- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Sequential Treatment with Nusinersen and Risdiplam in a Paediatric Patient with Spinal Muscular Atrophy: A Case Report
Pitarch-Castellano I1,2*, Ibáñez-Albert E3, Ñungo-Garzón NC1,4, Vázquez-Costa JF2,4,5 and Teresa Sevilla2,4,5
1Neuromuscular Referral Center ERN-EURO-NMD, Neuropediatric Department, UIP La Fe Hospital, Spain
2Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), Spain
3Physical Medicine and Rehabilitation Department, UIP La Fe Hospital, Spain
4Neuromuscular Referral Center ERN-EURO-NMD, Neurology Department, UIP La Fe Hospital, Spain
5Department of Medicine, Universitat de València, Spain
*Corresponding author:Inmaculada Pitarch-Castellano, Neuromuscular Referral Center ERN-EURO-NMD, Neuropediatric Department, UIP La Fe Hospital, Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), Spain
Submission: November 22, 2022; Published: December 05, 2022

ISSN 2637-7934 Volume3 Issue5
Abstract
Spinal Muscular Atrophy (SMA) is an autosomal recessive neuromuscular disorder that causes muscle atrophy and weakness. Although at present there is no cure, several effective disease-modifying treatments have become available in recent years. However, there are currently no recommendations on the management of therapy sequencing involving these new treatments. An 11-year-old girl with SMA type 2 was initiated on treatment with nusinersen resulting in significant improvement in her motor and respiratory function. However, after nine doses, treatment was changed to the orphan drug risdiplam due to its more suitable route of administration given the patient’s recent history of coxalgia related to the spinal fusion surgery she underwent in January 2019. Five weeks into treatment with risdiplam the patient developed medication-related leucocytoclastic vasculitis and treatment was switched back to nusinersen.
This case report provides insight on how a patient that required several changes in therapy was managed in real-life clinical practice.
Keywords:Spinal muscular atrophy; Treatment; Nusinersen; Risdiplam; Paediatric
Abbreviations: FVC: Forced Vital Capacity; HFMSE: Hammersmith Functional Motor Scale Expanded Capacity; SMA: Spinal Muscular Atrophy; SMN: Survival Motor Neuron; RULM: Revised Upper Limb Module
Introduction
Spinal Muscular Atrophy (SMA) is an autosomal recessive neuromuscular disorder characterised by an insufficient level of Survival Motor Neuron (SMN) protein, coded by the SMN1 gene, that causes degeneration of motor neurons in the spinal cord and brain stem, resulting in progressive muscle atrophy and weakness [1]. Successful preclinical approaches enhancing SMN [2-4] led to clinical trials in humans with nusinersen, an antisense oligonucleotide that increases the synthesis of SMN protein via SMN2, [1] a paralogous gene of SMN1 that naturally produces a small fraction ~10-15% of functional SMN [5-7]. In May 2017, nusinersen became the first drug capable of influencing the disease course of SMA to be approved by the European Medicines Agency [8] and to date, its efficacy and safety has been demonstrated in both children and adults [9-11]. Another treatment option that has recently become available is risdiplam, a small molecule that also increases the production of full- length SMN protein and that was approved by the United States’ Food and Drug Administration in August 2020 [12], becoming available through an Expanded Access Program in Spain. To date, there is limited evidence on the management of switches between these medications although a clinical trial is currently being conducted [13].
Case Presentation
In this case study, we report a paediatric female patient diagnosed with SMA type 2 at 20 months of age. She had good head control from 3-4 months and was able to sit unassisted by 7-8 months of age. At 10 months she presented difficulty crawling, could not transition from sitting to standing and was unable to walk independently. Examination by the paediatric neurologist at 20 months demonstrated absent reflexes and proximal predominant weakness that was most severe in the lower limbs. Molecular genetic testing revealed deletion of SMN1 and three copies of SMN2 which confirmed the diagnosis. Throughout the years, the patient presented a progressive disease course, losing the ability to sit without support and requiring assistance with all basic activities of daily living and the use of an electric wheelchair. In 2018, at age 11, the patient was considered suitable for treatment with nusinersen. After the first few doses, the patient’s parents reported an improvement in motor function in the upper limbs, confirmed by the functional motor tests results measured by the Revised Upper Limb Module (RULM) (see Table 1 for timeline and followup results). In January 2019, the patient underwent a planned posterior spinal arthrodesis. Despite the expected deterioration of the Hammersmith Functional Motor Scale Expanded (HFMSE) scores and Forced Vital Capacity (FVC) after the spinal fusion, a consistent improvement in the RULM scores was still observed. In July 2020, the patient was admitted to hospital with a coxalgia from a suspected infection related to the hardware inserted in the spinal fusion surgery, which resolved after receiving 12 weeks of antimicrobial therapy.
Table 1: Patient characteristics treatment and follow up periods.
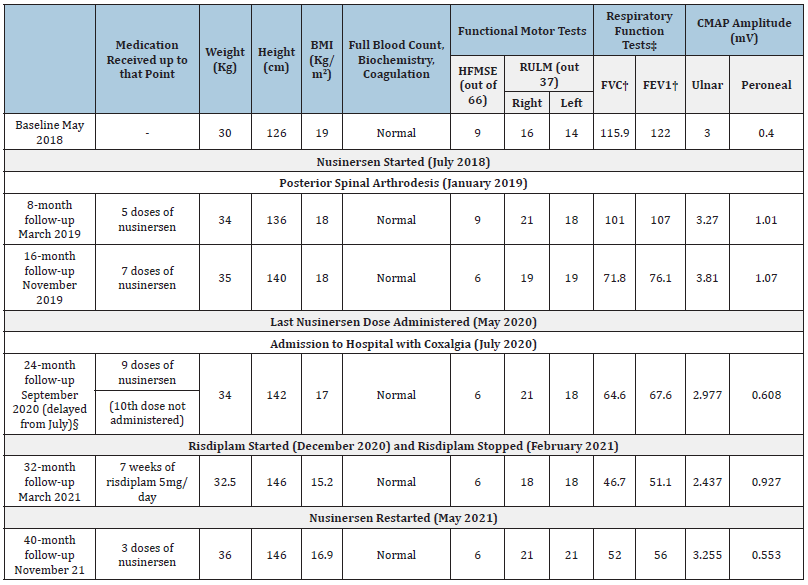
Source: BMI: Body Mass Index; CMAP: Compound Muscle Action Potential; HFMSE: Hammersmith Functional Motor Scale Expanded; FEV1: Forced Expiratory Volume in 1 second; FVC: Forced Vital Capacity; RULM: Revised Upper Limb Module. †: % of predicted value. §: The 24-month follow-up appointment was delayed because of an admission to hospital to treat a suspected infection resulting from the hardware inserted at the posterior spinal arthrodesis the patient underwent in January 2019.
Given these recent events, at the 24-month follow-up visit it was decided to change the patient’s treatment to risdiplam due to its more convenient oral administration. This decision was made despite the limited evidence available at the time regarding switching between different drugs. In December 2020, the patient started daily treatment with 5mg of risdiplam, a standard dose for all patients weighing ≥20Kg [14]. In line with the findings from clinical trials that have so far reported dermatological side effects to be very common [14], five weeks into this new treatment the patient developed lower limb palpable purpura and oedema. She was referred to dermatology and risdiplam was withheld. The histological findings from the lesions’ biopsy were compatible with a diagnosis of leucocytoclastic vasculitis. By the end of the second week of monitoring, both the lesions and the oedema had spontaneously resolved, and risdiplam was restarted. However, two weeks later the lower limb purpura returned, and risdiplam was permanently discontinued. The decision to restart nusinersen was made in May 2021, using a shorter two-dose loading regimen as suggested by pharmacokinetic data [15]. Administration was carried out via ultrasound-guided lumbar puncture. By November 2021, the patient’s positive response to nusinersen was evidenced by the regained improvement observed in RULM scores and respiratory function tests.
Our patient maintained motor stability after 12 months of discontinuing nusinersen, which leads us to wonder whether nusinersen had residual activity during this time. This case also showcases the deleterious effect of spinal fusion on HFMSE scores and FVC, which should be therefore interpreted with caution in this context. Thus, RULM scores should be the main outcome measure for monitoring disease progression in non-sitter patients that have undergone recent spinal surgery. Moreover, ultrasoundguided lumbar puncture is a feasible option to continue nusinersen treatment in these patients.
Conclusion
Despite the discontinuation of nusinersen caused by the challenge of an intrathecal injection in a complex spine due to the natural progression of the disease, treatment with nusinersen showed better improvement in motor scales and absence of adverse events in comparison with risdiplam. Using ultrasound-guided lumbar puncture to administrate nusinersen is a technique that can be used in complex spines to ensure delivery of the drug directly into the CNS as conducted in real world evidence studies worldwide [16-18]. In this exciting era in which different therapeutic options are available for SMA patients, there is still limited data on the management of patients that require therapy changes. This case report provides insight on how a patient that required therapy sequencing was managed in real-life clinical practice.
Acknowledgement
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain) with funding from Biogen. The manuscript was developed independently by the authors with no control or influence by Biogen. All authors have contributed significantly to the conception, design, or acquisition of data, or analysis and interpretation of data. All authors have participated in in drafting, reviewing, and/or revising the manuscript and have approved its submission
Conflict of Interest
IPC has been a consultant and speaker honoraria from Biogen, Roche and Novartis. EIA has been a consultant and speaker honoraria from Biogen and Roche. JFVC has received consultant and speaker honoraria from Biogen and Roche. The other authors declare no conflicts of interest.
References
- Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, et al. (2017) Control in infantile-onset spinal muscular atrophy. N Engl J Med 377(18): 1723-1732.
- Foust KD, Wang X, Mcgovern VL, Braun L, Adam K, et al. (2010) Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 28(3): 271-274.
- Hua Y, Sahashi K, Rigo F, Hung G, Horev G, et al. (2011) Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478(7367): 123-126.
- Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, et al. (2014) Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 345(6197): 688-693.
- Soler-Botija C, Cuscó I, Caselles L, López E, Baiget M, et al. (2005) Implication of fetal SMN2 expression in type I SMA pathogenesis: Protection or pathological gain of function? J Neuropathol Exp Neurol 64(3): 215-223.
- Boza-Morán MG, Martínez-Hernández R, Bernal S, Wanisch K, Also-Rallo E, et al. (2015) Decay in survival motor neuron and plastin 3 levels during differentiation of iPSC-derived human motor neurons. Sci Rep 5.
- Wirth B, Garbes L, Riessland M (2013) How genetic modifiers influence the phenotype of spinal muscular atrophy and suggest future therapeutic approaches. Curr Opin Genet Dev 23(3): 330-338.
- Spinraza (2021) European Medicines Agency.
- Coratti G, Cutrona C, Pera MC, Bovis F, Ponzano M, et al. (2021) Motor function in type 2 and 3 SMA patients treated with nusinersen: A critical review and meta-analysis. Orphanet J Rare Dis 16(1): 430-442.
- Hagenacker T, Wurster CD, Günther R, Schreiber-Katz O, Osmanovic A, et al. (2020) Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol 19(4): 317-325.
- Maggi L, Bello L, Bonanno S, Govoni A, Caponnetto C, et al. (2020) Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry 91(11) :1166-1174.
- Dhillon S (2020) Risdiplam: First approval. Drugs 80(17): 1853-1858.
- Clinical Trails (2022) A study to evaluate higher dose (HD) nusinersen (BIIB058) in participants with spinal muscular Atrophy Previously Treated With Risdiplam (ASCEND).
- Evrysdi (2021) European Medicines Agency. pp. 1-48.
- MacCannell D, Berger Z, East L, Mercuri E, Kirschner J, et al. (2021) Population pharmacokinetics-based recommendations for a single delayed or missed dose of nusinersen. Neuromuscul Disord 31(4): 310-318.
- Veiga-Canuto D, Cifrián-Pérez M, Pitarch-Castellano I, Vázquez-Costa JF, Aparici F (2021) Ultrasound-guided lumbar puncture for nusinersen administration in spinal muscular atrophy patients. Eur J Neurol 28(2): 676-680.
- Zhang J, Cui X, Chen S, Dai Y, Huan Y, et al. (2021) Ultrasound-guided nusinersen administration for spinal muscular atrophy patients with severe scoliosis: An observational study. Case Reports Orphanet J Rare Dis 16(1): 274-282.
- Özütemiz C, Karachunski P, Nascene DR (2020) Nusinersen injections in adults and children with spinal muscular atrophy: a single-center experience. Diagn Interv Radiol 26(6): 596-602.
© 2022 Pitarch-Castellano I. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






