- Submissions

Full Text
Experiments in Rhinology & Otolaryngology
The Role of the Nasal Inspiratory Flow Peak, the Clinical and of the Visual Analogue Scale in the Evaluation of Allergic Rhinitis
Ana Carolina Gonçalves Ribeiro de Carvalho1*, Cláudia Ribeiro de Andrade2, Cássio da Cunha Ibiapina2, Ricardo Neves Godinho1 and Renata Victória Tassara3
1 Department of Semiology and Otorhinolaringology, Universitary Center of Belo Horizonte, Brazil
2 Department of Pediatrics, Medical College of Federal University of Minas, Brazil
3 Department of Otorhinolaringology, Pontifical Catholic University of Minas, Brazil
*Corresponding author: Ana Carolina Gonçalves Ribeiro de Carvalho, Department of Semiology and Otorhinolaringology, Universitary Center of Belo Horizonte, Brazil
Submission: April 16, 2018; Published: May 11, 2018

ISSN 2637-7780 Volume1 Issue4
Introduction
Allergic rhinitis (AR) is a nose symptomatic affection induced by exposure to allergens, immunoglobulin E (IgE) mediated and characterized by inflammation of the nasal mucosa. The cardinal symptoms are the nasal obstruction, sneezing, and watery rhinorrhea. Furthermore, conjunctival hyperemia and nasal, oropharyngeal, and ocular pruritus are also part of these symptoms, which may be resolved spontaneously or by treatment [1,2]. Allergic rhinitis is a public health problem, and its prevalence has considerably increased in recent decades. It is the most prevalent chronic disease caused by allergy in childhood. Although it does not cause direct risk of life, it can cause a significant effect on patient’s quality of life, as well as exacerbate a large number of comorbidities, such as, asthma and sinusitis [1].
In Brazil, according to the International Study on Asthma and Allergy in Childhood (ISAAC), the prevalence of allergic rhinitis is 29.6% among adolescents and 25.7% among schoolchildren [1]. The diagnosis of allergic rhinitis is often simple. However, many cases are under diagnosed because patients do not realize the impact of symptoms in their daily activities, since they adapt to the degree of their nasal obstruction, ignoring what would be normal breathing [3]. As it seems to be a not too serious disease, the patients and health professionals do not consider it as a disease, impairing their identification and treatment. Allergic rhinitis can have an important impact on people quality of life, educational and labor productivity [4]. They can also suffer from emotional problems, such as stress and fatigue, and present sleep disorders according to the severity of rhinitis [4-6]. The majority of patients are diagnosed by a detailed clinical history and clinical examination of the nasal fossa. At this moment the clinical allergic rhinitis score can be used as a capable alternative of classifying AR according to the severity in mild, moderate or severe, based on the frequency and intensity of symptoms and their impact on the quality of life of the patient. The clinical score is useful both in diagnosis and in follow-up of patients with AR optimizing treatment with different treatment proposes for each classification. Nevertheless, clinical evaluation may be insufficient to perform routinely in the followup of these patients [1,7]. Therefore, in some cases the degree of nasal obstructive must be assessed by objective tests in order to monitor the patients and to evaluate treatment response. Examinations such as rhinomanometry, acoustic rhinometry or nasal inspiratory flow peak (NIFP) may help in clinical practice, with the first two being more restricted to research. Among the three methods, the NIFP has become the most attractive because of its simplicity, ease of handling, portability, low cost and capacity provide immediate results. Nonetheless, the definition of normal nasal patency is difficult, since the nose is directly exposed to the external environment and is subjected to temperature and humidity variations, as well as to air pollutants [8]. In addition, the nasal flow undergoes fluctuations once that nasal mucosa veins alternate congestion and decongestion in the so-called nasal cycle. Therefore, the passage of air through the nostrils is usually asymmetric. Other factors that influence the measurement of the nasal patency, such as sex, age, weight, height and ethnic differences [8]. All this makes it difficult to define reference values for nasal patency through any objective evidence of nasal function.
Studies in children with AR revealed that parents often have a poor perception of their children respiratory problems [3]. Therefore, it is very important to have good subjective methods in which children can see their own illness and that these may have a strong correlation with the disease itself, as well as with its severity.
The visual analogue scale (VAS) is a quantitative measure widely used to aid in the assessment of many diseases [4]. This scale is useful both in assessing the severity of rhinitis and in verifying the effectiveness of the therapeutic intervention. The objective of this article is to present a review of the correlation between the clinical score of allergic rhinitis, peak inspiratory flow rate (PIFR) and visual analogue scale (VAS) from non-systematic research in the Medline database, via Pubmed. In this way, we hope to collaborate with a greater understanding of allergic rhinitis, as well as the improvement in its diagnosis and management.
Definition
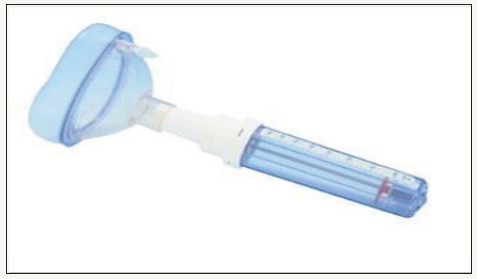
The PIFR measurement is performed by a portable instrument, developed by Youlten in 1980 that has become very attractive for its simplicity [9]. For its execution, the patient is instructed to do a maximum forced inspiration through the nose after proper nasal hygiene that removes any nasal discharge. The mask stays on the patient’s face, covering the entire area of the nose and mouth. For this, the patient should keep his mouth fully closed. This procedure is repeated three times and the greatest measure is adopted (Figure 1).
Figure 1:Peak inspiratory nasal flow meter (NIFP).
Use in Allergic Rhinitis
Although the clinical evaluation of AR patients is sufficient for the classification and treatment of the disease in most cases, it is recommended that, whenever possible, the objective evaluation of nasal patency is used to quantify the degree of nasal obstruction [10]. This measure assists in the diagnosis, follow-up and evaluation of the response to the proposed treatment. It is also very important to perform it in clinical researches when a quantitative study is desired [11]. Nasal obstruction is one of the most uncomfortable symptoms in patients with AR and can be quantified through rhinomanometry, acoustic rhinometry or PIFR. Rhinomanometry is the gold standard test for assessing nasal resistance to airflow [1,2,7]. It is a dynamic measure that measures the relationship between pressure and trans nasal airflow. It is relatively easy to perform, however it should be performed in the laboratory with highly trained personnel. This makes it not very accessible to be done in any office and therefore, it is not practical for the day to day of the doctor. Acoustic rhinometry allows the determination of the cross-sectional area of the nasal cavity at any point in this cavity through the reflection of ultrasound waves [12,13]. Although the technique is simple and depends on minimal collaboration, the measurement of nasal volume requires a complicated mathematical exercise [7]. It is a good exam, but it is more restricted to the research due to its high cost, and the dependence on experienced professionals, appropriate temperature and humidity control.
The equipment consists of sound conductor tube to which a microphone is coupled in the proximal portion of the patient in test and a speaker in the distal portion. Sound waves are generated by the speaker, travel the sound tube, and enter the nasal cavity where the sound energy is reflected to the sound-conducting tube which sends it to a programmed computer for recording and analyzing the data. In this way, for all the aforementioned characteristics its use in the daily clinic becomes non practical. Found a weak but statistically significant correlation of the NIFP with the rhinomanometry (r=0.35; p< 0.01) and a significant correlation was also shown between NIFP and forced inspiratory volume in the first second. In subclinical allergic rhinitis there is a report of poor correlation between the nasal congestion score and rhinomanometry and acoustic rhinometry measurements, which reinforces the need for more instruments such as the NIFP for the evaluation of AR.
Benefits
NIFP test is simple, economical, non-invasive and easy to perform the objective measurement of the nasal obstruction of the patient with AR [14]. This is because it is portable instrument and very simple handling.
Limitations
The result of the measurement of the NIFP can be altered by a deficient motor coordination, poor lung capacity and alar collapse. This last one may occur if the alar nasalis muscles contract during maximal inspiration. In a study of allergic patients, 2% of the PIFR measures were not obtained due to total occlusion of the nose [7]. The NIFP is directly dependent on the nasal patency and lung capacity of each patient. It is also necessary to emphasize that the results of the NIFP rely on the cooperation of the patient and the impression of the examiner who will observe if the patient was capable of performing the maneuver properly [11]. In addition, another limitation of the NIFP is that it evaluates only the nasal obstruction component of AR.
Clinical Scores
Definition
In order to improve AR patient evaluation performance, subjective parameters such as the intensity symptoms and clinical signs of such patients can be adopted to classify the disease. Based on this aspect, we use scales of points for signs and symptoms capable of characterizing the intensity of AR, called clinical scores of AR [15]. Given the importance of allergic rhinitis and its impact on quality of life, the ARIA (Allergic Rhinitis and its impact on asthma) classified it as mild and moderate or severe according to the intensity of the symptoms and in persistent or intermittent according to their duration. This classification aims to assist the diagnosis and establish a specific treatment for each subtype of AR. However, given the great heterogeneity of the group with moderate to severe AR, there is a need for differentiation of this group in patients with moderate or severe AR separately. According to the original ARIA, four items are analyzed in the individuals: sleep; daily activities, sports and leisure; school and work productivity; and bothering symptoms [1]. Allergic rhinitis is considered mild when there is no impairment in any of these items in the patient’s daily life and is moderate/severe when one or more of these items are involved. The modified ARIA suggests that AR is mild when none of the items described above is present, moderate when one, two or three items are found and is severe when four items are involved [16,17].
Valero et al. [18] in 2011 showed that there is a statistically significant difference in the quality of life of adult patients with moderate and severe AR using the modified ARIA. In this same year, Jáuregui et al. [19] studied 1,275 children and verified that the classification of ARIA once made for adults was also valid for children between 6 and 12 years old. Following this line of studies, Montoro et al. [16] in 2012 analyzed 1,269 patients aged 6 to 12 years for the validation of the modified ARIA in children and that this classification was able to discriminate well the moderate AR of severe in these patients. The total six symptom scale (T6SS) is a clinical score that evaluates nasal and extra-nasal symptoms such as sneezing, rhinorrhea, nasal pruritus, nasal congestion, ocular symptoms and pruritus in the ears and / or oropharynx [20]. For each symptom a score is assigned from zero to three, where zero means absence of symptoms and 1, 2 and 3 mean mild, moderate and severe symptoms respectively. In this way, the patient receives a score from zero to 18, which refers to the absence of symptoms in one extreme and the presence of very serious symptomatology at the other extreme. This score is not valid for use as well as no work was found to use it in the pediatrics.
The total four symptom scale (T4SS), is a clinical sub score of the T6SS that evaluates only the four nasal symptoms described in T6SS. After the evaluation, then, for each patient, a score of zero to twelve is obtained representing the AR rating of it. From 0 to 2 it is considered absence of symptoms, 3 to 6 mild AR, 7 to 9 moderate and 10 to 12 severe AR. This score is also not validated, but there are studies in the pediatrics that use it. The symptomatic global score (SGS), evaluates nasal obstruction, rhinorrhea, sneezing, nasal and ocular pruritus [21]. For each symptom, the patient assigns a from zero to four depending on the intensity referred to, where zero is the absence of symptoms, 1 characterized mild symptoms, 2 moderate, 3 severe and 4 very serious. The total is between 0 and 20. AR is considered to be severe when clinical score is higher than 12. As with the previously described scores, this is also not validated for use. No studies were found in pediatrics to use it. Meltzer et al. [15] proposed a clinical score that assesses signs and symptoms of patient’s carriers of AR. Eight items are evaluated: sneezing / pruritus, coryza, nasal obstruction, retro-nasal secretion, turbinate coloration, nasal secretion, edema of turbinates and pharyngeal inflammation. For each of them it is attributed a score of zero to three, as determined signal or symptom is absent or present and from the lowest to the highest intensity, respectively. Patients are classified into four groups according to the sum of points. Group I ranges from 0 to 6, group II from 7 to 12, group III from 13 to 18 and group IV from 19 to 24. The groups reflect in an increasing order the presence of mild to very serious AR. This clinical score is used both in the adult population and in the pediatric population although it has not been validated. Wilson et al. [9] described a system of score in which six signs or symptoms, namely: nasal obstruction, rhinorrhea, sneezing, nasal pruritus, oropharyngeal itching and ocular pruritus. For each of them a note from 0 to 3 is given according to the intensity. Thus, 0 is the absence of any signal or symptom; 1 is the case that the signs or symptoms are mild, well tolerated, without interfere with sleep and the activities of individuals; 2 when they cause discomfort and interfere with activities that demand high concentration; and 3 when they are of intense intensity and uncomfortable, capable of preventing sleep and activities of individuals. The score ranges from 0 to 18, namely: 1 to 6 indicates mild AR, 7 to 12, moderate AR and 13 to 18, severe AR. Like all other scores, this is also not validated. However, it is used in with the pediatric population.
Use in Allergic Rhinitis
These scales were created to minimize variations in clinic evaluations of AR intra and inter patient and even between institutions or protocols of study [22]. The use of clinical scores is useful in clinical practice to diagnosis of AR and follow-up of patients with such disease. They are used to classify allergic rhinitis according to its intensity, which allows a grouping of patients to perform therapeutic different according to the group. It also allows a clinical follow-up of the patients after treatment (Figure 2).
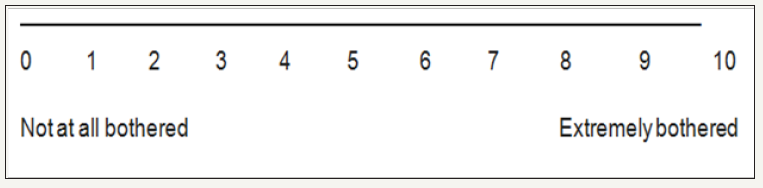
Figure 2:Visual analogue scale.
Benefits
The objective measures of nasal function require the use of specific equipment and its parameters are variable [15]. Subjective assessments such as an adequate clinical examination easily identify AR signs [11]. Clinical scores play an important role because they are easy to perform, depend on minimal patient collaboration and do not generate any liens. Besides, they do not rely on handsets or any other tool, since they use only the knowledge of who executes them.
Limitations
This is a subjective assessment and, therefore, is a dependent examiner. Thus, its reproducibility becomes smaller both intra and inter patient. In mild AR, when there is no doubt about the clinical picture, additional examinations may be waived. However, in the face of a patient with moderate or severe AR, it is recommended that other through further examinations. The existence of distinct clinical scores makes it difficult to standardize the evaluations and a comparison between different clinical research results. None of the scores described is validated for use. Despite this, they are used in several studies with adults, and it has a restrict use in pediatrics.
Visual Analogue Scale (Vas)
Definition
The visual analogue scale is a scale ranging from 0 to 10 where, in the evaluation of AR, 0 indicates absence of nasal symptoms, that is, the patient does not feel any discomfort and 10 indicates extreme nasal discomfort [23]. The scale is not usually used for isolated symptoms such as rhinorrhea, sneezing or pruritus, but for general symptoms. The patient is asked to indicate the number from 0 to 10 that corresponds to the degree of nasal discomfort he feels at that time, as illustrated below.
Use in allergic rhinitis
The VAS was validated initially as a scale for measuring the intensity of pain in cases of experimental and chronic pain. However, it has also been proposed in many other studies in several areas of knowledge. In allergic rhinitis, it is used both in assessing the severity of AR and in effectiveness of the therapeutic intervention. In a study aimed at comparing the visual analog scale with rhinomanometry, Mora et al. [4] found a statistically significant, strong and between the two variables (r=0.879, p< 0.001). We evaluated 50 adult patients before and after turbin ectomy. The same relationship described above was found after surgery, but with moderate correlation (r=0.567; p< 0.001). It is concluded that VAS and rhinomanometry correlate well in nasal diseases and may reflect changes in the nasal mucosa [4,24].
Allergic rhinitis may exacerbate a large number of diseases, including asthma. And since the association of these two diseases is quite common, it is estimated that part of the patients with AR are also carriers of asthma, even if subclinically. According to the ARIA, 10% to 40% of patients with AR have asthma, especially those with persistent AR, moderate / severe 2. In adults, the development of asthma in patients with AR is independent of the history of atopy, whereas in children both are often associated [2]. In this way, to evaluate the bronchus involvement in patients with AR, Ciprandi et al. [24] did a study with 1728 adults with AR aiming at the possibility of using VAS to define patients candidates for spirometry. The conclusion was that VAS values below 3.3 can identify patients with bronchial obstruction [24,25].
Benefits
The visual analogue scale as well as the clinical score is a subjective measure of the patient with RA. It is very simple because it does not require no calculation, easy to perform, does not require an experienced examiner and can be performed in any environment by any professional. It has good correlation with rhinomanometry as described above.
Limitations
Although VAS is easily applicable, no studies that used it in children and adolescents with AR were found.
For VASs intended for children, some differentiations are proposed. Some colorful versions are suggested, with the presence of happy and sad faces [26]. They are used in studies of allergic rhinitis. It is stated that only children over seven years of age are able to complete VASs with precision. The smaller ones extensively use the ends and the middle of the scale. Shields et al. [27], in a study with 40 children, observed that the age above 5.6 years associated with an IQ greater than or equal to 100 are predictors of the ability of the child in using VAS.
Correlation between Nifp, Clinical scores and VAS
Several studies were done aiming the correlation between the clinical score of AR, VAS, NIFP and other instruments associated to the AR assessment. It is important to mention that the visual analogue scale used in all these researches is the one described in the VAS subtitle. Teixeira et al. [28] in a study done with 78 adults, the average value of the NIFP in patients with allergic rhinitis is 114L/min and in non-carriers it is 154.3L/min, with p < 0.001. They realised a significant association between visual analogue scale and NIFP (p=0.002; r=-0.41). Regarding the age, the higher the age is the lower is the expected value for the NIFP. This is due to a probable fall of the nasal tip and narrowing of the nasal valve, both phenomena that occur with advancing age. Wilson et al. [29] in a study of 22 adults to compare NIFP with rhinomanometry and acoustic rhinometry after stimulation with histamine, found that NIFP is more sensitive than the other two in nasal obstruction. They also saw that the NIFP correlates well with the domiciliary symptoms of the patients evaluated by means of the clinical score of Wilson. Similarly, Hellgren et al. [30] observed that in adult patients NIFP is more sensitive in detecting small changes in the thickness of the nasal mucosa than rhinomanometry and acoustic rhinometry. The probable cause of being more sensitive than acoustic rhinometry is that the turbulence generated air flow through the NIFP produces a comparatively greater reduction of flow even with minor changes in the nasal cross-sectional area. As regards rhinomanometry, severe obstructions and excessive nasal secretion causes misinterpretation of results in this examination.
Wilson et al. [9] demonstrated in a study with 38 adults a significant correlation between the nasal symptoms reported by the patients and the NIFP (p< 0.01) measured in the morning (r=-0.51) and at night (r=-0.56). Nasal symptoms were subjectively evaluated through quality of life questionnaires and visual analogue scale. The correlation between NIFP and VAS was significant (p< 0.01) in both NIFP measured in the morning (r=-0.42) and measured at night (r=-0.48). Therefore, it is concluded that the NIFP can be useful in assessing response to treatments. Bousquet et al. [31] evaluated the usefulness of VAS in 3052 adults with AR and found that the severity of the symptoms of allergic rhinitis has more impact in VAS than the duration of the disease. In this study, they compared VAS and a quality of life questionnaire and found a significant correlation between the two (r=0.46, p< 0.0001) [23].
Bousquet et al. [31] in another study that compared the visual analog scale with the clinical score of AR (T4SS) and quality of life questionnaires (RQLQ) in 586 adult patients, found a significant (p< 0.001), but poor correlation between the three variables studied in paired form (RQLQ vs VAS r = 0.36; RQLQ vs T4SS r=0.40; T4SS vs VAS r=0.45). Rouve et al. [21] aiming also to compare the VAS with AR clinical score, did a study with 35,126 adults with uncomplicated and treated AR. A positive correlation was found between the two variables (r=0.4895; p< 0.001). The prevalence of severe allergic rhinitis was higher when using VAS. They concluded that VAS, that does not require any calculation, can represent the severity of RA through the perception of the patient as well as other criteria used, in this case the clinical score.
Table 1:Studies correlating NIFP, AR clinical score, VAS, quality of life questionnaire (RQLQ), rhinomanometry and acoustic rhinometry.

Note: *NIFP: Nasal Inspiratory Flow Pick; **RQLQ: Quality of Life Questionnaire; ***VAS: Visual Analogue Scale
In a study with 30 adult patients, Serrano et al. studied the presence of nasal and bronchial inflammation through noninvasive methods after allergic stimulation of these patients. The presence of nasal symptoms was assessed using the VAS and the clinical AR score (it was not mentioned which score was used). The result was a positive correlation of both variables that addition after 15 minutes, 2 and 24 hours after the stimulus [32]. All studies described above were performed in adults. It was identified only one study that evaluated the pediatric population. Aiming to verify if there is correlation between NIFP and AR clinical score, Gomes et al. [10] did a study with 52 children between 6 and 16 years. These children received fluticasone propionate and were followed up for eight weeks through biweekly evaluations. NIFP measurements and AR clinical score were made in each of the evaluations. It was observed that while reduction in the AR clinical score, there was an increase in the value of absolute NIFP in the eight weeks of study, that is, an inverse correlation moderate and statistically significant between NIFP and AR clinical score (r=-0.44, p< 0.001). This demonstrates that the NIFP does not replace the clinical score of AR but complements it in the evaluation of patients with AR 10 (Table 1).
Finally, another aspect that should be considered in pediatrics is from what age the procedure for obtaining NIFP can be performed correctly. In response to this questioning, JGCM et al. [33] did a study with children aged 4 to 13 years with the objective of evaluating whether they are capable and from what age this occurs. Measurements of NIFP were done at a first visit in the office and for one week in the patient residence twice a day. The results were written by the parents in a provided diary to each child. After a week they returned to the office where a new NIFP measure was made. The researchers found that after six years of age children have the ability to make a flow nasal airflow to generate a reliable NIFP value. There was no statistically significant difference between the NIFP measurement before and after one week in children after six years of age 33. These different results allow us to conclude that the evaluation of allergic rhinitis is not as simple and obvious as it may seem. It is necessary to use several means of evaluation to classify AR more trustworthy. It should also be mentioned in the majority of these studies, it was evaluated mostly or only a single aspect of allergic rhinitis, which is nasal patency. Nonetheless, AR is a disease that occurs with many others signs and symptoms in addition to nasal obstruction.
Conclusion
AR is a disease of high prevalence in Brazil. The clinical diagnosis is sufficient in many cases, however it is recommended to use instruments able to objectively and subjectively measure nasal patency for better classification and treatment of the disease. The NIFP is a simple instrument capable of objectively measuring obstruction nasal. It has a good correlation with rhinomanometry, acoustic rhinometry and scale visual analog. The maneuver for its use is considered satisfactory in patients over six years of age. The visual analogue scale has a high correlation with the clinical rhinitis score allergy and rhinomanometry. Therefore, it is a great and simple subjective measure of nasal obstruction capable of assisting in the diagnosis and treatment of patients.
Although several studies correlate these methods, there is a shortage of work in the pediatric population. In this way, further studies are needed to improve understanding and standardization of the use of VAS and NIFP in this population. Associating the NIFP with others instruments such as VAS and AR clinical score, one can have a good evaluation of patients with AR. Given the simplicity of the measures and subjectivity of information obtained from children and adolescents, it is concluded that this is a very important way to be followed in order to contribute significantly to the development of the study of allergic rhinitis and improve management in pediatric patients.
References
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. (2008) Allergic rhinitis and its impact on asthma (ARIA) 2008 Update (in collaboration with the World Health Organization, GA2LEN and Aller Gen). Allergy 63(86): 8-160.
- Bousquet J, Schünemann HJ, Samolinski B, Demoly P, Cagnani CE, et al. (2012) Allergic rhinitis and its impact on asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol 130(5): 1049-1062.
- Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR, et al. (1998) Measuring quality of life in children with rhino conjunctivitis. The journal of allergy and clinical immunology 101(21): 163-170.
- Mora F, Cassano M, Mora R, Gallina AM, Ciprandi G, et al. (2009) V.A.S. in the follow-up of turbinectomy. Rhinology 47(4): 450-453.
- Léger D, Annesi MI, Carat F, Rugina M, Chanal I, et al. (2006) Allergic Rhinitis and Its consequences on quality of sleep. Arch Intern Med 166(16): 1744-1748.
- Park CE, Shin SY, Lee KH, Cho JS, Kim SW (2012) The effect of allergic rhinitis on the degree of stress, fatigue and quality of life in OSA patients. Eur Arch Otorhinolaryngol 269(9): 2061-2064.
- Nathan RA, Eccles R, Howarth PH, Steinsvag SK, Togias A (2005) Objective monitoring of nasal patency and nasal physiology in rhinitis. J Allergy Clin Immunol 115(3): 442-459.
- Moore M, Eccles R (2012) Normal nasal patency: problems in obtaining standard reference values for the surgeon. J Laryngol Otol 126(6): 563- 569.
- Wilson A, Dempsey OJ, Sims EJ, Coutie WJR, Paterson MC, et al. (2000) Evaluation of treatment response in patients with seasonal allergic rhinitis using domiciliary nasal peak inspiratory flow. Clin Exp Allergy 30(6): 833-838.
- Gomes DL, Camargos PAM, Ibiapina CC, Andrade CR (2008) Nasal peak inspiratory flow and clinical score in children and adolescents with allergic rhinitis. Rhinology 46(4): 276-280.
- Ibiapina CC, Sarinho ESC, Camargos PAM, Andrade CR, Filho AASC (2008) Allergic rhinitis: epidemiological, diagnostic and therapeutic aspects. J Bras Pneumol 34(4): 230-240.
- Koo NG NKF, Young D, Mc Garry GW (2012) Reversible nasal airway obstruction: does change in nasal peak inspiratory flow following descongestion predict response to topical steroids in chronic rhinosinusitis patients? J Laryngol Otol 126(2): 1238-1240
- Clement PAR, Gordts F (2005) Consensus report on acoustic rhinometry and rhinomanometry. Rhinology 43(3): 169-179.
- Ottaviano G, Scadding GK, Coles S, Lund VJ (2006) Peak nasal inspiratory flow; normal range in adult population. Rhinology 44(1): 32-35.
- Júnior JFM, Castro FFM (1997) Allergic Rhinitis: Initial Approach. International archives of Otorhinolaryngology 1(2): 1-10.
- Montoro J, Cuvillo AD, Mullol J, Molina X, Bartra J, et al. (2012) Validation of the modified allergic rhinitis andits impact on asthma (ARIA) severity classification in allergic rhinitis children: the Pedrial study. Allergy 67(11): 1437-1442.
- Valero A, Izquierdo I, Sastre J, Navarro AM, Baró E, et al. (2013) ESPRINT-15 Questionnaire (Spanish Version): Reference Values According to Disease Severity Using Both the Original and the Modified ARIA Classifications. J Investig Allergol Clin Immunol 23(1): 14-19.
- Valero A, Muñoz Cano R, Sastre J, Navarro AM, Martí Guadaño E, et al. (2012) The impact of allergic rhinitis on symptoms, and quality of life using the new criterion of ARIA severity classification. Rhinology 50(1): 33-36.
- Jáuregui I, Dávila I, Sastre J, Bartra J, Cuvillo AD, et al. (2011) Validation of ARIA (Allergic Rhinitis and its Impacto n Asthma) classification in a pediatric population: The Pedrial study. Pediatr Allergy Immunol 22(4): 388-392.
- Bousquet PJ, Demoly P, Devillier P, Mesbah K, Bousquet J (2013) Impact of allergic rhinitis symptoms on quality of life in primary care. Int Arch Allergy Immunol 160(4): 393-400.
- Rouve S, Didier A, Demoly P, Jankowski R, Klossek JM, et al. (2010) Numeric score and visual analog scale in assessing seasonal allergic rhinitis severity. Rhinology 48(3): 285-291.
- La Banca RO, Corti ACR, Camelo NIC, Mallozi MC, Solé D (2011) Index of nasal congestion (CQ-7) in the assessment of nasal obstruction in adolescents with allergic rhinitis. Rev Bras Alerg Imunopatol 34 (1): 19- 22.
- Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Méchin H, et al. (2007) Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy 62(4): 367-372.
- Ciprandi G, Mora F, Cassano M, Gallina AM, Mora R (2009) Visual analog scale (VAS) and nasal obstruction in persistent allergic rhinitis. Otolaryngology Head and Neck Surgery 141(4): 527-529.
- Ciprandi G, Tosca MA, Signori A, Cirillo I (2011) Visual analogue scale assessment of nasal obstruction might define patients candidates to spirometry. Rhinology 49(3): 292-296.
- Prittis KN, Papadimitriou N, Anthracopoulos MB (2012) Should we evaluate objectively the nasal obstruction in children with chronic rhinitis? Jornal de Pediatria 88(5): 389-395.
- Shields BJ, Palermo TM, Powers JD, Grewe SD, Smith GA (2003) Predictors of a child’s ability to use a visual analogue scale. Child Care Health Dev 29(4): 281-290.
- Teixeira RUF, Zappelini CEM, Alves FS, Costa EA (2011) Peak nasal inspiratory flow evaluation as na objective method of measuring nasal airflow. Braz J Otorhinolaryngol 77(4): 473-480.
- Wilson AM, Sims EJ, Robb F, Cockburn W, Lipworth BJ (2003) Peak inspiratory flow rate is more sensitive than acoustic rhinometry or rhinomanometry in detecting corticosteroid response with nasal histamine challenge. Rhinology 41(1): 16-20.
- Hellgren J, Jarlstedt J, Dimberg L, Torén K, Karlsson G (1997) A study of some current methods for assessment of nasal histamine reactivity. Clin Otolaryngol 22(6): 536-541.
- Bousquet PJ, Combescure C, Klossek JM, Daures JP, Bousquet J (2009) Chenge in visual analog scale score in a pragmatic randomized cluster trial of allergic rhinitis. J Allergy Clin Immunol 123(6): 1349-1354.
- Serrano CD, Valero A, Bartra J, Roca Ferrer J, Muñoz Cano R, et al. (2012) Nasal and bronchial inflammation after nasal allergen challenge: assessment using noninvasive methods. J Investig Allergol Clin Immunol 22(5): 351-356.
- van Zoest JGCM, van der Weij AM, Duiverman EJ, Akerlund A, Kouwenberg JM (2000) Nasal peak inspiratory flow through Turbuhaler in children with symptomatic rhinitis and in healthy children. Pediatric Allergy and Immunology 11(4): 256-259.
© 2018 Ana Carolina Gonçalves Ribeiro de Carvalho. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






