- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Efficacy of Trans-mastoidal Vestibular Galvanic Stimulation in Improvement Gait Performance and Upright Postural Stability in Hemiplegic CP Children
Ahmed M Azzam*
Department of Physiotherapy for Developmental Disturbance and Pediatric Surgery, Egypt
*Corresponding author: Ahmed M Azzam, Department of Physiotherapy for Developmental Disturbance and Pediatric Surgery, Egypt
Submission: February 2, 2019;Published: May 06, 2019

ISSN 2637-7934 Volume2 Issue3
Abstract
Objectives: This work was carried out to investigate the efficacy of galvanic vestibular stimulation in improving gait performance and upright postural stability in hemiplegic cerebral palsy children.
Method: Thirty children were enrolled in this study and randomly assigned into two groups; group A (galvanic vestibular stimulation plus vestibular training program), and group B (vestibular training program). Stride length and time, walking speed tests and modified Ashworth, pediatric balance scales were used to detect and follow the walking performance and upright postural stability. This measurement was taken before initial treatment and after 12 weeks of treatment. The children parents in both groups A and B were instructed to complete 3 hours of the home routine program.
Results: Data analysis was available on the 30 hemiplegic cerebral palsied children participated in the study. The difference between pre and post-treatment results was significant representative in stride length and time and walking velocity, spasticity changes and pediatric balance scores in study groups while insignificant improvement in control groups.
Conclusion: The combined vestibular training program and trans-mastoidal vestibular galvanic stimulation are suggested in improving walking performance and upright postural stability in a static and dynamic situation. So, this selective physiotherapy approach may be used as a strong choice for improving walking and balance abilities in hemiplegic C.P children.
Keywords: Galvanic vestibular stimulation; Cerebral palsy; Walking performance
Introduction
Central nervous system lesion which occurs in cerebral palsy patients lead to impaired postural reaction mechanism, loss of reciprocal inhibition mechanism, muscle tone disturbance, impaired coordination mechanism and failure of posture stabilization. Poor balance mechanism is the direct source of the defective motor and functional skills acquisition and motor development delay [1-3] learning process through sensory feedback is the plasticity way for continuous modifications of neural connections. Environmental manipulation and external stimulation reshape the neural network patterns through synaptic competition mechanism with the greatest activated synaptic connections become the winner and gain the skill and the lowest activated become the loser. Throughout of life there is an alteration of the synapse’s functional characteristics according to the new experience and skills plus environmental elements and variant situations or occurring CNS lesion [4]. Brain plasticity can occur at every lifetime because the CNS is continually remodelling via synaptic reorganization. So vestibular restoration therapy relies on the active participation which produces anatomical and permanent changes in the synaptic network of the neural circuits which produce behavioral changes on the postural stability [5-10]. Utilization of vestibular galvanic electric stimulation on the mastoid process via placing of anode electrode on the mastoid process in the similar side of body sway and the cathode on the other side. This will activate Na+ channels opening lead to depolarization of vestibular afferent nerve which produce modulation of the afferent vestibular signals by raising firing rate of the vestibular afferent on the cathode side and reducing the excitation degree on the anodal side which leading to deviation of the posture toward anodal side [11-13]. GVS has a positive effect on the balance control via improve balance response time, decreasing the lateral and A-P sway in static balance together with improving dynamic balance performance and correcting the abnormal perception sway and motor response [14]. The anticipatory postural control was improved after application GVS via modulating the vestibulospinal tract signals and activation of vestibular-related sensory areas [15]. During walking the antigravity muscle tonus (ᵞ and α motor neurons) were modulated by the vestibulospinal and reticulospinal tracts so by the application of GVS will increase the vestibulospinal tract activity which transmitted to the antigravity muscles increasing their excitability leading to increasing of stride length and walking speed [16-27].
Material and Methods
Subjects
30 children from the two sexes with hemiplegic C.P. children were joined in this study, aged 5 to10 years at a time of enrollment due to the children in this age could participate in pediatric balance scale graduations. Children could walk with assistance, On the other hand, C.P. Children that run up against the involvement rules were derived out if they had: preceding BoNT-A dose in the L.L or U.L in the last 12 months or had surgical tendon or muscle lengthening operation. Children were selected randomly to the study group (A) taken vestibular galvanic electric stimulation plus vestibular training approach while the control group (B) taken vestibular training approach only. The individual-based vestibular galvanic electric stimulation treatment sessions of 30 minutes were conducted day after day for 3 months in a physiotherapy treatment room after the vestibular training period for the group (A). Also, children in the study group were subjected to home regular program 3 hours daily for the 3 months treatment period. The control group (B) received a vestibular training program only.
Outcome measurements
Gait speed test: It was used to evaluate the walking speed by determining 10 meters which evaluates functional vestibular abilities. Measuring tape and stopwatch tools were needed to measure the walking speed. Starting and finishing areas of one meter were used to build up the maximum speed and decelerate of the speed. The average of 3 trials results was recorded pre and post treatment [28].
Modified ashworth scale: It was used to evaluate and follow up the degree of muscle tone disturbance pre and post-treatment.
Pediatric balance scale: It was used to evaluate functional balance abilities. It consists of 14 items each one is scored on a 5-point scale with zero scores indicate to the child cannot achieve the task without assistance and 4 scores indicate to independence in performing the ability.
The highest score is 56 points (the summation of the scores in all items).
The points from 41-55 indicate to the independent child
The points from 21-40 indicate to the child need assistance
The points 20 or below indicate to the child need wheelchair [29,30].
Stride length and stride time measurements: Stride length is the extension from the toe of the foot (starting position) to the toe of same foot (ending position), or from the heel to heel of the same foot. The stride time was detected by stopwatch. 10 meter distance (about 8 steps) was calibrated to evaluate the stride length before and after treatment. Stride time could be calculated by counting time required to perform walking in the calibrated distance.
Intervention: For all children, the treatment was handling three times weekly, for 3 months. Each session persists for 90 minutes (30 minutes vestibular galvanic electric stimulation for study group plus 60 minutes for a vestibular training program for each group) in a physical therapy room, in addition to 3 hours of the home regular program, 7 days a week around the treatment duration.
Both groups (A and B) received a vestibular training program, like the following:
a) With the eyes closed: concentration on vestibular system training through an unstable surface to isolate proprioceptors and perform postural reaction training(righting +equilibrium+ protective reaction training) through medical balls and balance board of different sizes.
b) With the eyes opened then closed slow then rapid training. The child performs dynamic vestibular training forward to the mirror by walking sideways with feet followed each other then walk on one line then walk by passing foot to one another.
c) Static training to vestibular system by performing proximal stabilization in a quadruped, kneeling, half kneeling, standing.
d) Proprioceptive training with vestibular training by standing on one foot with eyes opened then closed.
e) Equal weight shift transfer by starting the big base of support then gradual decrease of BOS without disturbance then with disturbance-with eye opened then eye closed-with hand support then without.
f) Step forward including or excluding disturbance, with eyes opened then closed on different directions and surfaces.
g) Weight-bearing with upper extremity functional training as hand skills training (from sitting-standing-kneeling) as grasping, voluntary release, reaching, hand manipulative skills, bilateral hand use, and eye-hand coordination training.
h) Changing positions from non-weight bearing to weight bearing and opposite and from static to dynamic and opposite.
i) Upside training anteroposterior movement and lateral movement then rotatory movement.
j) Swing therapy anteroposterior movement and lateral movement then rotatory movement.
k) Biodex stability system training.
l) Perturbation with different positions (quadrupedkneeling- half-kneeling-standing, holding a big medical ball with maintaining posture stability, stand against the corner, stand with holding stand bar and manual support standing).
m) Jumping training with trampoline [28].
The experimental group (group A) received vestibular galvanic electric stimulation following: Vestibular galvanic electric stimulation is a noninvasive technique that sending a direct electric message to the vestibular receptors (3semicircular ducts and the two otolith organs utricle and saccule) aiming for enhancement of gait performance. By locating a target to the child and starting point on a sheet and placed the anodal electrode on the mastoid process on the side of paralysis and the cathode on the other mastoid process. The elastic headbands stabilized the two stimulating electrodes. The physiotherapist asks the child to go with holding the child from his upper parts of the shoulder in the similar time of turning on the direct current with the intensity of 0.5mA intensity with long latency more than 200ms for 30 minutes. There is deviation of the posture on the anodal side till the child reaches to the target or the child could use a pediatric treadmill withholding of hand support and ask the child to slowly walk with the similar time of turning the apparatus on.
Result
Patients characteristics
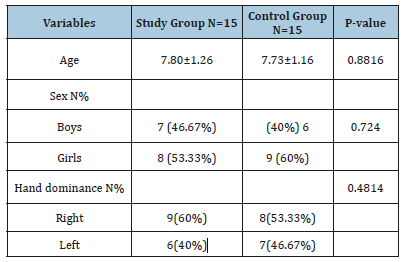
Table 1 display the demographic and analytic traits of all patients. There were 13 boys (43,33%) and 17 girls (56.67%) and in term of right-hand dominance reported in 17 patients (56.67%), and also13 patients (43,33%) were left-hand dominance. There was no representative change within both groups regarding age (p=0.8816), to sex (p=0.7240) and in term of hand dominance (p=0.4814).
Table 1:Patients charactaristics.
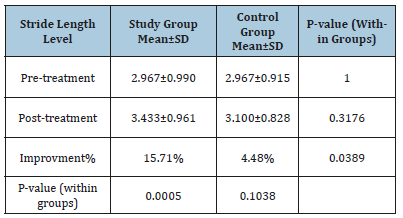
Changes in stride length
Mean test scores and SD for both groups are demonstrated in Table 2. The mean record of stride length level in the two groups at (pre- and post-treatment levels) was worthless (p>0.05). The average improvement of stride length level tended to be extremely representative’s improvement in the experimental group (3.433±0.961versus2.967±0.990, p=0.0005) while worthless representatives in the control group (3.100±0.828versus 2.967±0.915, p=0.1038). The percentage of improvement of stride length level was (15.706%) in the study group compared to the (4.483%) in the control group.
Table 2:The average test of stride length level in both groups.
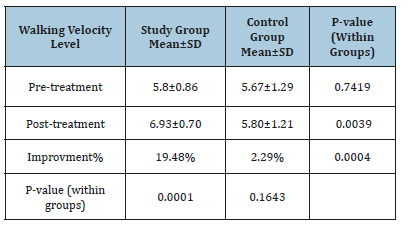
Changes in walking velocity
Mean test scores and SD for the both are demonstrated in Table 3. The mean record of walking velocity level in the two groups at (pre-treatment) was worthless (p>0.05) while the two groups had a representative’s improvement in walking velocity at post-treatment level(p<.05). The average improvement of walking velocity level tended to be extremely representative’s improvement in the study group (6.93±0.7 versus 5.8±0.86, p=0.0001) while worthless representatives regarding control group (5.80±1.21versus 5.67±1.29, p=0.1643). The percentage of improvement of walking velocity level was (19.48%) in the study group compared to the (2.29%) regarding the control group.
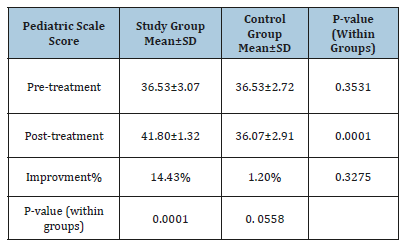
Changes in pediatric scale
Mean test scores and SD for both groups are presented in Table 4. The mean record of pediatric scale score in the two groups at pre-treatment was worthless (p>0.05) while the two groups had a significant improvement in pediatric scale score posttreatment( p<.05). The average improvement of pediatric scale score tended to be extremely representative’s improvement in the study group (41.80±1.32 versus 36.53±3.07, p=0.0001) than regarding control group (36.07±2.91versus 36.53±2.72, p=0.0558). The percentage of improvement of pediatric scale score was (14.43%) in the study group compared to the (1.2%) regarding control group.
Table 3:The average test of walking velocity level in both groups.
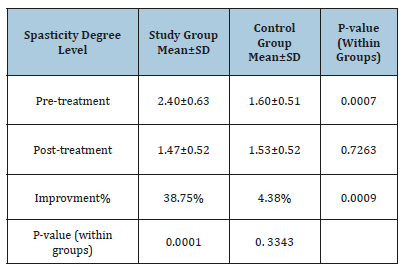
Changes in spasticity degree
Mean test scores and SD for both groups are demonstrated in the Table 5. The mean record of spasticity degree level in the two groups at (pre-treatment) had significant improvement (p<0.05) while both groups had a worthless improvement in spasticity degree at post-treatment level (p>0.05). The average improvement of spasticity degree level tended to be extremely representative’s improvement in the study group. (1.47±0.52 versus 2.40±0.63, p=0.0001) while worthless regarding control group (1.53±0.52versus 1.60±0.51, p= 0.3343). The percentage of improvement of spasticity degree level was (38.75%) in the study group compared to the (4.375%) regarding control group.
Table 4:The average test of pediatric scale score in both groups.
Changes in spasticity degree
Mean test scores and SD for both groups are demonstrated in the Table 5. The mean record of spasticity degree level in the two groups at (pre-treatment) had significant improvement (p<0.05) while both groups had a worthless improvement in spasticity degree at post-treatment level (p>0.05). The average improvement of spasticity degree level tended to be extremely representative’s improvement in the study group. (1.47±0.52 versus 2.40±0.63, p=0.0001) while worthless regarding control group (1.53±0.52versus 1.60±0.51, p= 0.3343). The percentage of improvement of spasticity degree level was (38.75%) in the study group compared to the (4.375%) regarding control group.
Table 5:The average test of spasticity degree level in both groups.
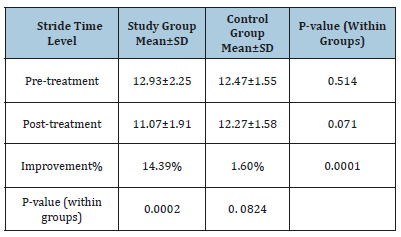
Changes in stride time
Mean test scores and SD for both groups are demonstrated in Table 6. The mean record of stride time level in the two groups at (pre-and post-treatment level) was worthless (p>0.05).The average improvement of stride time level had a tendency to be extremely representatives improvement in the study group (11.07±1.91 versus 12.93±2.25, p=0.0002) than regarding control group (12.27±1.58 versus 12.47±1.55, p= 0. 0824). The percentage of improvement of stride time level was (14.385%) in the study group compared to the (1.6%) regarding control group.
Table 6:The average test of stride time level in both groups.
Discussion
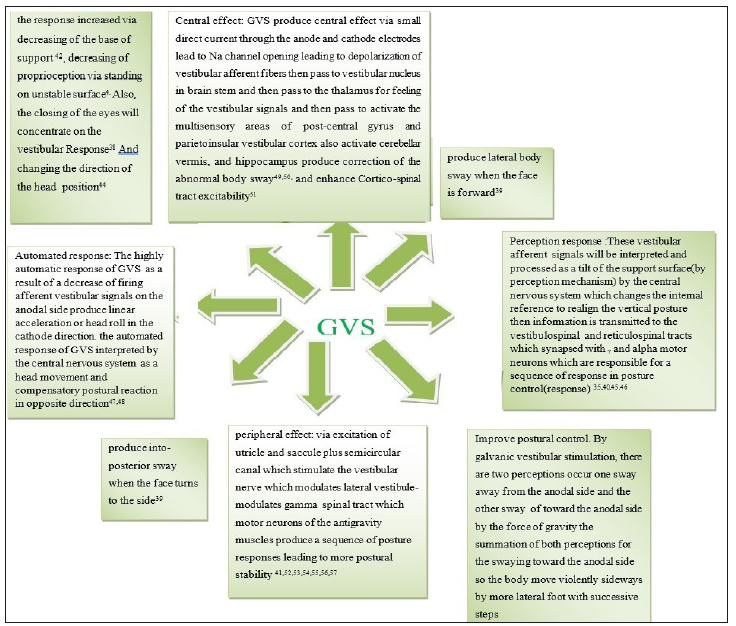
Utricle and saccule stimulation occur by placing galvanic stimulation on mastoid processes in which cathode on one side and anode on another side which lead to sway on the anodal side. The perception tilt sway occur toward the paralyzed side so the anode should be located on the mastoid process of the paralyzed side because it decreases the firing rates in vestibular afferent of anodal current this will cause shifting in subject perception sway(tilt) in a direction opposite to that turned in walking [13,31]. When a patient walks in a well-learned place he will have a pre-programmed strategy and neural motor circuits so when the patient makes a fast walking there is decrease in sway as a result of vestibular signals is less vital in fast walking as it depends on pre-programmed motor strategy and less of vestibular input while slow walking depends mainly on vestibular input so the sway in vestibular impairment is more in slowly walking than fast walking [32]. Patients with vestibular impairment make sway laterally, decrease walking speed and increase head movement as a compensatory mechanism [33]. Patients with unilateral vestibular impairment suffer from sway toward affected side [34]. The response of GVS is low when the patient is in standing but has a great effect during walking. This is an indicator that vestibular apparatus is very vital during walking more than during standing [35-40]. GVS could stimulate the semicircular canal which evoked by angular acceleration and head velocity also could stimulate the static utricle and saccule which evoked by linear acceleration and head tilt [41-57]. The static and kinetic labyrinthine provide the CNS with successive sensory input about the linear and rotatory acceleration of the head which activates postural control during head movement via vestibulospinal and reticulospinal tracts [58]. GVS is an electric stimulation passed through application over mastoids producing modulation of the vestibular hair cells and their afferent activity [12,59]. GVS could decrease the abnormalities in walking performance especially in slow walking because the vestibular apparatus has a great role in postural stability in slow walking than in fast walking [60-64]; (Figure 1).
Figure 1:Underlying mechanisms of vestibular galvanic electric stimulation.
Because the vestibular apparatus is a nonlinear fundamentally, so the numbers of the neural network units increase the spread of stimulation producing large correct neural response due to more complicated neural network were involved in dynamic balance than in static balance which leads to improvement of stride length and time and walking velocity [65,66]. The vestibular disorder clinical picture includes static symptoms which include vestibular nystagmus, head tilting, and body as a vestibulospinal sign, vertigo, perception sway and autonomic manifestations (nausea and vomiting) it needs short time for compensations to occur. Dynamic symptoms include impaired postural control and VOR deficits. It is poorly compensated and take a long of time [67]. The vestibular restoration therapy is aiming for formation new learned dynamic strategies that can adjust posture during walking to reach the best performance of daily activities. The vestibule-plasticity depends on changing of the traditional neural circuits to welldeveloped strategies. The interplay between the brain plasticity and vestibule-plasticity in developing re-synaptic connection is the way for reaching the optimum dynamic balance control [68]. The molecular and cellular responses of the CNS due to feedback input and feedforward command as a result of physiotherapy training which produces increasing of neurotrophins, neurogenesis and new motor strategies which improve CNS plasticity [69-73]; (Figure 2). The desensitization practices in vestibular restoration therapy is aiming for learning acquisition process to deal with difficult circumstances and responses could be used in vertigo treatment [74-78]. Vestibular restoration therapy should concentrate on adaptation mechanism for gaining the dynamic vestibular skill sensory substitution play a vital role in recovery of vestibular impairment by increasing the sensory feedback during close and open eyes, disturbance stimulus through different positions and manipulating the surface(unstable-rough-smooth-rubbery), upside down training. proprioceptive training through static weight bearing and dynamic approximation plus a sense of weights to gain improvement of postural sway [73,79,80].
Conclusion
The combined vestibular training program and transmastoidal vestibular galvanic stimulation are suggested in improving walking performance and upright postural stability in a static and dynamic situation. So, this selective physiotherapy approach may be used as a strong choice for improving walking and balance abilities in hemiplegic C.P children.
Acknowledgement
The author would like to acknowledge professor Dr. Emam Elnegmy for his support and advices.
References
- Kerem Günel M, Livanelioğlu A (2009) Cerebral palsy physiotherapy. New Uzbek Press, Ankara, Turkey.
- Berger W, Altenmueller E, Dietz V (1984) Normal and impaired development of children’s gait. Hum Neurobiol 3(3): 163-170.
- Liao HF, Jeng SF, Lai JS, Cheng CK, Hu MH (1997) The relation between standing balance and walking function in children with spastic diplegic cerebral palsy. Dev Med Child Neurol 39(2): 106-112.
- Hubel DH, Wiesel TN (1965) Binocular interaction in striate cortex of kittens reared with artificial squint. J Neuro Physiol 28(6): 1041-1059.
- Merzenich MM (2013) Soft wired: how the new science of brain plasticity can change your life. San Francisco, Parnassus Publishing, California, USA.
- Merzenich MM, Vleet VTM, Nahum (2014) Brain plasticitybased therapeutics. Front Hum Neuro Sci 8: 385.
- Allred RP, KimSO, Jonas (2014) Use it and/or lose it–experience effects on brain re-modelling across time after stroke. Front Hum Neurosci 8: 379.
- Hebb DO (1947) The effects of early experience on problem-solving at maturity. Am Psychol 2: 306-307.
- Alwis DS, Rajan R (2014) Environmental enrichment and the sensory brain: the role of enrichment in remediating brain injury. Front Syst Neurosci 8: 156.
- Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25(6): 292-298.
- Lowenstein O (1955) The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata). J Physiol 127(1): 104-117.
- Goldberg JM, Smith CE, Fernandez C (1984) The relation between discharge regularity and responses to externally applied galvanic currents in the vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51(6): 1236-1256.
- Courjon JH, Precht W, Sirkin DW (1987) Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp Brain Res 66(1): 41-48.
- Nardone A, Schieppati M (2006) Balance in parkinson’s disease under static and dynamic conditions. Mov Disord 21(9): 1515-1520.
- Samoudi G, Nissbrandt H, Dutia MB, Bergquist F (2012) Noisy galvanic vestibular stimulation promotes GABA release in the substantia nigra and improves locomotion in hemi-parkinsonian rats. PLoS One 7(1): e29308.
- Blouin JS, Dakin CJ, Vanden Doel K, Chua R, McFadyen BJ, et al. (2011) Extracting phase-dependent human vestibular reflexes during locomotion using both time and frequency correlation approaches. J Appl Physiol 111(5): 1484-1490.
- Dakin CJ, Inglis JT, Chua R, Blouin JS (2013) Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J Neurophysiol 110(1): 86-94.
- Brandt T, Strupp M, Benson J (1999) You are better off running than walking with acute vestibulopathy. Lancet 354(9180): 746.
- Schniepp R, Mohwald K, Wuehr M (2017) Gait ataxia in humans: vestibular and cerebellar control of dynamic stability. J Neurol 264(1): 87-92.
- Schniepp R, Wuehr M, Neuhaeusser M, Kamenova M, Dimitriadis K, et al. (2012) Locomotion speed determines gait variability in cerebellar ataxia and vestibular failure. Mov Disord 27(1): 125-131.
- Matsuyama K, Drew T (2000) Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. walking on a level surface. J Neurophysiol 84: 2237-2256.
- Mori S (1987) Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol 28(2): 161-195.
- Minor LB, Lasker DM, Backous DD, Hullar TE (1999) Horizontal vestibule-ocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol 82(3): 1254-1270.
- Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE (2007) Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27(4): 771-781.
- Bent LR, Inglis JT, McFadyen BJ (2004) When is vestibular information important during walking? J Neurophysiol 92(3): 1269-1275.
- Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D (2008) The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 131(11): 2913-2927.
- Ilg W, Golla H, Thier P, Giese MA (2007) Specific influences of cerebellar dysfunctions on gait. Brain 130(3): 786-798.
- Kembhavi G, Darrah J, Magill EJ, Loomis J (2002) Using the berg balance scale to distinguish balance abilities in children with cerebral palsy. Pediatr Phys Ther 14(2): 92-99.
- Kavlak E, Ünal A, Tekin F, Altuğ F (2018) Effectiveness of bobath therapy on balance in cerebral palsy. Cukurova Medical Journal 43(4): 975-981.
- Franjoine MR, Gunther JS, Taylor MJ (2003) Pediatric balance scale: a modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr Phys Ther 15(2): 114-128.
- Goldberg JM, Fernandez C, Smith CE (1982) Responses of vestibular nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Research 252(1): 156-160.
- Richard CF, Daniel LW, Janet LT (1999) Effects of galvanic vestibular stimulation during human walking. J Physiol 517( 3): 931-939.
- Glasauer S, Amorim MA, Vitte E, Berthoz A (1994) Goal-directed linear motion in normal and labyrinthine-defective subjects. Experimental Brain Research 98(2): 323-335.
- Halmagyi GM, Baloh RW (1996) Overview of common syndromes of vestibular disease. In disorders of the vestibular System. Oxford University Press,UK, pp. 291-299.
- Coates AC (1973) Effect of varying stimulus parameters on the galvanic body-sway response. Annals of Otology, Rhinology and Laryngology 82(1): 96-102.
- Nashner LM, Wolfson P (1974) Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Research 67(2): 255- 268.
- Smetanin BN, Popov KE, Gurfinkel VS, Shlykov VY (1988) Effect of real and illusory movements on the human vestibule-motor reaction. Neirofiziologiia 20: 250-255.
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, et al. (1993) Postural electro-myographic responses in the arm and leg following galvanic vestibular stimulation in man. Experimental Brain Research 94(1): 143-151.
- Fitzpatrick R, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. Journal of Physiology 478(2): 363-372.
- Inglis JT, Shupert CL, Hlavacka F, Horak FB (1995) Effect of galvanic vestibular stimulation on human postural responses during support surface translations. Journal of Neurophysiology 73(2): 896-890.
- Yang Y, Pu F, Lv X, Li S, Li J, et al. (2015) Comparison of postural responses to galvanic vestibular stimulation between pilots and the general populace. Biomed Res Int 2015: 567690.
- Day BL, Severac CA, Bartolomei L, Pastor MA, Lyon IN (1997) Human body-segment tilts induced by galvanic stimulation: a vestibular driven balance protection mechanism. J Physiol 500(3): 661-672.
- Fitzpatrick R, McCloskey DI (1994) Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol 478(1): 173-186.
- Lund S, Broberg C (1983) Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand 117(2): 307-309.
- Popov KE, Smetanin BN, Gurfinkel VS, Kudinova MP, Shlykov VYu (1986) Spatial perception and vestibulo motor responses in man. Neurophysiol 18: 548-553.
- Hlavacka F, Mergner T, Krizkova M (1996) Control of body vertical by vestibular and proprioceptive inputs. Brain Res Bull 40(5-6): 431-435.
- Guerraz M, Thilo KV, Bronstein AM, Gresty MA (2001) Influence of action and expectation on visual control of posture. Cogn Brain Res 11(22): 259-266.
- Guerraz M, Day BL (2001) Human body response to galvanic vestibular stimulation is not affected when the stimulus is selftriggered. J Physiol 531: 142.
- Utz KS, Dimova V, Oppenlander K, Kerkhoff G (2010) Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology review of current data and future implications. Neuropsychologia 48(10): 2789-2810.
- Stephan T, Deutschlander A, Nolte A, Schneider E, Wiesmann M, et al. (2005) Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage 26(3): 721- 732.
- Moliadze V, Atalay D, Antal A, Paulus W (2012) Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimul 5(4): 505-511.
- Uchino Y, Kushiro K (2011) Differences between otolith- and semicircular canal activated neural circuitry in the vestibular system. Neurosci Res 71(4): 315-327.
- Lowrey CR, Bent LR (2009) Modulation of the soleus H-reflex following galvanic vestibular stimulation and cutaneous stimulation in prone human subjects. Muscle Nerve 40(2): 213-220.
- Kennedy PM, Inglis JT (2001) Modulation of the soleus H-reflex in prone human subjects using galvanic vestibular stimulation. Clin Neurophysiol 112(11): 2159-2163.
- Roerdink M, Hlavackova P, Vuillerme N (2011) Effects of plantar-flexor muscle fatigue on the magnitude and regularity of center-of-pressure fluctuations. Exp Brain Res 212(3): 471-476.
- Hlavackova P, Pradon D, Vuillerme N (2012) Control of bipedal posture following localized muscle fatigue of the plantar-flexors and fingerflexors. Eur J Appl Physiol 112(2): 789-793.
- Cathers I, Day BL, Fitzpatrick RC (2005) Otolith and canal reflexes in human standing. J Physiol 563(1): 229-234.
- Baloh RW, Honrubia V (2001) Clinical neurophysiology of the vestibular system. Oxford University Press; New York 2001.
- Aw ST, Todd MJ, Aw GE, Weber KP, Halmagyi GM (2008) Gentamicin: vestibule-toxicity impairs human electrically evoked vestibular-ocular reflex. Neurology 71(22): 1776-1782.
- Fujimoto C, Yamamoto Y, Kamogashira T, Makoto Kinoshita, Naoya Egami, et al. (2016) Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep 6: 37575.
- Iwasaki S, Yamamoto Y, Togo F, Kinoshita M, Yoshifuji Y, et al. (2014) Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology 82(11): 969-975.
- Iwasaki S, Karino S, Kamogashira T, Togo F, Fujimoto C, et al. (2017) Effect of noisy galvanic vestibular stimulation on ocular vestibularevoked myogenic potentials to bone conducted vibration. Front Neurol 8: 26.
- Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, et al. Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 86(23): 2196-2202.
- Wuehr M, Nusser E, Krafczyk S, Straube A, Brandt T, et al. (2016) Noise-enhanced vestibular input improves dynamic walking stability in healthy subjects. Brain stimulation 9: 109-116.
- Collins JJ, Chow CC, Imhoff TT (1995) Stochastic resonance without tuning. Nature 376: 236-238.
- Collins JJ, Imhoff TT, Grigg P (1996) Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J Neurophysiol 76(1): 642-645.
- Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, ToddMJ, et al. (1990) The human horizontal vestibulo-ocular reflex in response to high- acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res 81(3): 479-490.
- Lacour M, Demanze LB (2015) Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy: 10 recommendations for optimal functional recovery. Front Neurol 5: 285.
- Cotman CW, Berchtold NC (2002) Exercise: behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25(6): 292-298.
- TravoC, Gaboyard NS, Chabbert C (2012) Plasticity of Scarpa’s ganglion neurons as a possible basis for functional restoration within vestibular end organs. Front Neurol 3: 91.
- Vande BR, Guinand N, Stokroos R, Guyo JP, Kingma H (2011) The vestibular implant: quovadis? Front Neurol 2: 47.
- Borel L, Lopez C, Peruch P, Lacour M (2008) Vestibular syndrome: a change in internal spatial representation. Clin Neurophysiol 38(6): 375- 389.
- Horak FB (2010) Postural compensation for vestibular loss. Resto Neurol Neurosci 28(1): 57-68.
- Norré ME, Becker AM (1988) Vestibular rehabilitation training. Specificity of a de-quate exercises. Arch Otolaryngol Head Neck Surg 114(8): 883-886.
- Brandt T, Daroff RB (1980) Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol 106(8): 484-485.
- Tee LH, Chee NW (2005) Vestibular rehabilitation therapy for the dizzy patient. Ann Acad Med Singapore 34(4): 289-294.
- Bronstein AM, Golding FJ, Gresty MA (2013) Vertigo and dizziness from environ- mental motion: visual vertigo, motionsickness and drivers’ disorientation. Semin Neurol 33(3): 219-230.
- Bittar RS, Barros CG (2011) Vestibular rehabilitation with bio feedback in patients with central imbalance. BrazJ Otorhinolaryngol 77(3): 356-361.
- Dozza M, Chiari L, Hlavacka F, Cappello A, Horak FB (2006) Effects of linear versus sigmoid coding of visual or auditory biofeedback for the control of upright stance. EEE Trans Neural Syst Rehabil Eng 14(4): 505- 512.
- Dozza M, Horak FB, Chiari L (2007) Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp Brain Res 178(1): 37-48
© 2019 Handan Ankarali. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






