- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Ultrasound-Guided Pulsed Radiofrequency Neuromodulation of Scapular Dorsal and Spinal Accessory Nerves: Report of a Chronic Pain Clinical Case
Ana João Costa1*, Adriana Pascoal2, Luís Oliveira1, Eugénio Gonçalves3, André Borges1, Mariana Martins4, Tiago Lopes1 and José Luís Carvalho1
1North Rehabilitation Center-Hospital Center Vila Nova de Gaia/ Espinho, Portugal
2Regional Rehabilitation Medicine Center-Rovisco Pais, Portugal
3Hospital Center Vila Nova de Gaia/ Espinho, Portugal
4University Hospital Center from Lisbon Central, Portugal
*Corresponding author:Ana João Costa, North Rehabilitation Center-Hospital Center Vila Nova de Gaia/ Espinho, Avenida Infante de Sagres 349. 4405-565 Vila Nova de Gaia, Portugal
Submission: January 23, 2023; Published: February 03, 2023

ISSN 2637-7934 Volume4 Issue1
Abstract
Introduction: Pulsed radiofrequency is emerging as a valid therapeutic option in chronic pain approach.
The addition of ultrasound guidance allows the precise identification of nerve structures, that otherwise
could not be easily approached.
Case report: A 49-year-old woman with severe postoperative located nociceptive and neuropathic
pain after undergoing right partial scapulectomy for osteosarcoma of the scapula. For several years, the
patient had been treated with a combination of a conservative rehabilitation program, oral and topical
analgesics, and interventional techniques, with minimal results, baseline VNS of 8/10. After a successful
dorsal scapular and spinal accessory block under ultrasound guidance, she underwent PRF regarding
upper trapezius myofascial pain syndrome and dorsal neuropathic pain. Six months after PRF, there was
a 50% reduction of pain, and the procedure was repeated for a long-lasting effect.
Conclusion: Postoperative nerve and muscle structural changes are a frequent source of chronic pain.
Non-invasive treatment options are limited and oftentimes ineffective. Interventional treatments
performed under ultrasound guidance allow the dynamic application of therapies such as radiofrequency
ablation, with a pain neuromodulation effect.
Keywords:Spinal accessory nerve; Dorsal scapular nerve; Chronic pain; Neuropathic pain; Pulsed radiofrequency; Scapular osteosarcoma; Myofascial pain syndrome
Introduction
Persistent Neuropathic Pain (NP) is an important part of chronic postoperative pain in many surgeries, being a source of considerable disability for patients [1]. One of the major pathophysiological mechanisms of chronic postoperative NP is nerve damage during surgery, having the more extensive ones an higher chance of developing postoperative NP [2,3]. Surgery for sarcoma of the extremities or pelvis often requires extensive tissue dissection, commonly violating the internervous plane. Because of this, patients who undergo these types of surgery may be prone to developing postoperative NP [2]. Literature on orthopaedic postoperative NP is scarce [2]. In shoulder replacement surgery, the prevalence of NP was reported as 13% [4]. Pulsed Radiofrequency (PRF) has been used for many chronic pain conditions [5,6]. PRF was developed with the goal of pain reduction using electromagnetic fields in the absence of neural injury [7]. Such radiofrequency variant has been shown to exert a biological effect unrelated to any thermal damage, but potentially focusing on both small-diameter and C and A-delta nociceptive fibres. Vallejo et al. [8] showed that electromagnetic energy applied by PRF influences the reversal of behavioural and molecular effects of hypersensitivity developed from a peripheral nerve injury. The results indicate that gene expression changes not only occur at the site of PRF treatment, but also along the nociceptive painful pathways.
Clinical-Case
A forty-nine year old woman, with relevant medical history of chronic sarcoidosis, hypothyroidism, chronic gastritis, depression, supraspinatus tear of left shoulder and a resection surgery of right scapular osteosarcoma twelve years ago, was referred to a Interventional Physiatry consultation in our rehabilitation centre. Since the right partial scapulectomy, the patient developed cervical and dorsal chronic pain and was being followed in a chronic pain unit. Over the years, the pain was not significantly reduced after the combination of a conservative rehabilitation program, oral analgesics, including opioids and cannabinoids, and lidocaine and capsaicin patches. By that time, in chronic pain unit, she was also submitted to a suprascapular nerve block, muscular paravertebral block, scapular trigger point block, intercostal nerve block and PRF, and T4 to T7 thoracic roots PRF. All these procedures did not produce significant and sustained pain relief. In our evaluation, the patient presented with persistent cervical and right dorsal pain with neuropathic descriptors irradiating to the right parietotemporal region of the head and half superior arm, scoring 8/10 in Visual Numeric Scale (VNS). She was medicated with tapentadol 250mg twice a day, etoricoxib 60mg and 10mg morphine sulfate in periods of more severe pain. She was also using daily transcutaneous electrical nerve stimulation (TENS) at home.
\On clinical examination, there was severe amyotrophy of the right shoulder girdle, regarding supraspinatus, infraspinatus, and deltoid muscles. There was also allodynia in right scapular and dorsal region, including the scar and skin around it. Right shoulder had limited Active Range of Motion (AROM), 80° in lateral elevation and 60° in anterior elevation, with marked dyskinesia associated. Passive mobilization of the shoulder was painful above the mentioned AROM. At palpation, there was a contracture of the upper fibres of the trapezius, without palpable taut bands. Considering the symptoms and clinical examination findings, combined with the failure of previous treatments, it was proposed an ultrasound-guided blockage of right spinal accessory nerve and right dorsal scapular nerve, targeting the upper trapezius myofascial syndrome and dorsal neuropathic pain associated. The blockage was performed with 5ml of ropivacaine 2mg/ml and there was a significant pain reduction after the technique. Following the procedure, the patient experienced immediately pain relief, followed by approximately 50% reduction for a week (VNS 4/10), referring the initial level pain three weeks later (VNS 8/10). Because of the small duration of pain relief, we performed ultrasound guided PRF ablation of the of dorsal scapular and spinal accessory nerves, four months later.
Spinal accessory nerve
Using a posterior approach, spinal accessory nerve PRF was carried out in long-axis view, passing superficial to the splenius capitis muscle towards the trapezius muscle (Figure 1). It was used sensorial and motor nerve stimulation to confirm spinal accessory nerve identity, eliciting contraction of the trapezius muscle, whilst ensuring no contraction of the sternocleidomastoid muscle.
Figure 1:Ultrasound image and corresponding line diagram depicting the anatomy of the spinal accessory nerve,
the in-plane needle and surrounding structures. Coronal plane.
Source: The spinal accessory nerve is indicated by the ▲ symbol; The needle is indicated by the ↓ symbol. Tr:
Trapezius Muscle; EC: Esplenius Capitis.

Dorsal scapular nerve
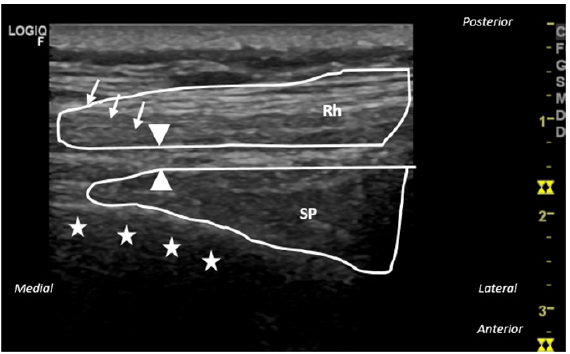
Using a posterior approach, the dorsal scapular nerve was identified as a monofascicular anechoic fascicle with a hyperechoic border, lying between rhomboid major muscle and serratus posterior muscle (Figure 2). It was used sensorial and motor nerve stimulation to confirm dorsal scapular nerve identity. It was used 22-G, 6cm-10mm Injection Electrode (Boston Scientific, Valencia CA, USA). PRF protocol used was the following: 6 mins of PRF on each nerve, applying 45V, with temperature control at 42 °C, and with a pulse width of 20ms. After the PRF application, a total volume of 5ml of local anaesthetic (ropivacaine 2mg/ml) was deposited at treated levels. The patient was reassessed 1 and 6 months later, reporting a pain reduction of 70% and 50%, respectively. She was able to reduce her pain medication and perform her daily activities more easily. We then decided to repeat the ultrasound-guided PRF neuromodulation of dorsal scapular and accessory nerves, to control the pain. All the procedures were explained, considering risks and benefits. The patient gave her informed consent to perform the intervention, and her consent to be included in this report. No immediate or late adverse effects were reported in all procedures described.
Figure 2:Ultrasound image and corresponding line diagram depicting the anatomy of the dorsal scapular nerve, the
in-plane needle, and surrounding structures. Transversal plane.
Source: The dorsal scapular nerve is indicated by the ▲ symbol; The needle is indicated by the ↓ symbol; The costal
arch is indicated by the star symbols. Rh: Rhomboid Major Muscle; SP: Serratus Posterior.
Discussion
Even though patients who undergo partial scapulectomy are likely to have preservation of at least a portion of the rotator cuff and deltoid [9], the scapulohumeral rhythm is inevitably disturbed, either by inappropriate pattern of muscle activation or inappropriate force of muscle contraction and scapular stabilizers muscle resection [10]. The kinematic alterations in scapular motion may increase the upper trapezius and levator scapulae muscle activity, with muscular overload, causing myofascial pain syndrome [11]. All these conditions may cause a chronic pain syndrome, with miofascial and neuropathic contribute.
The pathophysiology of myofascial pain is not completely understood. Traditional theory describes pain beginning after an initial insult to muscle fibres, resulting in the release of inflammatory substances which may activate nociceptors and cause reflex muscle contraction forming trigger points [12]. More recently, it has been suggested that myofascial pain is better explained as secondary hyperalgesia of peripheral neural origin [12]. The positive effect of our blockade of the dorsal scapular and spinal accessory nerves may be explained by these both mechanisms, resulting in relaxation of this involuntary muscle contraction and reduced pain transmission. PRF has been emerging as a minimally invasive treatment modality for musculoskeletal painful conditions, frequently refractory to previous medical management, such as physical therapy and trigger point injections [10,13]. Some studies identify the PRF target by trigger points palpation, by clinical response to anaesthetic bloc, or by sensory stimulation with the PRF cannula [10,13]. However, we did not find any article describing ultrasound-guided spinal accessory nerve PRF directed to upper trapezius myofascial pain syndrome. We just found descriptions of ultrasound-guided spinal accessory nerve block for intractable trapezius muscle-related pain [12,14]. Townsley P et al. [12] described a similar approach, obtaining a short-axis view of the spinal accessory nerve. We believe and hypothesize that accurate nerve identification and needle placement can give more time of pain relief until nerve sprouting occurs.
Surgical damage of nerve structures is a frequent source of chronic pain. Persistent neuropathic pain is a significant contributor to poor physical function, impaired quality of life, and greater use of health care services [15]. In our clinical case, the dorsal scapular nerve seemed to be involved not only in nociceptive pain but also in the neuropathic pain described. Levator scapulae is a muscle usually involved in scapular dyskinesia by parasitic overactivity ad subsequent myofascial syndrome. The anaesthetic block confirmed our suspicion, by reducing significantly and immediately the allodynia. As the dorsal scapular nerve branches from the C5 or C6 nerve roots of the brachial plexus, it plays a significant role in sensory innervation of the scapula, along with the suprascapular and long thoracic nerves [16]. However, somatic innervation of the overlying skin is not covered with selective scapular dorsal nerve block, and paravertebral or intercostal nerve blocks may be good adjuncts to a selective dorsal scapular nerve block to target the somatic skin dermatomes [16]. In the future, to optimize the pain control, these additional nerve blocks may be discussed.
Selective blockades using anterior and posterior approaches have been conventionally used for diagnosing and treating dorsal scapular nerve entrapment neuropathy [17,18]. The anterior approach is through the posterior triangle of the neck, while the posterior approach involves injection below the levator scapulae or rhomboid muscles in the back [19]. We used the posterior approach through the interscapular region, which has been regarded to be a relatively safer method for dorsal scapular nerve blockade because of the simplicity of the structures close to the levator scapulae and rhomboid muscles [18]. The dorsal scapular artery identification may facilitate dorsal scapular nerve block based on anatomical close relationship [18].
Some important limitations need to be notice. To assess the effectiveness of the technique, we only used the VNS. It would be important to evaluate the impact on daily living activities, sleep, and quality of life.
There were no immediate or late adverse effects after PRF sessions. Despite severe complications from PRF are rare, they may include neural trauma; injection into vessels; haematoma formation; local, intra-articular or systemic infection. Most side effects are local sweeling, pai at the site of needle insertion and in the extremities, usually self-limited [20]. Our case report demonstrates that the spinal accessory and dorsal scapular nerves are possible targets for ultrasound guided PRF for chronic postoperative pain. It is also important to emphasize that PRF should not be an isolated strategy in management of chronic pain. It is also important to combine other therapeutical modalities. In this case, a combination of a rehabilitation program is essential to optimize shoulder and scapular cinetics and back stabilization and mobility. Peripheral nerve stimulation may also be suggested for long lasting effects. We intend to continue the patient follow-up, to evaluate long-term results of pain relief and late adverse effects. It is important to evaluate pain relief, but also the return to the previous suspended or limited activities and to measure quality of life before and after the procedure.
Conclusion
Ultrasound guided PRF of dorsal scapular and spinal accessory nerve was effective in pain reduction in this clinical case of chronic pain after partial scapulectomy.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki.
References
- Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH (2003) Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 104(1-2): 1-13.
- Park JW, Kim HS, Yun JY, Han I (2018) Neuropathic pain after sarcoma surgery: Prevalence and predisposing factors. Medicine 97(21): e10852.
- Steegers MAH, Snik DM, Verhagen AF, Drift MA, Wilder-Smith OHG (2008) Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 9(10): 955-961.
- Bjørnholdt KT, Brandsborg B, Søballe K, Nikolajsen L (2015) Persistent pain is common 1-2 years after shoulder replacement. Acta Orthop 86(1): 71-77.
- Magistroni E, Ciclamini D, Panero B, Verna V (2014) Ultrasound-guided pulse-dose radiofrequency: treatment of neuropathic pain after brachial plexus lesion and arm revascularization. Case Rep Med 2014: 429618.
- Cahana A, Zundert JV, Macrea L, Kleef M, Sluijter M (2006) Pulsed radiofrequency: Current clinical and biological literature available. Pain Med 7(5): 411-423.
- Cosman ER (2005) A comment on the history of the pulsed radiofrequency technique for pain therapy. Anesthesiology 103(6): 1312.
- Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, et al. (2013) Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician 16(5): E601-E613.
- Vahanan NM, Mohanlal P, Bose JC, Gangadharan R, Karthisundar V (2007) The functional and oncological results after scapulectomy for scapular tumours: 2–16-year results. Int Orthop 31(6): 831-836.
- Niraj G (2012) Ultrasound-guided pulsed radiofrequency treatment of myofascial pain syndrome: A case series. British Journal of Anaesthesia 109(4): 645-646.
- Saxena A, Chansoria M, Tomar G, Kumar A (2015) Myofascial pain syndrome: An overview. J Pain Palliat Care Pharmacother 29(1): 16-21.
- Townsley P, Ravenscroft A, Bedforth N (2011) Ultrasound-guided spinal accessory nerve blockade in the diagnosis and management of trapezius muscle-related myofascial pain. Anaesthesia 66(5): 386-389.
- Tamimi MA, McCeney MH, Krutsch J (2009) A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med 10(6): 1140-1143.
- Herbst MK, Sorkin R (2022) Ultrasound-guided spinal accessory nerve block for intractable trapezius pain. The American Journal of Emergency Medicine 52: 268.e3-268.e7.
- O’Connor AB (2009) Neuropathic pain: Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 27(2): 95-112.
- Auyong DB, Cabbabe AA (2014) Selective blockade of the dorsal scapular nerve for scapula surgery. Journal of Clinical Anesthesia 26(8): 684-687.
- Haim K, Urban BJ (1993) Dorsal scapular nerve block: Description of technique and report of a case. Anesthesiology 78(2): 361-362.
- Cho H, Kang S, Won HS, Yang M, Kim YD (2019) New insights into pathways of the dorsal scapular nerve and artery for selective dorsal scapular nerve blockade. Korean J Pain 32(4): 307-312.
- Chang KV, Lin CP, Lin CS, Wu WT, Karmakar MK, et al. (2017) Sonographic tracking of trunk nerves: essential for ultrasound-guided pain management and research. J Pain Res 10: 79-88.
- Facchini G, Spinnato P, Guglielmi G, Albisinni U, Bazzocchi A (2017) A comprehensive review of pulsed radiofrequency in the treatment of pain associated with different spinal conditions. Br J Radiol 90(1073): 20150406.
© 2023 Ana João Costa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






