- Submissions

Full Text
Forensic Science & Addiction Research
Harm of Heroin Substitution for Cocaine in Opioid Naïve Patients
Therese Becker1, Evangelia Papathomas2 and Betty S Chan1*
1Emergency Physician & Clinical Toxicologist, Prince of Wales Hospital, Australia
2Department of Clinical Chemistry, Liverpool Hospital, Australia
*Corresponding author: Betty S Chan, Emergency Physician & Clinical Toxicologist, Prince of Wales Hospital, Australia
Submission: January 12, 2018; Published: February 19, 2018

ISSN: 2578-0042 Volume2 Issue4
Abstract
Recreational drug use is known to cause multiple complications especially with intravenous administration. We describe four patients who developed multi-organ failure including severe hypoxic encephalopathy, rhabdomyolysis, hepatitis and renal failure. This is following intranasal drug use that was subsequently found to be heroin. One patient was a regular user of heroin while the other three had purchased what they believed to be cocaine and was opiate naive. Heroin substitution for cocaine can cause significant harm when used via the intranasal route in opiate users as well as naive patients.
Introduction
Heroin has traditionally been used either via inhalation or intravenous route. An increase in the purity of heroin has resulted in an escalation of the use of heroin via non parenteral routes [13]. These include intranasal route (snorting), inhalation of heated fumes ('chasing the dragon'), smoking or swallowing of heroin [2]. The intranasal route provides similar pharmacological effects to the parenteral route without the use of needles and risk of infection [1]. The pharmacokinetics of intranasal heroin are similar to intramuscular heroin use with peak blood concentrations achieved within 5 minutes of administration across mucous membranes [2-4]. The potency is approximately 50% and this is particularly concerning in naive users [1]. Heroin (di-acetylmorphine) is often not detectable as it is rapidly metabolized to morphine and has a half-life of only a few minutes. Detection times for morphine were generally 24-36h while 6-mono-acetylmorphine (heroin metabolite) was about 2-3h [1] (Figure 1).
We describe 4 cases of recreational intranasal opiate use that result in significant morbidity. In this case series, heroin was sold as cocaine to three opiate naive patients resulting in serious morbidities. All four patients developed multi organ involvement including severe hypoxic encephalopathy with bilateral globus pallidus infarct, rhabdomyolysis, hepatitis and renal failure.
Case 1
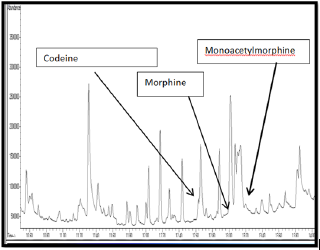
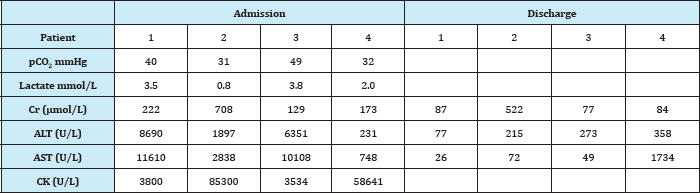
A 27-year-old male last seen 36 hours earlier, was found unconscious lying on his rightsidewith anemptypacketofquetiapine. There was evidence that he had vomited and was incontinent of urine. He was brought into the emergency department with a GCS 5 (E1 M2 V2). Vital signs: HR 78, BP 163/107, RR 15, saturating 97% on 10L/min O2. Pupils were 5 mm, equal and reactive; BSL was 7.7mmol/L. Clinical examination showed pressure areas on his right leg and elbow and increased tone and clonus in both lower limbs. He was found to have acute kidney injury (AKI) with a Cr 223μmol/L, hepatitis, and rhabdomyolysis (Table 1). Paracetamol concentration was negative. He was intubated and ventilated for airway protection. CXR showed signs of aspiration pneumonia with bilateral lower lobe consolidation more so on right than left side. CT brain was normal. He was commenced on ceftriaxone and metronidazole which was later changed to tazocin. He failed to improve neurologically and remained somnolent. EEG performed on day 3 of admission supported the diagnosis of encephalopathy. MRI showed bilateral globus pallidus and watershed infarcts. He was extubated on day 6. He has significant cognitive impairment. Assessment on day 12 showed that he has Addenbrooke's Cognitive Examination-III (ACE-III) score of 56/100 (memory 18/26, language 21/26, and verbal fluency 2/14). He admitted to a 3-year history of intravenous heroin use and on this occasion he snorted heroin. Initial urine drug screen (immunoassay) was positive for opiate. Subsequent Gas Chromatography Mass Spectrophotometry (GCMS) on urine from admission detected 6-monoacetylmorphine (heroin metabolite), morphine and codeine which are indicative of heroin use (Figure 2). The substance used by the patient was analyzed by GCMS and was found to be heroin with codeine. Patient was transferred to rehabilitation and discharged after one month of hospitalization.
Figure 1: A diagrammatic illustration of how heroin and codeine are metabolized to morphine. Heroin (di-acetylmorphine) is metabolized to its unique metabolite, 6 monoacetylmorphine and then morphine.
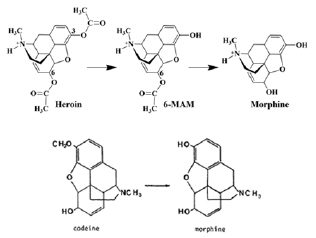
Figure 2: Gas Chromatography and Mass Spectrophotometry (GCMS) of urine from patient 1 which showed morphine, 6 monoacetyl-morphine and codeine.
Case 2
Figure 3: Brain MRI of patient 2 demonstrating bilateral globus pallidus infarct.
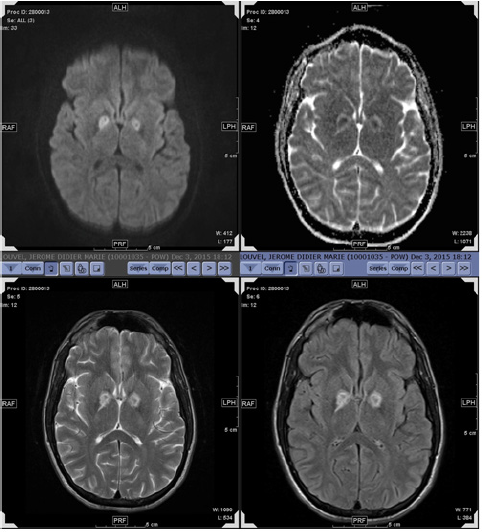
A 28-year-old man presented to the Emergency Department with lethargy, drowsiness, chest and right shoulder pain 2 days following a history of snorting cocaine. On examination, he had a painful swollen right shoulder. He was drowsy with slow mentation. He was hemodynamically stable. Investigation showed that he has acute kidney injury with Cr 732μmol/L with rhabdomyolysis CK was 85300U/L. Renal ultrasound was normal. CT and MRI brain showed bilateral globus pallidus and watershed infarct (Figure 3). EEG showed mild global encephalopathy. During his admission, his renal function deteriorated with a peak creatinine 940μmol/L on day 4 of admission. Urine drug screen was positive for opiates. GCMS analysis on urine detected the presence of codeine only. He was able to pass urine and his cognitive function continued to improve. He discharged against medical advice on day 9 of hospital admission. He was urged to have follow up for further neuropsychiatric testing.
Case 3
A 43-year-old woman presented with an altered level of consciousness after allegedly drinking alcohol and snorting cocaine the night before with 3 other women. They all became ill with recurrent vomiting and were in and out of consciousness. The next morning, she was found unrousable. Ambulance officers found her to be hypoglycemic with BSL 2.6mmol/L and have pin-point pupils. She was given 1.6mg intravenous naloxone. She became combative over the next 5 minutes and was sedated with midazolam (3mg). On arrival at the Emergency Department, vital HR 105, BP 90/60, O2 sat 94% on non-rebreathing mask. She required further doses of midazolam to control her agitation (1.5mg). She was then noted to develop generalized erythema, hypotension and bronchospasm. She was treated with bolus dose of metaraminol and then adrenaline infusion (400μg/h). She was intubated and ventilated for airway protection. CXR showed bilateral aspiration pneumonia and was treated with ceftriaxone. CT brain was normal. Urine drug screen was positive for opiates and benzodiazepines. GCMS analysis of the urine detected the presence of heroin (diacetylmorphine), 6-monoacetylmorphine, morphine and codeine. Patient had persistent confusion and agitation and required sedation. She was extubated on day 3 in ICU but remained delirious for another 1-2 days. Venous Doppler performed on day 5 of hospital admission showed thrombosis in basilica and cephalic vein up to the axillary vein and was managed with anti-coagulant therapy. She was discharged on day 8 to be followed up by neuropsychiatric testing and hematology.
Case 4
A 46-year-old male used what he believed was cocaine via the intranasal route. He could not recall events from that time and was found 18 hours later with a GCS of 13. He responded to an initial dose of naloxone and then maintained a GCS of 15. He had an acute kidney injury (Cr 173μmol/L), rhabdomyolysis (CK 58641U/L) and a mild elevation of transaminase. His urine GCMS was positive for diacetylmorphine, 6-monoacteylmorphine, morphine, codeine. He was discharged on day 4 with full thickness pressure areas to both heal and a left ulnar nerve neuropraxia.
Discussion
All four cases did not present with a clear diagnosis but a clinical syndrome of multi-organ dysfunction with altered level of consciousness, hypoxic brain damage, hepatitis, acute kidney injury and rhabdomyolysis. It was subsequently confirmed that the patients took heroin via the intranasal route. Intranasal use of heroin is not a new route of administration. In Stockholm 18 of 239 (7.5%) fatal heroin intoxication involved non-parenteral administration (either inhalation or intranasal use). Of these 18 cases, 7 were opiate naive and 4 had relapsed after periods of abstinence [5]. Media reports from Amsterdam in 2015 reported 3 people dying and 17 becoming seriously ill following intranasal use of heroin that was sold as cocaine. (http://www.businessinsider. com/white-heroin-is-being-sold-as-cocaine-in-amsterdam-2015- 2?IR=T)
Our 4 patients were found to have laboratory evidence of heroin use. The presence of 6- monoacetylmorphine, morphine and codeine in patients 1 and 3 and 4 is conclusive evidence of heroin use. The detection of heroin in the urine of patient 3 suggested ongoing intranasal absorption of heroin via the intranasal route and could explain the delay onset of generalized erythema and bronchospasm, possibly from histamine release caused by morphine that was metabolized from heroin. The detection of codeine in the second patient is also indicative of heroin use. Codeine is often present as a cutting agent or by-product or an impurity of the manufacturing process of heroin. Patient 2 presented two days after the use of intranasal substance, heroin would have been metabolized by then but codeine could still be detected for up to 3 days.
Three patients demonstrated varying degrees of encephalopathy. This was confirmed on EEG in Patients 1 & 2. Both patients demonstrated bilateral global pallidus and watershed infarcts on MRI (Figure 3). This is consistent with report findings that bilateral ischemia of the globus pallidus has been considered to be a hallmark of the ischemia-hypoxic process [6-8]. Patient 3 was confused and agitated requiring intubation with a prolonged delirium that lasted 5 days. An EEG and MRI were not performed on her as she clinically improved.
Rhabdomyolysis and acute kidney injury occurred in all four patients (Table 1). The pathophysiology of rhabdomyolysis in heroin addiction is multifactorial. Acidosis, systemic hypoxia, immunological/hypersensitivity reaction of the drug or a contaminant, a direct myotoxic effect or prolonged immobility causing muscle injury are thought to contribute [9]. Determining the exact cause can be difficult. Both patients 1 & 2 had evidence of direct muscle injury presumably from prolonged immobility. However only patients 2's CK rose to an extreme level and he subsequently had the most severe renal impairment.
All four patients developed acute hepatitis (Table 1). Whilst infective hepatitis is well reported amongst intravenous drug users, the cause of acute hepatitis in these cases is not clear. This may be from global hypoxia and/or ischemia or the direct toxic effects of the opiate. In intravenous heroin users, the liver demonstrates enzyme induction causing metabolic destruction. A study involving hypertrophy of the smooth endoplasmic reticulum, but these were autopsies in 40 heroin addicts demonstrated hyperplasia and chronic users many with chronic active hepatitis [10].
Table 1: Biochemistry profile of patients.
Conclusion
Intranasal use of heroin produces similar pharmacological toxic effect to parenteral administration. Our case series demonstrate multi-organ failure can occur when heroin is substituted for cocaine especially in opioid naive patients.
References
- Cone EJ, Jufer R, Darwin WD, Needleman SB (1996) Forensic drug testing for opiates. VII. Urinary excretion profile of intranasal (snorted) heroin. J Anal Toxicol 20(6): 379-392.
- Darke S, Hetherington K, Ross J, Lynskey M, Teesson M (2004) Noninjecting routes of administration among entrants to three treatment modalities for heroin dependence. Drug Alcohol Rev 23(2): 177-183.
- Comer SD, Collins ED, MacArthur RB, Fischman MW (1999) Comparison of intravenous and intranasal heroin self-administration by morphine- maintained humans. Psychopharmacology 143(4): 327-338.
- Zuckerman GB, Ruiz DC, Keller IA, Brooks J (1996) Neurologic complications following intranasal administration of heroin in an adolescent. The Annals of pharmacotherapy 30(7-8): 778-781.
- Thiblin I, Eksborg S, Petersson A, Fugelstad A, Rajs J (2004) Fatal intoxication as a consequence of intranasal administration (snorting) or pulmonary inhalation (smoking) of heroin. Forensic science international 139(2-3): 241-247.
- Cone EJ, Holicky BA, Grant TM, Darwin WD, Goldberger BA (1993) Pharmacokinetics and pharmacodynamics of intranasal "snorted” heroin. J Anal Toxicol 17(6): 327-337.
- Vila N, Chamorro A (1997) Ballistic movements due to ischemic infarcts after intravenous heroin overdose: report of two cases. Clinical neurology and neurosurgery 99(4): 259-262.
- Caplan LR, Hier DB, Banks G (1982) Current concepts of cerebrovascular disease--stroke: stroke and drug abuse. Stroke 13: 869-872.
- Gupta A, Khaira A, Lata S, Agarwal SK, Tiwari SC (2011) Rhabdomyolysis, acute kidney injury and transverse myelitis due to naive heroin exposure. Saudi J Kidney Dis Transpl 22(6): 1223-1225.
- Ilic G, Karadzic R, Kostic-Banovic L, Stojanovic J, Antovic A (2010) Ultrastructural changes in the liver of intravenous heroin addicts. Bosn J Basic Med Sci 10(1): 38-43.
© 2018 Therese Becker, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






