiac aging and lifespan in humans remains unclear.
Nutrient signaling in cardiac aging
The PI3K/AKT/mTOR pathway lies at the intersections of numerous signaling pathways, thus positioning this pathway as a vital mediator of aging and the cardiovascular system. Its inhibition activates autophagy, allowing for the destruction of defective molecules and organelles and promoting health of cardiovascular tissues.
Deletion of TOR or treatment with rapamycin has been observed to extend lifespan in yeast, worms, flies, and mammals. Inhibition of mTOR signaling in the heart represses cardiac hypertrophy mediated by pressure overload. Further, the relevance of the mTOR pathway to cardiac health is indicated by studies showing that rapamycin is protective in models of cardiac hypertrophy and heart failure.
Studies show that inhibition of the mTOR pathway in Drosophila attenuates the aging-related decline in cardiac function. Administration of rapamycin inhibits Ang II-induced increases in protein synthesis in cardiac myocytes and can cause regression of established pressure-overload induced LVH and left ventricular fibrosis in mice with hypertrophy and heart failure [38]. Rapamycin also attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice [39].
SIRT1 has the closest amino acid sequence homology to yeast SIR2, which has been reported to mediate the extension of replicative lifespan in response to reduced glucose availability. The three namely Sirt3, Sirt4, and Sirt5 are localized within the mitochondria. SIRT1 is localized in the nucleus and regulates the AMPK pathway, the Insulin/IGF-1 pathway and the IGF-1 signaling pathway. It also interacts with TSC1/2, inhibiting mTOR activity. In S cerevisiae (yeast), C. elegans and Drosophila, extra copies of the Sirt genes are associated with an extended lifespan.
In experiments, mice deficient in SIRT1 displayed several developmental defects and shortened lifespan, whereas mice with an extra copy of Sirt1 demonstrated a metabolic phenotype similar to CR mice. Cardiac-specific SIRT1 over expressing mice show delayed age-dependent cardiomyopathies and reduction of stress-induced apoptosis. However, extensive SIRT1 over expression, say ~20 fold, resulted in oxidative stress, apoptosis, and cardiomyopathy. SIRT1 has been observed in mice to regulate blood vessel growth and arterial stiffness. The Sirt 3 knockout mice show accelerated signs of aging in the heart, including cardiac hypertrophy and accelerated fibrosis.
Sirt3 expression is reported to be reduced with age in sedentary individuals, but improves by exercise conditioning in both young and old persons. The SIRT7 knockout mice develop cardiac hypertrophy and inflammatory cardiomyopathy and show increased fibrosis. Further, the cardiomyocytes from SIRT7 knockout mice have decreased resistance to oxidative stress and an increase in apoptosis. The involvement of SIRTs in nutrient signaling and aging implicates histone deacetylation and epigenetic regulation of DNA expression. Various recent studies highlight the potential role of epigenetic modification in cardiac aging and CVDs.
AMPK is cardio protective during ischemia and reperfusion. In mouse models, activation of AMPK by met for min reduces pressure overload-induced cardiac hypertrophy. Being involved in energy sensing, AMPK may be a mediator of the positive effects of CR and resveratrol on both longevity and CVD. Many of the effects of resveratrol are lost in AMPK knockout mice [43].
The mammalian p66shc: It is a splice variant of the Shc locus and functions as a redox enzyme modulating mitochondrial ROS. Loss of p66shc leads a decrease in ROS and lifespan extension. In the cardiovascular system, loss of p66shc blocks the decline in cardiac progenitor cell senescence, decreases DNA damage, necrosis, and apoptosis, and preserves LV volume and function, thus reducing heart failure. Loss of p66shc also prevented Angiotensin II-induced hypertrophy and apoptotic cell death. Furthermore, consistent with a central role of ROS in the pathogenesis of atherosclerosis, loss of p66sch protected mice from aortic lesions when placed on a highfat diet.
Cardiac stem cells and cardiac cellular aging
There occurs a progressive decrease of cardiomyocyte turnover from 1% per year at the age of 25 to 0.45% per year at the age of 75 in older adults [44]. Endomyocardial biopsies of hearts from older adults with dilated cardiomyopathy show decreased telomere length, increased levels of senescence markers, and increased apoptosis. The shortened telomeres, increased expression of senescence markers and apoptosis indicate that senescence may play a role in heart disease and cardiac aging.
Though there exists a pluripotent population of cells in the human cardiac tissue, it is incapable of a significant regenerative response after acute ischemic injury or during the cardiac aging process. Also, till date the role of cardiac stem cells for therapeutic interventions remains debatable. But, in light of current research and advances, it seems plausible that in near future, cardiac stem cells will play an important role in the treatment and prevention of cardiac dysfunction and cardiac aging.
Understanding from premature aging syndromes
The patients suffering from the human progeroid syndromes, like Werner syndrome (adult progeria) and Hutchinson- Gilford syndrome (childhood progeria) progressively develop atherosclerosis, and myocardial infarction or cerebrovascular events occur at an early age [45]. The accelerated senescence of vascular cells may underlie the occurrence of premature atherosclerosis in these diseases [46].
In experiments, the primary cultured cells from patients with premature aging syndromes, such as Werner syndrome and Bloom syndrome, are known to have a shorter lifespan than cells from age-matched healthy persons, which supports the notion that cellular senescence is associated with aging. With age, there occurs telomere shortening in human vasculature [47]. This is evident in the endothelial cells of the abdominal aorta and iliac arteries. Telomere shortening is also more advanced in coronary artery endothelial cells from patients with coronary heart disease compared with cells from healthy subjects.
Vascular cell senescence in diabetic patients
Insulin resistance and hyperinsulinemia are essential features of T2DM. The basal level of tissue Akt activity is higher in diabetic patients than in normal subjects, and the Akt activity is markedly reduced in the target organs of insulin such as adipose tissue [48].The findings suggest that diabetes promotes the senescence of endothelial cells via the insulin/Akt pathway and/or the high glucose-induced signaling pathway, resulting in the development of diabetic vascular complications. Increased plasma and tissue levels of proinflammatory cytokines and prothrombogenic factors have been demonstrated to exacerbate insulin resistance and to contribute to diabetic complications.
From diabetic mice studies [49], reported that endothelial cell senescence and expression of cell cycle proteins, such as p53 and p16, is induced in the endothelium of these animals. Further, these animals display evidence of vascular dysfunction, such as impaired endothelium-dependent relaxation and reduced angiogenesis.
Impact of Aging Research: Emerging Strategies for Cardiac Aging and CVDS
The concept of genome stability and aging
The age-dependent changes occur in both the heart and vasculature, which undergo numerous alterations at cellular and sub cellular levels as a result of deregulation of various molecular longevity pathways [50]. A number of diverse stimuli induce senescence. But, they appear to converge on certain pathways that influence cell cycle regulation, DNA repair and apoptosis, and the process of cellular senescence (Figure 3).
Figure 3: The concept of genome stability and aging.
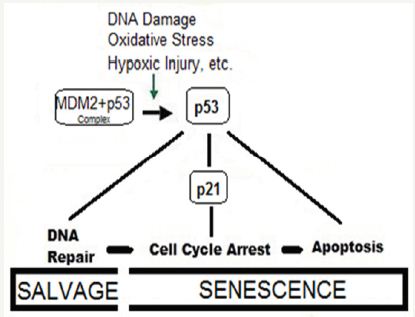
These pathways are regulated by the tumor suppressor proteins p53 and pRb. The p53 is a crucial mediator of the cellular response to damaged DNA and dysfunctional telomeres, and in turn activates the cyclin-dependent inhibitor p21. It is considered that senescence occurs via the p53 pathway in response to DNA damage and telomere dysfunction [51], whereas the p16/pRb pathway mediates senescence caused by oncogenic stimuli, chromatin disruption, and other cellular stresses.
The chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. Many of these genes and their related pathways have been subsequently studied in model organisms like, Saccharomyces cerevisiae (yeast), the Caenorhabditis elegans (nematode), Drosophila melanogaster (fruit fly), and Mus musculus and other mice. Angiogenesis, another critical mechanism, is responsible for repairing tissues after damage caused by daily wear and tear and events such as myocardial ischemia and infarction and cerebrovascular stroke [52].
The age-related impairment of angiogenesis contributes to increased end-organ damage and affects cardiovascular health. Processes and pathways contributing to impairment of angiogenesis, include cellular senescence, telomere attrition, oxidative damage, NO, hypoxia, and vascular growth factors.
Reducing ROS to retard aging
The modalities to prevent, delay or treat CV changes that accompany aging will reduce the prevalence of CVDs. But, retarding the rate of progression of CVDs at subclinical level need to be considered before the clinical disease becomes manifest. There are strategies aiming to reduce the oxidative stress which exacerbates aging process and leads to debility to human body in general and CVS in particular. The most promising data comes from experimental biology highlightening calorie restriction as a tool for reduction in oxidative stress and increasing longevity. The life-style interventions in form of regular physical exercise, smoking cessation and intervention in sleep disorders, are known to mitigate oxidative damage and exert a beneficial effect on CVS. Certain medications or humoral factors are known to reduce CVDs are a practical ways to prevent and treat atherosclerosis and CVD. Further, a multitude of therapeutic interventions relating to various pathways involved in cellular aging hold promise.
Calorie restriction and CR mimetics
The dietary modification in form of calore restriction with adequate nutrition (CRAN) and adding antioxidants as nutraceuticals is an important and simple intervention. The natural antioxidants are found in plants, fruits and vegetables, namely alkaloids and polyphenols. The CRAN diet combined with exercise appears to increase the health span by stimulating the expression of ‘longevity genes’ that promote cellular defence against aging and age-related diseases (Figure 4).
Figure 4: Pathways linking CR to health and longevity.
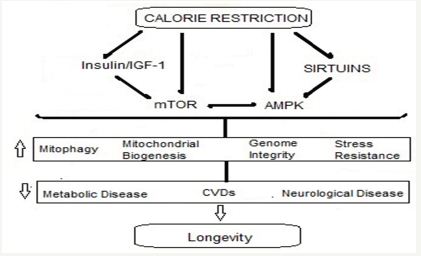
There are some fundamental molecular processes involved in CR-mediated protection of CVS. CR modulates various factors in the longevity network and alters cellular responses to oxidative stress. It reduces oxidative stress and inflammation in the vasculature by suppressing the activity of vascular adhesion molecules, prostanoids, and inflammatory cytokines. It improves endothelial function and reduces atherosclerosis and arterial stiffness [53]. With regard to cardiac function, CR delays the age-related decline in diastolic function accompanied by reductions in inflammation, myocardial degeneration, cardiac fibrosis, and cardiomyopathy.
The recent research highlights that the CR not only reduces metabolic rate and damage caused by ROS; it also triggers an active defence response that promotes a network of redundant pathways involving the longevity genes lying dormant through the evolutionary process. These so-called ‘longevity regulatory pathways’, include insulin/insulin-like growth factor 1 (IGF-1), the mammalian target of rapamycin (mTOR), AMP-activated kinase (AMPK), and nicotinamide adenine dinucleotide (NAD)+- dependent deacetylases (sirtuins). The CR is extensively-studied longevity intervention and has been shown to increase lifespan in various model organisms, from yeast and the nematode, C. elegans to mice, rats, and rhesus monkeys [54,55].
The CR involving a moderate dietary restriction (35% reduction in calorie intake) attenuates a variety of age-related pathologies and is protective against CVDs in mice and other nonhuman primates [56]. In addition to its protective effects of long-term, the CR for a brief period of 10wk was able to reverse the preexisting cardiac hypertrophy and diastolic dysfunction in old mice, and that this was accompanied by proteomic and metabolomic remodeling to a more youthful state [57].
A study in human volunteers undertaking CR for a mean of 6.5yr showed reduced blood pressure and systemic oxidative stress, and improved diastolic function [58]. Similar improvements in diastolic function have been reproduced in individuals maintained on 1-yr CR and in human trials of alternate-day fasting, demonstrating that even transient activation of the pathways involved in the CR response has beneficial effects.
In real life situations, the data indicate that Japanese Okinawans who eat a moderately restricted diet have lower rates of coronary heart disease and in general live longer. Similarly, individuals on CR diets for 0.5-8 years, have reduced triglycerides, lower blood pressure, reduced inflammatory markers and decreased oxidative stress, and a relative protection from CVDs. On the other hand, the increased adiposity with weight gain and a sedentary lifestyle negatively affect the cardiovascular system by adding oxidative stress and inhibiting the natural defence and longevity pathways.
Multiple mechanisms have been implicated in the beneficial effects of CR including inhibition of mTOR signaling, normalization of mitochondrial biogenesis, attenuation of mitochondrial ROS production and the subsequent ROS-induced signaling, and increased SIRT1 signaling [59]. Studies suggest several synergistic mechanisms that work with CRAN. There occurs an increase in NO with a combination of reduced ROS is both neuro and cardio protective, due to activation of the Nrf2 antioxidant pathway. The CR reduces oxidative stress-induced induction of pro inflammatory markers, like NF-κB-mediated cytokine synthesis, protection from endothelial damage and reversal of the progression to atherosclerosis.
Further, a reduction in myocyte size and cell death via apoptosis is observed in calorie-restricted aged hearts, a mechanism attributed to the protection of mitochondria from membrane collapse. The changes seen due to CR can be attributed to SIRT1 and PPAR. Further, RAS inhibition and CR seem to have converging effects in their mechanism, both mediated by PPAR up regulation.
The CR mimetics: The protective effects of CR in age-related disorders including cardiac aging are established. But, the use of the CR may appear challenging. Now, whether it is possible to mimic the beneficial effects of diet regulation and exercise by tweaking the right pathways, using certain neutraceuticals or pharmaceuticals, so called ‘CR mimetics’? This is a fertile area for the study of pharmacologic interventions through the CR mimetics to improve human health span.
The use of CR mimetics such as resveratrol and metformin, which activate the SIRT1-AMPK system, and rapamycin, which inhibits mTOR, show that it is possible for a rodent to be obese and sedentary while maintaining the physiology of a lean animal. Further, the recent research has identified a hormone, irisin [60-63], which when increased, induces energy expenditure in the absence of exercise, positively influencing obesity and glucose homeostasis.
References
- (2006) Incidence of CVD by age and sex, Incidence and prevalence 2006; Incidence and prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Nat Heat, Lung and Blood Inst.
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. (2011) Executive summary: heart disease and stroke statistics-2011 update: a report from the american heart association. Circulation 123(4): e18-e209.
- North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110(8): 1097-1108.
- Mahmood SS, Levy D, Vasan RS, Wangb TJ (2014) The framingham heart study and the epidemiology of cardiovascular diseases: a historical perspective. Lancet 383(9921): 999-1008.
- Chantler PD, Lakatta EG (2012) Arterial-ventricular coupling with aging and disease. Front physiol 3: 90.
- Strait JB, Lakatta EG (2012) Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 8(1): 143-164.
- Thomas S, Rich MW (2007) Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin 3(4): 381-387.
- Rich MW (2001) Heart failure in the 21st century: a cardio-geriatric syndrome. J Gerontol A Biol Sci Med Sci 56(2): M88-M96.
- Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, et al. (2014) Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 64(6): 1219-1227.
- Loffredo FS, Nikolova AP, Pancoast JR, Lee RT (2014) Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 115: 97-107.
- Minamino T, Komuro I (2007) Vascular cell senescence: contribution to atherosclerosis. Circu Res 100(1): 15-26.
- Seals DR, Kaplon R, Gioscia-Ryan RA, LaRocca TJ (2014) you’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiolog 29(4): 250-264.
- Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a “set up” for vascular disease. Circulation 107(1): 139-146.
- Lakatta EG, Levy D (2003) Part II: The aging heart in health: links to heart disease. Circulation 107(2): 346-354.
- Hopkins PN (2013) Molecular biology of atherosclerosis. Physiol Rev 93(3): 1317-1542.
- Le Couteur DG, Lakatta EG (2010) A vascular theory of aging. J Gerontol A Biol Sci Med Sci 65(10): 1025-1027.
- Thomas Sydenham (2010) How old are your arteries? Harvard health publications, Harvard Medical School, USA.
- Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, et al. (1996) Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation 94(3): 359-367.
- Chiao YA, Rabinovitch PS (2015) The aging heart. Cold Spring Harb Perspect Med 5(9): a025148.
- Dai D, Chen T, Johnson SC, Szeto H, Rabinovitch PS, et al. (2012) Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 16(12): 1492-1526.
- Sverdlov AL, Ngo DTM, Chan WPA, Yuliy Y Chirkov, John D Horowitz, et al. (2014) Aging of the nitric oxide system: are we as old as our no? vascular medicine. J Am Heart Assoc 3: e000973.
- Afjeh SSA, Sayyed Mohammad Hossein Ghaderian (2013) The role of microRNAs in cardiovascular disease. Int J Mol Cell Med 2(2): 50-57.
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, et al. (2013) MicroRNA- 34a regulates cardiac ageing and function. Nature 495(7439): 107-110.
- Quiat D, Olson EN (2013) Micro RNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J Clin Invest 123(1): 11-18.
- Venkataraman K, Khurana S, Tai TC (2013) Oxidative stress in agingmatters of the heart and mind. Int J Mol Sci 14(9): 17897-17925.
- Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS (2014) Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3: 6.
- Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, et al. (2012) Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovasc Res 93(1): 79-88.
- Dai DF, Rabinovitch PS, Ungvari Z (2012) Mitochondria and cardiovascular aging. Circ Res 110(8): 1109-1124.
- Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E, et al. (2012) Contribution of impaired mitochondrial autophagy to cardiac aging: Mechanisms and Therapeutic Opportunities. Circ Res 110: 1125-1138.
- Lopez-Lluch G, Irusta PM, Navas P, de Cabo R (2008) Mitochondrial biogenesis and healthy aging. Exp Gerontol 43(9): 813-819.
- Mammucari C, Rizzuto R (2010) Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev 131(7-8): 536-543.
- Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, et al. (2009) An attempt to prevent senescence: A mitochondrial approach. Biochim Biophys Acta 1787(5): 437-461.
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, et al. (2009) Disruption of the ANG II type 1 receptor promotes longevity in mice. J Clin Invest 119(3): 524-530.
- Inserra F, Romano L, Ercole L, De Cavanagh EM, Ferder L, et al. (1995) Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension 25(3): 437-442.
- Fontana L, Vinciguerra M, Longo VD (2012) Growth factors, nutrient signaling, and cardiovascular aging. Circ Res 110(8): 1139-1150./a>
- Cruzen C, Colman RJ (2009) Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med 25(4): 733- 743.
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, et al. (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325(5937): 201-204.
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, et al. (2011) Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50(1): 117-127.
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, et al. (2012) Rapamycin slows aging in mice. Aging Cell 11(4): 675-682.
- Flynn JM, O’Leary MN, Zambataro CA, Emmeline CA, Michael PP, et al. (2013) Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12(5): 851-862./a>
- Oellerich MF, Potente M (2012) Foxos and Sirtuins in vascular growth, maintenance and aging. Circ Res 110(9): 1238-1251.
- Sheydina A, Riordon DR, Boheler KR (2011) Molecular mechanisms of cardiomyocyte aging. Clin Sci 121(8): 315-329.
- Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, et al. (2006) Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ Res 98(4): 532-539.
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, et al. (2008) A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 3(6): e2264.
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, et al. (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923): 98-102.
- Martin GM (2005) Genetic modulation of senescent phenotypes in homo sapiens. Cell 120(4): 523-532.
- Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, et al. (2004) Essential role of limiting telomeres in the pathogenesis of werner syndrome. Nat Genet 36(8): 877-882.
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, et al. (2003) Lamin a truncation in hutchinson-gilford progeria. Science 300(5628): 2055.
- Aviv H, Khan MY, Skurnick J, , Okuda K, Kimura M, et al. (2001) Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis 159(2): 281-287.
- Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, et al. (2004) Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24(3): 546-550.
- Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, et al. (2004) Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J 23(1): 212-220.
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, et al. (2005) DNA repair, genome stability, and aging. Cell 120(4): 497-512.
- Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, et al. (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117(Pt 11): 2417-2426.
- Moslehi J, De Pinho RA, Sahin E (2012) Telomeres and mitochondria in the aging Heart. Circ Res 110(9): 1226-1237.
- La ̈hteenvuo J, Rosenzweig A (2012) Effects of aging on angiogenesis. Circ Res 110(9): 1252-1264.
- Riordan MM, Weiss EP, Meyer TE, Ehsani AA, Racette SB, et al. (2008) The effects of caloric restriction-and exercise-induced weight loss on left ventricular diastolic function. Am J Physiol Heart Circ Physiol 294(3): H1174-H1182.
- Neumann B, Chen Y, Issa H, Silber RE, Rohrbach S, et al. (2010) Caloric restriction delays cardiac ageing in rats: role of mitochondria. Cardiovasc Res 88(2): 267-276.
- Mattison JA, Roth GS, Beasley TM, Edward MT, April HH, et al. (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489(7415): 318-321.
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, et al. (2011) Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50(1): 117-127.
- Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, et al. (2014) Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13: 529- 539.
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, et al. (2006) Longterm caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47(2): 398-402.
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, et al. (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A 103(6): 1768-1773.
- Erickson HP (2013) Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte 2(4): 289-293.
© 2017 Vinod Nikhra. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






