- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
A Good Therapeutic Approach for Patients with Paraparesis due to Spinal Arteriovenous Fistula
Sebastian Ștefan1*, Cosmin Argintaru1, Athena Ribigan1, Andreea Marinescu3, Bogdan Dorobat2 and Florina Antochi1
1Department of Neurology, Bucharest Emergency University Hospital, Romania
2Department of Interventional Neuroradiology, Bucharest Emergency University Hospital, Romania
3Department of Radiology, Bucharest Emergency University Hospital, Romania
*Corresponding author:Sebastian Ștefan, Department of Neurology, Bucharest Emergency University Hospital, Bucharest, Romania
Submission: July 24, 2023; Published: August 24, 2023

ISSN 2637-7934 Volume4 Issue3
Abstract
Introduction: Although Spinal Dural Arteriovenous Fistula (SDAVF) is the most common arterio-venous
malformation of the spinal cord, it is an extremely rare pathology. It is usually an acquired condition
that may cause congestive myelopathy through chronic venous hypertension. The clinical picture is
non-specific but the diagnosis can be inferred from Magnetic Resonance Imaging (MRI) and confirmed
through Digital Subtraction Angiography (DSA).
Case Presentation: We report four cases that presented in our clinic in the past twelve months and in
which SDAVF was suspected based on spinal MRI. The mean age was 54. Three of them presented with
severe motor deficits and two of them had sphincteric disturbances. Sensory deficits varied. Three of
the patients had a subacute progression, while one of them evolved rather acutely. The diagnosis was
confirmed through DSA with selective catheterization and endovascular occlusion of the feeding artery
by injection of liquid embolic material was performed with no periprocedural incidents. There was no
further progression of deficits and three of the patients presented partial improvement by the time of
discharge.
Discussion: One of the main challenges posed by these patients is correctly identifying the feeding artery
of the SDAVF, which may be achieved by spinal Magnetic Resonance Angiography (MRA) and elaborate
DSA. Another aspect relates to the recovery of neurologic deficits which includes motor rehabilitation
programmes, as well as management of associated symptoms and pathologies.
Conclusion: It is our experience that shunt obliteration should be always attempted as SDAVF is a rare
but recuperable cause of severe neurologic deficits.
Keywords:Spinal dural arteriovenous fistula; Paraparesis; Paraplegia; MRI; DSA; Embolization; Neuroradiology; Rehabilitation
Abbreviations:DSA=Digital Subtraction Angiography; MRA=Magnetic Resonance Angiography; MRI=Magnetic Resonance Imaging; MRC=Medical Research Council; SDAVF=Spinal Dural Arteriovenous Fistula
Introduction
Although Spinal Dural Arteriovenous Fistula (SDAVF) is the most common arteriovenous malformation of the spinal cord, accounting for ~70% of cases [1], it is an extremely rare pathology [2]. There is no precise information on the incidence and prevalence of this condition, although it is recognized that it predominantly affects males and typically manifests in the sixth decade of life [2,3]. First described by Foix and Alajouanine in 1926, SDAVF is classically defined as an arteriovenous shunt between a radiculo meningeal artery and a radicular vein, leading to increased pressure in the perimedullary venous plexus consequently causing congestive myelopathy due to chronic medullary venous hypertension [4]. Unlike other spinal vascular malformations, SDAVFs are most often acquired, and the most frequent pathogenic mechanisms involved in their development are local venous thrombosis and trauma [4]. Although the clinical picture is nonspecific, the diagnosis is supported by spinal cord imaging – mainly Magnetic Resonance Imaging (MRI) and confirmed interventionally by Digital Subtraction Angiography (DSA) [5].
We report four cases - two women aged 50 and 63 years-old respectively, and two men aged 44 and 59 years-old, who were admitted in different services before presenting in our clinic.
Case Presentation
Case 1
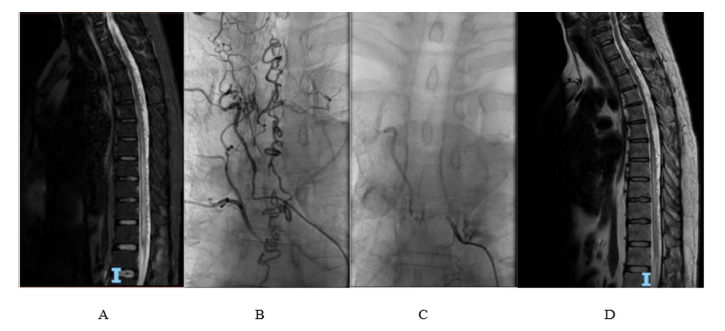
A 44-year-old male patient presents with spastic paraplegia and asymmetric sensory disturbance involving proprioception and vibratory sense in the lower limbs (more pronounced on the left side). Additionally, he experiences sphincteric disturbances consisting of neurogenic bladder and chronic constipation. The condition started nine months prior to presentation when the patient experienced low back pain radiating to the left buttock and the posterior aspect of the left thigh. The motor deficit initially developed in the left leg one month prior to presentation in the context of an infectious episode following COVID-19 contact; there was no other past medical or family history. Spinal MRI depicted serpiginous flow-voids with gadolinium enhancement, located predominantly posterior in the epidural space of the inferior cervical, thoracic and superior lumbar spinal canal (Figure 1A). DSA is performed three times, the third confirming the presence of SDAVF with significant dilatation of the perimedullary venous plexus after selective catheterization of the right T6 intercostal artery (Figure 1B).
Embolization of the fistula using Phil 25% material is performed with subsequent favorable evolution, including significant improvement in motor deficit (Figure 1C). At discharge, the patient is able to walk with bilateral support, has a bilateral lower limb muscle strength of 3/5 according to Medical Research Council (MRC), and shows improvement in sensory deficits. On follow-up, nine months later, the patient presents slightly improved muscle strength and walks unassisted with crutches providing bilateral support (Figure 1D). Notably, in this case, there was a relatively rapid progression of symptoms following suspected SARS-CoV-2 infection.
Figure 1:A. Spinal MRI: T2 hyperintensity of the spinal cord from the level of T7 vertebra to the conus medullaris, as well as perimedullary “flow-voids” from the inferior cervical to the superior lumbar spinal canal B. DSA: significant dilation of perimedullary veins following selective injection of the feeding artery originating in the right T6 intercostal artery. C. DSA: no dilated veins are visible following embolization of the feeding artery. D. Follow-up MRI (nine months later): slight reduction in the size of congestive myelopathy, as well as in the number of perimedullary “flow voids”.
Case 2
A 63-year-old female patient with a known history of back injury five years prior and type II diabetes mellitus under oral antidiabetic medication, presents with symptoms that started in the past two years and have progressed gradually. The clinical examination reveals asymmetric spastic paraparesis with muscle strength of 3/5 according to MRC scale in the left lower limb and 4/5 in the right lower limb, tactile hypoesthesia in the left lower limb without impairment of deep sensation, and overactive bladder with urgency, frequency and urge incontinence. MRI examination demonstrates T2/STIR hyperintensity at the level of T9-T12 vertebrae, and multiple serpiginous epidural flow-voids suggestive of congestive myelopathy due to venous hypertension (Figure 2A). Furthermore, multiple disc protrusions and disc herniations are identified at the cervical-thoracic level. Spinal MR angiography (MRA) reveals vascular dilation with early filling around the left L5 nerve root (Figure 2B). As a result, selective injection of the branches of the internal iliac arteries is performed, identifying an arterial feeder originating from the right iliolumbar artery and slow drainage into a tortuous vein located in the spinal canal (Figure 2C). Embolization of the feeding artery is carried out without procedural complications (Figure 2D), resulting in partial improvement of symptoms upon discharge.
Figure 2:A. Spinal MRI: T2 high signal enlargement of the spinal cord at the level of T9-T12 vertebrae, as well as perimedullary “flow-voids”. B. Spinal MRA: vascular dilation with early filling around left L5 nerve root. C. DSA: arterial feeder originating in the right iliolumbar vein with slow drainage into a tortuous vein located in the spinal canal. D. DSA: embolic material inside the feeding artery.
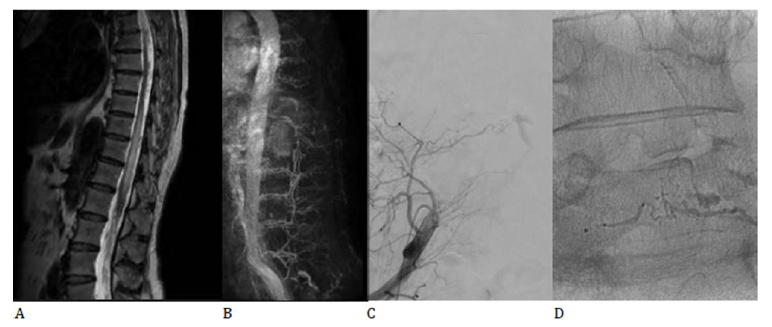
The peculiarities of this case lie in the unusual location of the feeding artery (where angio-MRI sequences proved essential in guiding standard angiography) and the associated pathologies, including diabetes mellitus and multiple disc pathologies, which raise issues regarding the differential diagnosis and prognosis for recovery.
Case 3
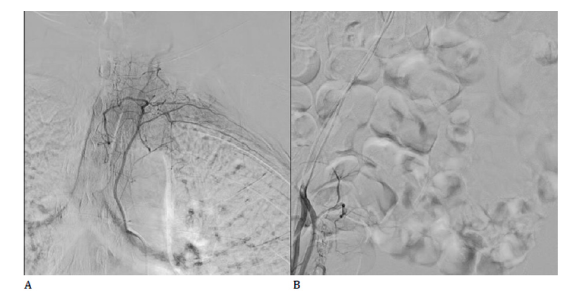
A 50-year-old female patient with a history of surgical procedures on the right knee, hypertension, smoking, dyslipidemia, and no prior treatment, presents with symptoms that started approximatively seven years prior and had slow progression. The neurologic exam revealed asymmetric spastic paraparesis – right crural muscle strength of 0/5 and left crural muscle strength of 3/5 according to MRC scale; tactile hypoesthesia at the L1 level, along with bilateral distal crural paresthesia and abolished proprioceptive sensation in both lower limbs. The diagnosis was supported by a previous spinal MRI which was performed five years prior, but the patient refused any further interventional investigation at that time. During spinal catheter angiography performed in our clinic, selective injection of the branches of the internal iliac arteries identifies an arterial branch originating in the left lateral sacral artery and causing early filling of a radicular vein (Figure 3A). Occlusion of the fistulous tract is performed using Phil 30% embolic material (Figure 3B). Besides yet another case of arterial feeder originating in the internal iliac artery branches, the particularity of this case lies in the prolonged evolution of the condition, as well as in the presence of multiple uncontrolled vascular risk factors that require aggressive management in order to prevent cardiac, cerebrovascular or peripheral artery disease that may pose challenges regarding the prospects of subsequent motor recovery.
Figure 3:A. DSA: Arterial feeder originating in the left lateral sacral artery with drainage in a tortuous vein. B. DSA: Post-embolization aspect with no drainage vein.
Case 4
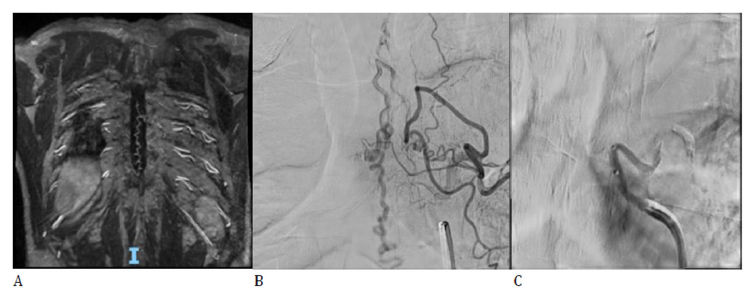
A 59-year-old male patient, known with a history of arterial hypertension and dyslipidemia, no prior treatment, presents with spastic paraplegia, with no associated sensory deficits or sphincteric disturbances, symptomatology progressing in the last two years. Spinal MRI revealed intramedullary hyperintensity at the level of T8-L1 vertebrae and conus medullaris associated with multiple serpentine extramedullary vessels on the posterior aspect of the spinal cord at the level of T5-L2 vertebrae. Spinal MRA was also performed showing dilated vein inside the spinal canal with possible arterial feeder at the level of left T6 spinal artery (Figure 4A). DSA with selective catheterization permitted precise identification of an arterial feeder originating in the left T6 spinal artery (Figure 4B) and endovascular occlusion with Phil 25% embolic material was further performed with no periprocedural incidents (Figure 4C). The clinical evolution was favorable with slight recovery of motor deficits upon discharge.
Figure 4:A. Spinal MRA: Feeding artery is suggested at the level of T6 intercostal artery. B. DSA: identification of the arterial feeder originating in the left T6 spinal artery. C. DSA: post-embolization aspect with no drainage vein.
Discussion
One of the main challenges posed by these patients is correctly identifying the feeding artery through DSA for subsequent exclusion (endovascular or surgical). In our experience, this can be achieved by MRA which may provide clues about the location of the fistula and by elaborate DSA which may require injection of arteries as far as internal iliac branches. Even cases with feeder originating in more direct aortic branches may require repeating the DSA. As such, the diagnosis of SDAVF is easily overlooked [6] and delayed, even when DSA is performed by an experienced neuroradiologist. Another important aspect relates to the recovery of neurologic deficits. Patient perseverance in attending motor rehabilitation programs, as well as careful management of associated symptoms and pathologies are key factors in patient recovery.
Conclusion
Patients with SDAVF usually present with slowly progressive non-specific neurologic symptoms suggestive of extensive spinal cord lesion, but 5-15% of the cases may experience acute exacerbations. Spinal MRI should be the primary imaging investigation and MRA should also be performed to guide further DSA. It is our strong opinion that regardless of the onset and severity of neurologic symptoms, the complete obliteration of the shunt should be attempted to prevent deficit progression and even reduce disability, as SDAVF is a rare but recuperable cause of severe neurologic deficits.
References
- Maimon S, Luckman Y, Strauss I (2016) Spinal dural arteriovenous fistula: A review. Adv Tech Stand Neurosurg (43): 111-137.
- Rosenblum B, Oldfield EH, Doppman JL, Chiro GD (1987) Spinal arteriovenous malformations: A comparison of dural arteriovenous fistulas and intradural AVM's in 81 patients. J Neurosurg 67(6): 795-802.
- Jellema K, Tijssen CC, Gijn J (2006) Spinal dural arteriovenous fistulas: A congestive myelopathy that initially mimics a peripheral nerve disorder. Brain 129(Pt 12): 3150-3164.
- Sim SY (2022) Pathophysiology and classification of intracranial and spinal dural AVF. J Cerebrovasc Endovasc Neurosurg 24(3): 203-209.
- Krings T, Lasjaunias PL, Hans FJ, Mull M, Nijenhuis RJ, et al. (2007) Imaging in spinal vascular disease. Neuroimaging Clin N Am 17(1): 57-72.
- Jonathan M (2012) Morris, imaging of dural arteriovenous fistula. Radiologic Clinics of North America 50(4): 823-839.
© 2023 Sebastian Ștefan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






