- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Neuropsychological Interviews: A Strategic Tool for Alzheimer’s Disease Initial Cognitive Impairment Identification and Patient Empowerment: A Case Study
Letteria Tomasello1,2*, Claudio Zaccone1 and Santi Galletta3
1Department of Clinical and Experimental Medicine, University of Messina, Italy
2Faculty of Medicine and Dentistry, Sapienza University of Rome, Italy
3Neurology and Neurorehabilitation Department, Neuchâtel Hospital Network (RHNe), Switzerland
*Corresponding author:Letteria Tomasello, Department of Clinical and Experimental Medicine, University of Messina, Faculty of Medicine and Dentistry, Sapienza University of Rome, Italy
Submission: September 11, 2023; Published: October 02, 2023

ISSN 2637-7934 Volume4 Issue4
Abstract
Alzheimer’s Disease (AD) typically presents as declarative memory deficits, slow progressive temporal disorientation, executive and speech impairment [1]. This association is collectively referred to as “amnestic-dysexecutive syndrome”. The diagnostic process for AD relies heavily on clinical examination and neuropsychological interviews. These initial evaluations, aimed at identifying cognitive deficits, are crucial and often prove invaluable in establishing a clear trajectory for diagnosis, without delay. As demonstrated in the following case report, neuropsychological interviews not only serve as an essential first step in the diagnostic process but also empower patients with knowledge about their cognitive abilities. This approach provides patients with necessary insights for making informed social choices, anticipating future arrangements, and even writing advanced directives. By focusing on patients’ cognitive capacities rather than the pathological processes affecting them, this ethically sound approach significantly enhances their quality of life [2,3].
Keywords:Mild cognitive impairment; Alzheimer’s disease; Neuropsychological examination.
Introduction
F.T., a 63-year-old man holding an executive position, accompanied by his wife, consulted our dementia clinic due to his declining interest in daily activities and social withdrawal for the past three years. His only outings are for work. His wife also notes unusual memory lapses without temporal or spatial disorientation. The patient reports feeling fatigued and stressed by his work, which he still regularly performs, and denies having memory or attention disorders. He is a non- smoker and does not consume alcohol or use substances. His only medication is sertraline, which has had no beneficial effects on his neuropsychological symptoms (Table 1).
Table 1:Initial neuropsychological interview.
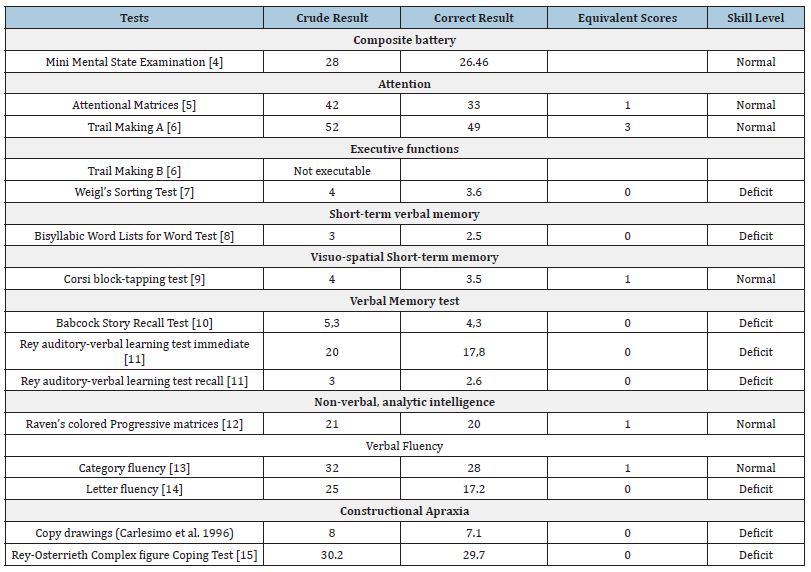
The patient was alert, cooperative, and well oriented in time, space, and personally. His speech was fluent and correct, without any anomias. His interaction with the examiner was appropriate and he demonstrated good insight. His Mini Mental State Examination (MMSE) test score was 26.46, within the normal range. His Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scores were also normal. The interview then focused on individual cognitive functions, revealing deficits in short-term, long-term, and verbal memory, phonemic verbal fluency, and constructive apraxia. Specifically, during the Trail Making Test B (TMT-B), the patient struggles to complete the task, unable to manage the attention shift, leading to task discontinuation. His processing speed is notably slow, and cognitive hesitation is present.
Based on the neuropsychological interview, we initiated further instrumental investigation. Routine laboratory exams are within normal limits, except for a slight elevation in cholesterol. Electroencephalogram (EEG) shows an alpha-activity of 8Hz of mean amplitude, well- modulated and reactive in post-rolandic regions. Fast and hypovoltated activities are observable in fronto-temporal regions. Brain electrical activity is mostly within range. Brain MRI with 1.5 Tesla and Angio-MRI sequences reveal minor white matter hyperintensities on T2 and Fluid Attenuated Inversion Recovery (FLAIR) detectable in periventricular regions, nonspecific signs of gliosis; minimal and nonspecific signs of atrophy are detectable in frontal and temporal lobes. Brain Positron Emission Tomography (PET) images, acquired 90 minutes after the administration of Florbetapir (18F) a tracer for β-amyloid, reveal a widespread elevated uptake in both hemispheric cortices. Significant loss of differentiation between grey and white matter in the temporal lobes is apparent. The parietal and frontal lobes bilaterally do not show a normal distribution of the radiopharmaceutical tracer. We also detected an abnormal accumulation of the radiopharmaceutical tracer in the posterior cingulum. Combining the patient’s history, neuroimaging results, and cognitive test findings satisfies the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association Alzheimer’s (NINCDS-ADRDA) criteria for probable Alzheimer’s disease (Table 2).
Table 2:Final neuropsychological interview.
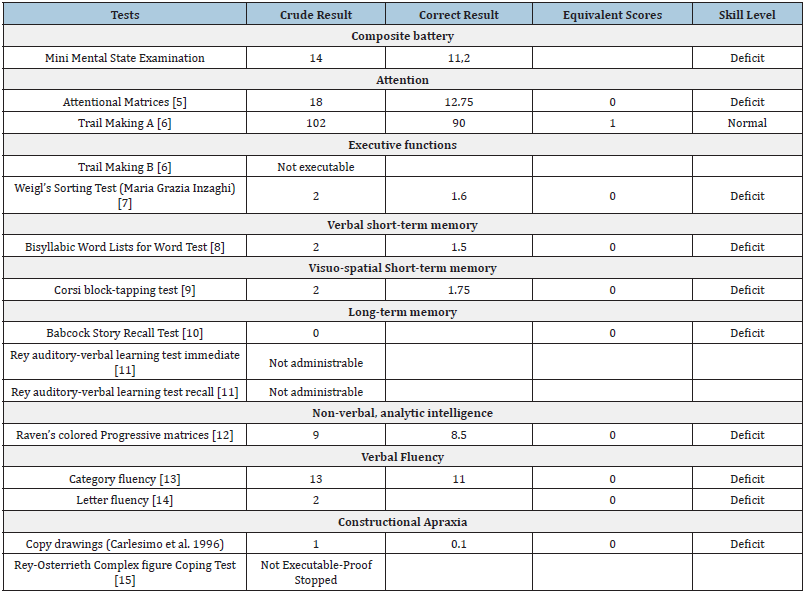
Two years later, he returned for a follow-up neuropsychological interview after having started a therapy with Donepezil and Memantine, with dosages gradually increased over the years. His wife reports worsening memory deficits for recent events, topographic disorientation, decreased attention to personal hygiene, and impairment of instrumental daily activities. The examination now reveals the patient’s disorientation in time and space, although he remains cooperative and motivated throughout, with visible signs of cognitive hesitation. Expressive language is limited and anomias are now present during naming tasks. The patient exhibits ideomotor slowdown, compromised judgment ability, and poor insight at the emotional-behavioural level. A severe decrease was observed in the patient’s ADL and IADL scores. There was further impairment in attention and memory functions (both short and long-term). Selective and divided attention components was defective. Difficulties in organizing, planning, and integrating visuo-spatial stimuli are evident, along with severe constructive apraxic deficits. He exhibited impaired access to the semantic lexicon, and his logical-deductive reasoning was significantly compromised. Trail Making Test B and Weigl’s Test are now not administrable. The patient struggles to understand instructions despite repeated explanation and exhibits difficulties in storing and retrieving contextual information. Furthermore, his information processing speed was extremely low. Difficulties in recalling memories from distant episodes and daily events are prominent, inhibiting their use in various situations. The diagnosis of AD dementia was clinically confirmed. The healthcare team advised the patient to stop driving and recommended him to consider early retirement.
Discussion and Conclusion
Even though diagnosis of dementia has become more efficient and precise in the recent past years [2], there is still stigma and lack of dementia awareness that poses significant barriers to accurate diagnosis. It is worth noting that even subclinical alterations in mood and anxiety, often classified as “Mild Behavioural Impairment” (MBI), should be considered during initial evaluations as they can present earlier than memory disorders associated with AD or even neuroimaging markers [3].
This case report demonstrates how the integration of clinical history and supplementary neuropsychological tests, which are cost-effective and reproducible, facilitated a swift initiation of the specific diagnostic process. Not only does the neuropsychological examination provide insight into the patient’s cognitive performance, empowering him/her to make informed social choices and anticipate future arrangements, but it also equips him/ her with the necessary knowledge to write advanced directives. This approach is ethically sound as it focuses on informing the patient about his/her cognitive capabilities rather than emphasizing the pathological processes affecting them, ultimately leading to an improved quality of life for the patient and their caregiver.
References
- Vreese LP, Neri M, Fioravanti M, Belloi L, Zanetti O (2001) Memory rehabilitation in Alzheimer's disease: A review of progress. Int J Geriatr Psychiatry 16(8): 794-809.
- Howard R, Schott JM (2021) When dementia is misdiagnosed. Int J Geriatr Psychiatry 36(6): 799-801.
- Ismail Z, Agüera OL, Brodaty H, Cieslak A, Cummings J, et al. (2017) NPS Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA of ISTAART). The Mild Behavioral Impairment Checklist (MBI-C): A Rating scale for neuropsychiatric symptoms in Pre-Dementia Populations. J Alzheimers Dis 56(3): 929-938.
- Measso G, Cavarzeran F, Zappalà G, Lebowitz BD, Crook TH, et al. (1993) The mini-mental state examination: Normative study of an Italian random sample. Dev Neuropsyc 9(2): 77-85.
- Spinnler H, Tognoni G (1987) Italian standardization and classification of Neuropsychological tests. Italian Journal of Neurological Sciences 6(8): 47-50.
- Giovagnoli AR, Pesce M, Mascheroni S, Simoncelli M, Lacaiona M, et al. (1996) Trail making test: Normative values from 287 normal adult controls. The Italian Journal of Neurological Sciences 17(4): 305-309.
- Hobson P, Meara J, Taylor C (2010) The weigl colour-form sorting test: A quick and easily administered bedside screen for dementia and executive dysfunction. Int J Geriatr Psychiatry 22(9): 909-915.
- Spinnler H, Tognoni G (1987) Italian standardization and classification of Neuropsychological tests. 6(8): 23-24.
- Orsini A, Grossi D, Laiacona MCE, Papagno C, Vallar G (1987) Verbal and spatial immediate memory span: Normative data from 1355 adults ans 1112 children. The Italian Journal of Neurological Sciences 8(6): 539-548.
- Spinnler H, Tognoni G (1987) Italian standardization and classification of Neuropsychological tests Italian. Journal of Neurological Sciences 6(8): 44-46.
- Carlesimo GA, Caltagirone C, Fadda L, Marfia G, Gainotti G and the Group for the standardization of the mental deterioration battery (1995) Qualitative cognitive impairments. Archive of Psychology, Neurology and Psychiatry 56(4): 489-502.
- Basso A, Capitani E, Laiacona M (1987) Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Functional Neurology 2(2): 189-194.
- Novelli G, Papagno C, Capitani E, Laiacona M, Vallar G, et al. (1986) Three clinical tests of research and lexical production. Calibration on normal subjects. Archives of Psychology Neurology and Psychiatry 4(47): 477-506.
- Caltagirone C, Gainotti G, Carlesimo GA, Parnetti L (1995) The difference between the standard group and the standardization of the mental deterioration battery Part I neuropsychological diagnostic description. Archives of Psychology, Neurology and Psychiatry 56(4): 461-470.
- Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey-osterrieth complex figure: Normative values in an Italian population sample. Neurological Sciences 22(6): 443-447.
© 2023 Letteria Tomasello. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






