- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Utilization of Laser Wavelengths in Periodontitis and Periimplantitis
Pawelczyk-Madalińska M1* and Dembowska E2
1Department of Periodontology, PUM Poland and FAN-DENT Centrum Stomatologii i Periodontologii Gdansk, Poland
2Department of Periodontology, PUM Poland
*Corresponding author: Magdalena Pawelczyk-Madalińska, Department of Periodontology, PUM Poland and FAN-DENT Centrum Stomatologii i Periodontologii Gdansk, Juliusza Słowackiego 71/2, 80-257 Gdansk, Poland
Submission: October 10, 2022; Published: October 20, 2022

ISSN 2637-7934 Volume3 Issue5
Abstract
The healthy status of the periodontal pocket and gingiva is mandatory for tooth survival, as well as, systemic health in general. Only 10% of the global adult population appears to be resistant to it, whereas 10-15% is highly sensitive. Due to the limitations of Scaling and Root Planing (SRP), the researches have focused to utilize additional therapies to enhance the conventional treatments in periodontitis and periimplantitis. Utilization of appropriate laser wavelengths with suitable protocols can probably improve the periodontal status for a more extended period and limit the use of antibiotics.
Keywords: Periodontitis; Periimplantitis; Laser; Diode; Erbium laser; Laser parameters; Decontamination
Abbreviations: SRP: Scaling and Root Planing; BoP: Bleeding on Probing; PD: Pocket Depth; PBM: Photobiomodulation; aPDT: Antimicrobial Photodynamic Therapy; PS: Photosensitizer; SPC: Supportive Periodontal Care
Introduction
The healthy status of the periodontal pocket and gingiva is mandatory for tooth and implant survival and systemic health in general [1,2]. The primary differentiation between clinically healthy and diseased periodontium is spontaneous or provoked by bleeding on probing. Periodontal disease is now believed to be caused by an excessive host immuneinflammatory response as a factor caused by pocket dysbiosis. The composition of microorganisms in the oral cavity ecosystem is one of the most sophisticated in the human body. It contains approximately 700 microorganisms in saliva, gingival fluid, biofilm and gingival pockets1. Some of them belong to the normal oral flora, and others are conditionally pathogenic, but pathogenic also can be found there. The protective mechanisms of the human organism have a considerable effect on the virulence of the conditionally pathogenic and pathogenic microorganisms in each habitat. The impact of the progression and severity of periodontitis is due to habits like smoking, drugs etc., and general health like diabetes etc., stress, and socioeconomic conditions [3-5]. In order to transition from early plaque-induced gingivitis to stabilized gingivitis and then to periodontitis, it is necessary to shift the immuneinflammatory response from an adequate, controlling, even relatively high biomass of the plaque into an excessive and uncontrolled biofilm pocket. Uncontrolled inflammation in the tissues leads to structural and functional changes in the biofilm. This is characterized by a decrease in Lacto- and Bifidobacteria content there [6]. That disruption of the relationship between bacteria in a healthy, average gingival pocket and pathogenic or potentially pathogenic bacterial flora leads to the development of dysbacteriosis. The primary role in that process belongs to Porphyromonas gingivalis, Treponema denticola, Tannerella forsythensis (red-complex), Fusobacterium spp., and other microorganisms and probably viruses [5- 10]. Periimplantitis is characterized by inflammatory lesions in peri-implant tissues and associated loss of supporting bone [4]. Peri-implant mucositis is the first step to developing peri-implantitis, and the management of it is considered a preventive measure for the onset of periimplantitis [10-12].
While periimplantitis and Periodontitis share many clinical features, structural differences, such as epithelial attachment as opposed to periodontal ligament fibers, and the absence of blood vessels in the supporting tissues between implants and teeth, can influence the host’s response to infection. Compared to normal, Aggregatibacter actinomycetemcommitans and Prevotella intermedia were more frequently identified when periimplantitis was diagnosed in healthy tissues surrounding the implant [6]. The peri-implant biofilm microbiota seems to be similar to the subgingival biofilm in Periodontitis but differs between implants placed in edentulous patients and partially edentulous patients. This second ones has more pathogenic peri-implant microflora than edentulous ones [1,13]. Also, the implant design, implant material used and roughness of the surface, lack of control of bacterial biofilm and history of Periodontitis and also improper postoperative care and no recall phase, poor patient compliance, smoking and cement left in the pocket, as well as improperly planned and manufactured prosthetic construction may facilitate biofilm formation [3,14].
Discussion
Periodontitis and periimplantitis can be managed with nonsurgical and surgical procedures, but the second ones need additive bone material grafting due to tissue remodelling during the healing phase. Flapless treatment is more gentle for tissues and can boost natural healing processes but requires more knowledge, supportive technics and equipment. After the non-surgical pocket periodontal procedure, we must remember that biofilm recolonization occurs three weeks after each mechanotherapy: scaling root planing or periodebridement. The recolonization can come from the saliva, supragingival dental plaque, mucosal surfaces or digestive system, and another person [15]. Periodontal bacteria affect the success or failure of the colonization process and, thus, maintenance of the host’s health or illness. A patient’s response to bacteria is based on the host genotype, which is considered an additional risk factor for periodontal disease. This situation significantly affects the course of the disease progression and determines the quality and strength of the immune response [1,4,5,13]. Non-surgical treatment of Periodontitis is Scaling and Root Planning (SRP), the standard mechanical instrumentation for bacterial debridement of the pocket. This can be achieved either; with manual or mechanical curettes or by ultrasound or sound instruments. In the era of increased antibiotic resistance, scientists’ efforts are aimed at finding technologies and procedures that would allow limiting their use in all possible cases. The answer to this need is certainly the possibility of using broadly understood laser therapy in the treatment of periodontal diseases in order to reduce the amount of bacteria in pathological pockets and restore the immune balance in the pocket while allowing the healing phase to be accelerated and enhanced moreover to release muscle stress and pain after the therapy used.
One of the main variables used to evaluate the severity and progress of Periodontitis; likewise, the clinical success of periodontal therapy is Bleeding on Probing (BoP), and another one is periodontal Pocket Depth (PD). In non-surgical treatment, the baseline of treatment is SRP (manual or mechanical, long enough to remove most of the biofilm-about 30sec per tooth surface is recommended), which can be supported with local or general antibiotics supplements or some the use of lasers [4,9-12,16,17-21]. Pockets 3-4mm deep can be easily treated with mechanotherapy and air-polishing; it is highly effective and safe [17], but periodontal pockets with a depth ≥5, 6mm are considered „critical” and present a risk factor for both the progression of periodontal disease, tooth loss and periimplantitis next to adjacent teeth [4,5,18]. These deep pockets are difficult to access for SRP in the form of curettes or mechanical units. Subgingival use of sandblasting is also not recommended in the case of inflamed tissues, as it may cause tissue emphysema [19] when you perform it without due diligence. These and other limitations lead to using different wavelength lasers in closed pocket therapy as an alternative in combined SRP+laser treatment [20-24]. Mechanical instrumentation of periodontal tissues is a gold standard in dentistry but followed by diode laser application leads to complete removal of pocket epithelium compared with conventional SRP. Wide methodological heterogeneity makes the literature on the clinical effects of laser treatment in Periodontitis, both as monotherapy and adjunct to non-surgical therapy, challenging to interpret. It may be due to the errors in the methodology of research works, as many of them used split-mouth blindness to compare. This will not work because saliva is the means of transport for, among other things, periodontal pathogens from the pocket to the pocket. The same will be true of testing the effect of decontamination of only two or a few pockets, while not all of them have been decontaminated [7,25].
Another problem is with complete laser procedure documentation; sometimes, it is not duplicatable, which is why it is a need for further research. There seem to be two schools of thought about the advantage of Energy (fluence-J/cm2) versus power (intensity, irradiance-W/cm2) for an effective dose; the correct dose must also include two other intrinsic, critical variables, namely time treatment and biological context. The diversity in the mechanisms is related to the current paradigms of periodontal biofilm behaviour, tissue response to laser therapy is dependent on tissue type (melanin is the primary chromophore for 800nm- 980nm dental diode lasers), and health (inflammation tissue has more chromophores for the laser beam light-haemoglobin, peptides and also water). The successful therapeutic treatment window is mandatory for the result to be specific to the target tissue, biofilm composition, laser wavelength, and laser energy delivered [26,27]. Beam profile and its emission mode (pulse, super pulse or continuous), pulse shape, and emission cycle play an essential role in the expected effect that needs to be carefully vetted for specific clinical use concerning the appropriate biological molecular interactions. Inside the periodontal pocket for non-surgical therapy, we use a diode near-infrared laser with a wavelength between 800nm-980nm for coagulating gingivae (epithelial ablation inside the pocket) as an adjunct to SRP. Additionally, it results in complete removal of the inflamed epithelium and decreases the amount of pro-inflammatory agents [9,12,28-36]. Appropriate laser wavelengths, with suitable protocols, probably can improve the periodontal status for a more extended period and likely limit the use of antibiotics. The diode use (non-surgical treatment) as an adjunct to the conventional treatment has proven to reduce the bacterial load in the pocket [37] (Figure 1).
Figure 1:Diode tip in periodontal pocket decontamination procedure.

The photothermal properties of diode laser photonic energy facilitate ablation of the graduation tissue and inflamed periodontal tissue (sulcular debridement) and coagulation simultaneously, which can be achieved at 60°C, leading to protein denaturation and reduction in the proinflammatory cytokines [20,26]. As the diode laser family is predominantly absorbed by mainly haemoglobin and pigmented bacteria and poorly absorbed by hydroxyapatite in teeth and bone, it is considered a safe and suitable treatment modality in sulcular debridement [38,39]. The photonic energy of an ablative diode laser in the diseased periodontal pocket results in various beneficial effects [35,37,40-45].
The diode laser in dentistry is utilized to treat diseased inflamed tissue, causing a significant bacterial reduction and removal of the inflammatory products, resulting in optimal haemostasis. A flexible fiber delivery system of the diodes allows easy access into the pocket, and the clinician controls the direction of the fiber in the pocket; therefore, it is safe. Traditional methods like curettes and ultrasonic devices used during maintenance are responsible for irreversible hard tissue damages ensuing from the repeated mechanical scraping of tooth surfaces or unproper handling. In order to achieve the intended result, we have to deliver the exact number of photons with dedicated energy transfer in one of them to the target tissue and then repeat it once or multiple times. To obtain this, we have to follow specific rules leading to the optimal absorption of laser light by the target tissue and choose the laser wavelength, emission mode, time of exposure, distance from the tip, its angulation towards the surface, and tissue composition and given thickness. In Periodontitis, the heat generated by the laser beam can additionally deactivate some bacterial enzymes leading to their apoptosis via photothermal effect [26,36,40,46,47]. In addition, the inflamed socket soft tissue can be debrided induced by laser beam coagulation at 60ᵒ Celsius; in this process, the blood vessels and lymphatic connections can be coagulated to enable healing. As a result, of the limitations of the current non-surgical treatment strategies, which are regarded as the conventional line of treatment in the management of Periodontitis, several adjunctive therapies have been proposed in (Table 1).
Table 1: Periodontitis is caused by disturbances between the host’s immune response and bacteria in the tooth’s biofilm, therefore in the maintenance phase we use preparations and behaviors that support and modulate the immune response (some of them are recommended by EFP*).
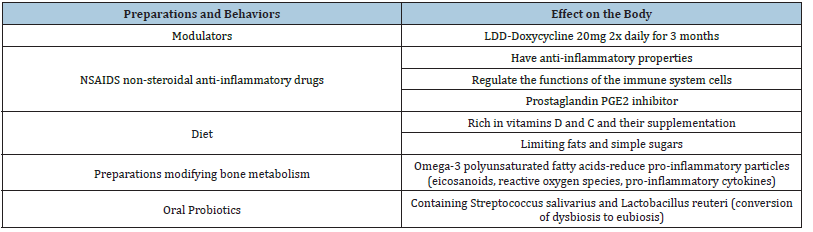
Source: *EFP European Federal of Periodontology.
Some of the periopathogenes are sensitive to the temperature of about 50 ᵒC, for example, A.a., because of photocoagulation which occurs in moderate temperatures of 45-65 ᵒC. Brown/black pigmented anaerobes like Porphyromonas spp., Prevotella spp., and Tonnerella spp. are also susceptible to diode laser photoablation due to pigmentation. That treatment is performed mainly with the “hot tip” technique. Diode laser (810nm,940nm,980nm) tip can be activated to ‘‘hot tip” to achieve higher temperature, but in this case, it should be used by more trained doctors who can understand the requirements for this kind of procedure with such significant hit transfer in the pocket tissue to avoid overheating of tissue and destruction. These wavelengths generate a photothermal phenomenon with ablation and additionally haemostasis due to microvessels construction inside the pocket. Some part of the light is absorbed superficially, but some are reemitted as longer wavelengths with more superficial penetration, which causes the complete removal of the contaminated by intracellular periodontal pathogens gingival epithelium [32,34,37,45-49].
You can also provide decontamination without tip activation with the same diode lasers. The tip diameter for all periodontal procedures with diodes is 300, 320 or 400nm regarding the equipment. The desired clinical effect can be achieved when brown/black anaerobic bacteria absorb the photonic energy of a specific laser wavelength. Quick transfer of the photonic energy results in the destruction of the biological target and generates photomechanical (photoacoustic) effects, which can disrupt superficially surrounding tissue [26,49]. This procedure also has a cleansing effect by removing inflammatory products and reducing inflammatory markers so it can increase cell proliferation and lymphatic circulation, improving a periodontal attachment (regenerative effect). You are reducing the volume of Aggregatibacter actinomycetemcomitans (A.a) (periodontal pocket violet complex) and Porphyromonas gingivalis (P.g) (redcomplex), which can easily penetrate the gingival epithelium without damaging the underlying connective tissues (bactericidal activity), which are the primary pathogens in Periodontitis. In soft tissue, you can also expect postoperative pain relief with the quasi Photobiomodulation (PBM) effect [21,26,36].
Another additional to SRP therapy which can improve the results in periodontal treatment (in opposite to AAP guidelines published in 2015 with changes in 2018 for the treatment of Periodontitis) [1] can be the Antimicrobial Photodynamic Therapy (aPDT). It is a low dose, non-thermal light treatment with the final in the form of destruction of a biological target. aPDT reaction occurs when a settled concentration of the photosensitizer dye the microorganisms for a predictable time and a compatible wavelength with proper emission mode, power output, energy, fluence and irradiance into the pocket without distance is utilized. Antimicrobial photodynamic therapy involves light activating the Photosensitizer (PS) dye in the presence of molecular oxygen in tissue or liquid to elicit cell death. It can be noticed that multiple sessions 1,7,21 day seems to be better than a single session to achieve the best results. A.a is highly sensitive to TBO and MB (blue dyes). For this kind of treatment aPDT, you can use 660nm laser with phenotiazine chloride 10mg/mL or Methylene blue, 670- 690nm with Toluidine blue or Phenothiazine chloride [40] and 810 with indocyan green for herpes virus [27].
Diode laser with hydrogen peroxide can produce decontamination inside the pockets is the utilization of the 940nm or 980nm laser just after mechanical therapy SRP. The irradiation with a laser beam aims to enhance the efficacy of disinfection of the root surface against the biofilm and delay the epithelial migration to the bottom of the pocket, simultaneously providing deepithelialization inside the periodontal pocket. The laser irradiation during the procedure coagulates the blood, which may lead to better pocket wound healing. It will delay the reattachment of the junctional epithelium. Moreover, the laser irradiation provokes the release of collagen and fibrin inside this tissue after six hours of radiation, increasing the bonding of the pocket soft tissue to the freshly cleaned root surface [28,29].
Since the increase in surface roughness and surface free energy facilitates biofilm formation on the dental implant and abutment surface, the cloud reactions on the exposed implant surface and the implant-abutment configuration’s design features also play a significant role in biofilm formation [49-51]. For implant surface decontamination, as we have similar pathogens like in periodontitis, so probably, we can utilize the same protocols, but we should be careful with the implant surface temperature because diode lasers heat it; if this elevation exceeds about 47 ᵒC, we can damage the surrounding bone structure losing the ability to repair tissue around the implant [38,39].
Erbium lasers (Er,Cr: YSGG 2780nm and Er: YAG 2940nm) appear to give better decontamination results in the treatment of periodontitis and periimplantitis because they can be used to remove biofilm from the root or implant surface without damaging them. The laser beam acts on the surface is precise and does not penetrate the surrounding tissues. Their photonic energy can be used in contact or non-contact mode to provide treatment. Due to their high water absorption, erbium lasers induce sudden photothermal evaporation of the water contained in the mineralized biofilm, bone and hydroxyapatite, leading to micro-explosions and tissue ablation [33]. Calculus can be easily removed due to the exact parameters settled at the laser with a specific wavelength without damaging the surrounding tissue. With Er:YAG to limit undesired removal of root tissues, a combination of pulsed emission at frequency >10Hz and low energy (40-100mJ/pulse, energy density 10-20J/cm2) is recommended [31,51]. The long-term clinical efficacy of Er:YAG laser is also associated with its ability to eliminate periodontal bacteria and their endotoxins [9,23,31,42,44]. This property may positively influence immuno-microbial balance and reduce inflammation in the periodontium. Contrary to root photoablation, supragingival laser scaling on enamel is contraindicated since complete removal of tartar without affecting the enamel with this wavelength is almost impossible [44,52]. Er, Cr:YSGG laser is similar because its primer target is water, then hydroxyl ions (OH-) and secondary minerals. This wavelength has a penetration depth of 2,28μm, which is why it can be safely used on the root surface [31,53-55]. Due to the differences between absorption in water and hydroxyapatite, we should modify the settings on Er,Cr: YSGG compared to Er:YAG. (in the literature: RFPT tip, 1,5-1,75W, 30- 50Hz, 30-60%water, 60% air, 60μs) [53,55].
Erbium lasers are often used for perimucositis and periimplantitis treatment; as for the first one, it can be a perfect solution due to the precise photo vaporisation process of the inflamed soft tissue and biofilm. On the contrary, in flapless procedures with periimplantitis, we can expect some difficulties due to the technical limitations. Laser light is transported to the infected pocket via the tip. The optical fiber is at the end obliquely cut to redirect the laser light to the side because we want it to act on the walls of the pocket (radial faring tip RFT), the root canal or its infected surface. Despite the beam divergence, we cannot transmit electromagnetic energy into the deeply concave surfaces such as the threads of the infected implants. That’s why we should consider surgical access during this kind of treatment instead. Erbium lasers are often used for perimucositis and periimplantitis treatment; as for the first one, it can be a perfect solution due to the precise photo vaporisation process of the inflamed soft tissue and biofilm; on the contrary, in flapless procedures with Periimplantitis, we can expect some difficulties due to the technical limitations. Even with Nd: YAG, we can utilize LANAP, which is an effective procedure in periodontitis; we still lack evidence for photoablation usefulness with that wavelength. The target chromophore for this laser is mainly melanin and other pigments, but it is not an absorber in water, so the penetration depth in soft tissue is deep [24,56]. It is helpful for 0,3-0,8mm soft tissue cut with excellent hemostasis and perfects quick healing time. In non-surgical periodontal treatment, we do not have enough data and research even that it has significant potential for decontamination in deeper layers of soft tissue without damaging the surface. Nd: YAG lasers are used to treat periimplantitis, but the impact of the beam described in the literature may or may not damage the surface (implant surface fusion) [57] with appropriate laser settings and selected tips [56,58].
Each periodontal treatment needs to be supported and controlled after. During the appointment, we have to check the periodontal status, BoP, and PD at six points at each tooth with furcation status and the implant. We have to consider the prosthetic parts and contamination on them as risk factors for the future. Supportive Periodontal care (SPC) is mandatory to the patient. Periodontal risk assessment is a good tool for the healed teeth tissue; the BoP should be below 10%, and the PD (pocket depth) should be less than 3mm or 4mm in the absence of bleeding in this place. With implants we have to be sure what is the shape of the transgingival status, so it is difficult to point out the exact correct deep. Bleeding on probing during the maintenance phase increases the risk of losing a specific site attachment 2-3 times. Lack of bleeding proves the stability of the periodontal tissues. In the presence of pockets >4mm with concomitant bleeding in the maintenance phase examination, there is a risk of further loss of connective tissue attachment [14,25,32,36,42,45,59].
Conclusion
Utilization of different laser wavelengths in Periodontitis and Periimplantitis can significantly improve the periodontal tissue status for longer time with proper maintenance.
References
- Larsen T, Fiehn NE (2017) Dental biofilm infections-An update. APMIS 125(4): 376-384.
- Marsh PD (2003) Are dental diseases examples of ecological catastrophes? Microbiology 149(2): 279-294.
- Schmidt JC, Jajjo E, Berglundh T, Zitzmann NU (2020) Periodontitis lesions in smokers and non-smokers. Eur J Oral Sci 128(3): 196-203.
- Berglundh T, Zitzmann NU, Donati M (2011) Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol 38(11): 188-202.
- Velden UV, Abbas F, Armand S, Loos BG, Timmerman MF, et al. (2006) Java project on periodontal diseases. The natural development of periodontitis: Risk factors, risk predictors and risk determinants. J Clin Periodontol 33(8): 540-548.
- Sahrmann P, Gilli F, Wiedemeier DB, Attin T, Schmidlin PR, et al. (2020) The microbiome of peri-implantitis: A systematic review and meta-analysis. Microorganisms 8(5): 661.
- Adriaens PA, De Boever JA, Loesche WJ (1988) Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans. A reservoir of periodontopathic bacteria. J Periodontol 59(4): 222-230.
- Teles RP, Haffajee AD, Socransky SS (2006) Microbiological goals of periodontal therapy. Periodontol 2000 42: 180-218.
- Cobb CM (2017) Lasers and the treatment of periodontitis: The essence and the noise. Periodontology 2000 75(1): 205-295.
- Maisch T (2009) A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini-Reviews in Medicinal Chemistry 9(8): 974-983.
- Kamma JJ, Vasdekis VG, Romanos GE (2009) The effect of diode laser (980nm) treatment on aggressive periodontitis: Evaluation of microbial and clinical parameters. Photomed Laser Surg 27(1): 11-19.
- Sgolastra F, Severino M, Gatto R, Monaco A (2013) Effectiveness of diode laser as adjunctive therapy to scaling root planning in the treatment of chronic periodontitis: A meta-analysis. Lasers Med Sci 28(5): 1393-1402.
- Waal YC, Winkel EG, Meijer HJ, Raghoebar GM, Winkelhoff AJ (2014) Differences in peri-implant microflora between fully and partially edentulous patients: A systematic review. J Periodontol 85(1): 68-82.
- Tonetti MS, Muller-Campanile V, Lang NP (1998) Changes in the prevalence of residual pockets and tooth loss in treated periodontal patients during a supportive maintenance care program. J Clin Periodontol 25(12): 1008-1016.
- Graves D, Oskoui M, Volejnikova S, Naguib G, Cai S, et al. (2001) Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J Dent Res 80(10): 1875-1879.
- Dederich DN (2002) Laser curettage: An overview. Compend Contin Educ Dent 23(11): 1097-1103.
- Müller N, Moëne R, Cancela JA, Mombelli A (2014) Subgingival air-polishing with erythritol during periodontal maintenance: Randomized clinical trial of twelve months. J Clin Periodontol 41(9): 883-889.
- Haffajee AD, Socransky SS, Goodson JM (1983) Clinical parameters as predictors of destructive periodontal disease activity. J Clin Periodontol 10(3): 257-265.
- Rybalov OV, Dolgina LM (1977) Case of subcutaneous emphysema of the face occurring in the treatment of periodontitis. Stomatologiia (Mosk) 56(6): 77.
- Partovi F, Izatt JA, Cothren RM, Kittrell C, Thomas JE, et al. (1987) A model for thermal ablation of biological tissue using laser radiation. Lasers Surg Med 7(2): 141-154.
- Porteous MS, Rowe DJ (2014) Adjunctive use of the diode laser in non-surgical periodontal therapy: Exploring the controversy. J Dent Hyg 88(2): 78-86.
- Fontana CR, Kurachi C, Mendonca CR, Bagnato VS (2004) Microbial reduction in periodontal pockets under exposition of a medium power diode laser: An experimental study in rats. Photomed Laser Surg 35(4): 263-268.
- Krohn-Dale I, Boe OE, Enersen M, Leknes KN (2012) Er: YAG laser in the treatment of periodontal sites with recurring chronic inflammation: A 12-month randomized, controlled clinical trial. J Clin Periodontol 39(8): 745-752.
- Slot DE, Kranendonk AA, Paraskevas S, Weijden FV (2009) The effect of a pulsed Nd: YAG laser in non-surgical periodontal therapy. J Periodontol 80(7): 1041-1056.
- Diao J, Yuan C, Tong PY, Ma ZK, Sun XY, et al. (2021) Potential roles of the free salivary microbiome dysbiosis in periodontal diseases. Front Cell Infect Microbiol 11:
- Arany PR (2020) Photoimmunotherapy: A novel field with overlapping light treatment approaches. Photobiomodul Photomed Laser Surg 38(9): 524-526.
- Namvar MA, Vahedi M, Abdolsamadi HR, Mirzaei A, Mohammadi Y, et al. (2019) Effect of photodynamic therapy by 810 and 940nm diode laser on herpes simplex virus 1: An in vitro Photodiagnosis Photodyn Ther 25: 87-91.
- Zeinoun T, Nammour S, Dourov N, Aftimos G, Luomanen M (2001) Myofibroblasts in healing laser excision wounds. Lasers Surg Med 28(1): 74-79.
- Marques MM, Pereira AN, Fujihara NA, Nogueira FN, Eduardo CP (2004) Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med 34(3): 260-265.
- Lin GH, Amo FSL, Wang HL (2018) Laser therapy for treatment of peri-implant mucositis and peri-implantitis: An American academy of periodontology best evidence review. J Periodontol 89(7): 766-782.
- Akiyama F, Aoki A, Miura-Uchiyama M, Sasaki KM, Ichinose S, et al. (2011) In vitro studies of the ablation mechanism of periodontopathic bacteria and decontamination effect on periodontally diseased root surfaces by erbium: yttrium-aluminum-garnet laser. Lasers Med Sci 26(2): 193-204.
- Giannelli M, Materassi F, Fossi T, Lorenzini L, Bani D (2018) Treatment of severe periodontitis with a laser and Light-Emitting Diode (LED) procedure adjunctive to scaling and root planing: A double-blind, randomized, single-center, split-mouth clinical trial investigating its efficacy and patient-reported outcomes at 1 year. Lasers Med Sci 33(5): 991-1002.
- Giannelli M, Formigli L, Lorenzini L, Bani D (2012) Combined photoablative and photodynamic diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized split-mouth clinical trial. J Clin Periodontol 39(10): 962-970.
- Dalvi S, Khetal N, Ansari S, Benedicenti S, Hanna R (2021) Utilization of 810nm diode laser treatment in periodontitis as an alternative to surgical debridement approach. Photochem Photobiol 97(3): 566-573.
- Dalvi S, Benedicenti S, Sălăgean T, Bordea IR, Hanna R (2021) Effectiveness of antimicrobial photodynamic therapy in the treatment of periodontitis: A systematic review and meta-analysis of in vivo human randomized controlled clinical trials. Pharmaceutics 13(6): 836.
- Pawelczyk-Madalińska M, Benedicenti S, Sălăgean T, Bordea IR, Hanna R (2021) Impact of adjunctive diode laser application to non-surgical periodontal therapy on clinical, microbiological and immunological outcomes in management of chronic periodontitis: A systematic review of human randomized controlled clinical trials. J Inflamm Res 14: 2515-2545.
- Dembowska E, Samulak R, Jędrzychowska A, Dołęgowska B (2022) Effects of a 980nm diode laser as an adjunct to nonsurgical periodontal therapy on periodontal status and inflammatory markers in patients after myocardial infarction: A randomized controlled trial. Photobiomodul Photomed Laser Surg 40(8): 532-542.
- Rios FG, Viana ER, Ribeiro GM, González JC, Abelenda A, et al. (2016) Temperature evaluation of dental implant surface irradiated with high-power diode laser. Lasers Med Sci 31(7): 1309-1316.
- Valente NA, Mang T, Hatton M, Mikulski L, Andreana S (2017) Effects of two diode lasers with and without photosensitization on contaminated implant surfaces: An ex vivo Photomed Laser Surg 35(7): 347-356.
- Atieh MA (2010) Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med Sci 4: 605-613.
- Shah R, Thomas R, Mehta DS (2017) Neutrophil priming: Implications in periodontal disease. J Indian Soc Periodontol 21(3): 180-185.
- Smiley CJ, Shanon LT, Elliot A, Michalowicz BS, John MT, et al. (2015) Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. Journal of the American Dental Association 146(7): 508-524.
- Haidary D, Franzen R, Gutknecht N (2016) Root surface temperature changes during root canal laser irradiation with dual wavelength laser (940 and 2780nm): A preliminary study. Photomed Laser Surg 34(8): 336-344.
- Coluzzi DJ, Parker SPA (2017) Lasers in dentistry-Current concepts. Textbooks in Contemporary Dentistry (TECD), 1st (Edn.), Springer Publishers, USA, 14(4): 291-293.
- Samulak R, Suwała M, Dembowska E (2021) Nonsurgical periodontal therapy with/without 980nm diode laser in patients after myocardial infarction: A randomized clinical trial. Lasers Med Sci 36(5): 1003-1014.
- Hanna R, Dalvi S, Sălăgean T, Pop ID, Bordea IR, et al. (2021) Understanding COVID-19 pandemic: Molecular mechanisms and potential therapeutic strategies. An evidence-based review. J Inflamm Res 14: 13-56.
- Üstün K, Erciyas K, Sezer U, Şenyurt SZ, Gündoğar H, et al. (2014) Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: A randomized split-mouth clinical trial. Photomed Laser Surg 32(2): 61-66.
- Grössner-Schreiber B, Griepentrog M, Haustein I, Müller WD, Lange KP, et al. (2001) Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implants Res 12(6): 543-551.
- Dembowska E, Jaroń A, Homik RA, Gabrysz TE, Bladowska J, et al. (2022) Comparison of the treatment efficacy of endo-perio lesions using a standard treatment protocol and extended by using a diode laser (940nm). J Clin Med 11(3): 811.
- Subramani K, Jung RE, Molenberg A, Hammerle CH (2009) Biofilm on dental implants: A review of the literature. Int J Oral Maxillofac Implants 24(4): 616-626.
- Mailoa J, Lin GH, Chan HL, MacEachern M, Wang HL (2014) Clinical outcomes of using lasers for peri-implantitis surface detoxification: A systematic review and meta-analysis. J Periodontol 85(9): 1194-1202.
- Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 2000 36: 59-97.
- Ciurescu CE, Cosgarea R, Ciurescu D, Gheorghiu A, Popa D, et al. (2019) Adjunctive use of InGaAsP and Er,Cr:YSGG lasers in nonsurgical periodontal therapy: A randomized controlled clinical study. Quintessence Int 50(6): 436-447.
- Ishizaki NT, Matsumoto K, Kimura Y, Wang X, Kinoshita J, et al. (2004) Thermographical and morphological studies of Er,Cr:YSGG laser irradiation on root canal walls. Photomed Laser Surg 22(4): 291-297.
- Apel C, Meister J, Ioana RS, Franzen R, Hering P, et al. (2002) The ablation threshold of Er:YAG and Er:YSGG laser radiation in dental enamel. Lasers Med Sci 17(4): 246-252.
- Fenelon T, Bakr MM, Walsh LJ, George R (2020) Effects of lasers and their delivery characteristics on machined and micro-roughened titanium dental implant surfaces. Bioengineering 7(3): 93.
- Melanie N, Mobadder M, Magnin D, Peremans A, Verspecht T, et al. (2019) Q-Switch Nd:YAG laser-assisted decontamination of implant surface. Dentistry Journal 7(4): 99.
- Mertens B, Orti V, Vialaret J, Gibert P, Relaño-Ginés Aet al. (2018) Assessing a multiplex-targeted proteomics approach for the clinical diagnosis of periodontitis using saliva samples. Bioanalysis 10(1): 35-45.
- Gregg RH (2013) Lasers. Dent Today 32(10): 16.
© 2022 Pawelczyk-Madalińska M. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






