- Submissions

Full Text
Evolutions in Mechanical Engineering
Synthesis of Differently Shaped Nontoxic AgNPs Stabilized by PVP and TSC
Lívia Mačák1*, Oksana Velgosova1 and Elena Čižmárová2
1Institute of Materials and Quality Engineering, Faculty of Materials Metallurgy and Recycling, Technical University of Košice, Letná, Slovakia
2Department of Materials Engineering, Faculty of Mechanical Engineering, Czech Technical University in Prague, Czech Republic
*Corresponding author:Lívia Mačák, Institute of Materials and Quality Engineering, Faculty of Materials Metallurgy and Recycling, Technical University of Košice, Letná, Slovakia
Submission: March 26, 2024;Published: April 08, 2024

ISSN 2640-9690 Volume5 Issue3
Abstract
Adjusting the shape of nanoparticles is a fundamental issue in achieving the desired properties. We have proven that it is possible to synthesize the nanoparticles with the desired shapes (spherical, triangular, and colloids with a mixture of shapes) by a suitable combination of reagents. Subsequently, the size and shape of the nanoparticles affect the color of the solution. UV-VIS spectroscopy was used to evaluate the colloidal solutions, and Transmission Electron Microscopy (TEM) to indicate the shape and size of the nanoparticles. Combination of electrostatic repulsion (TCS) and steric stabilization (PVP) secure stability of nanoparticles and act also as good caping agents. AgNPs show very low antimicrobial/antibiofilm activity against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria and one-cell algae Chlorella kessleri (Ch. kessleri).
Keywords:Silver nanoparticles; Chemical synthesis; UV-VIS spectroscopy; TEM; Toxicity
Introduction
Silver nanoparticles are used in various fields such as electronics, healthcare and consumer products. The healthcare industry is the second significant consumer of AgNPs, where nanoparticles are used in medical devices, wound dressings and other applications. There are several methods of nanoparticle synthesis. One of the simplest is chemical, this procedure involves reduction reactions in a metal precursor solution. The precursor ions are reduced using a suitable reducing and the addition of stabilizing agents ensures the stability of the nanoparticles. By changing the reagents’ concentration and other processing conditions it is possible to prepare nanoparticles with different shapes. The shape of the silver nanoparticles is an important factor in determining the optical properties of the colloid and can be tuned to produce a wide range of colors. The shape of AgNPs has important implications for a variety of applications including sensing, imaging and catalysis [1,2]. The shape of AgNPs also impact their interaction with biological systems. For example, rod-shaped AgNPs have been shown to have improved cellular uptake and enhanced therapeutic efficacy compared to spherical AgNPs due to their ability to penetrate cell membranes [3]. Octahedral AgNPs have been shown to exhibit greater stability than spherical AgNPs due to their higher surface energy and more stable crystal structure. Silver is well known for its toxic properties but in some applications, it is needed to suppress this property. Nontoxic AgNPs can find applications in medicine, can be functionalized, or loaded with various active ingredients such as drugs, antimicrobial agents. Non-toxic AgNPs allow for controlled and targeted release of these substances, ensuring effective delivery while minimizing potential toxicity to healthy cells. Overall, the need for different shapes of AgNPs arises from the desire to tailor their properties to specific applications. The aim of this work was to develop an easy technique to synthesize silver nanoparticles of different shapes with low toxicity. We analyzed the impact of NaBH4 volume on shape formation and proved the PVP and TSC capability to ensure a good capping/stabilizing effect of prepared nanoparticles. The toxic tests were performed on bacteria E. coli, S. aureus and one-cell algal Ch. kessleri.
Materials and Methods
Silver nitrate (>98%), sodium borohydride (≥98%), sodium citrate (TSC) (≥99%), hydrogen peroxide (H2O2 30%) and Polyvinylpyrrolidone (PVP) were purchased from Mikrochem Ltd., Pezinok, Slovakia. De-ionized water was used for preparing all solutions. Silver nanoparticles were synthesized by chemical method, the concentration and volume of used solutions were as follow: AgNO3-0.11mM, 100ml; TSC-30mM, 8.56ml; PVP-2% w/w, 8.56ml; H2O2-30%, 0.28ml; NaBH4-0.1M, different volumes: 0.35, 0.37, 0.4, 0.47, 0.58 and 0.7ml. All reagents were freshly prepared and in 1min intervals, added to AgNO3 solution under constant steering in followed order (except sample Ag4): TSC, PVP, H2O2 and NaBH4. Subsequently, solutions were cowered and stirred for 25min in the dark. The labeling of solutions: Ag1, Ag2, Ag3, Ag5 and Ag6- all reagents added, volume of NaBH4 0.35, 0.37, 0.4, 0.58 and 0.7ml, respectively. Ag4 was prepared similarly but without TSC, and H2O2 was added, volume of NaBH4 was 0.47ml. Synthesized AgNPs were analyzed by UNICAM UV-VIS Spectrophotometer UV4. The size and morphology of the nanoparticles were studied by means of TEM (JEOL model JEM-2000FX). The image analysis (Image J software) was used for the analysis of Ag nanoparticles’ size.
Toxicity
The toxicity of colloidal AgNPs was evaluated using the agar well diffusion and the standard disk-diffusion method. Three microorganisms were used for testing: E. coli, S. aureus, and Ch. kessleri. In agar disk-diffusion test, agar plates were inoculated with the microorganism Ch. kessleri. Then, sterile swabs (ø6mm) containing the test compound (~15μl) were placed on the agar surface. The agar well diffusion method was performed on bacteria E. coli and S. aureus. The agar plate surface was inoculated by spreading the microbial inoculum over the agar surface. Then, a hole with a diameter of 6mm was aseptically punched, and a volume (20μL) of the tested agent was introduced into the well. In both tests, Petri dishes were incubated under suitable conditions and the diameters of inhibition zones were measured. As a control, the AgNO3 solution was used.
Result and Discussion
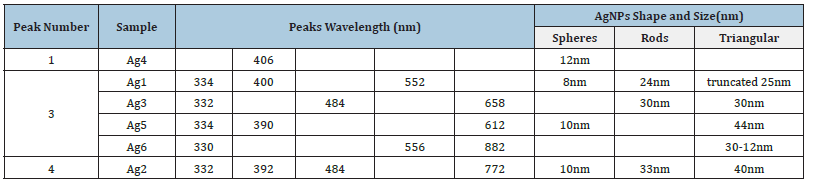
After adding all the ingredients and stirring for 25 minutes in the dark, colloids of different colors were formed, Figure 1, which indicates the presence of differently shaped nanoparticles. Based on the UV-VIS spectra, Figure 1a)-f), it is possible to divide all colloids into three groups: solutions with one (Ag4), three (Ag1, Ag3, Ag5, Ag6) and four peaks (Ag2). The TEM micrographs of AgNPs colloids are in Figure 1g)-l). Regarding UV-VIS spectra and TEM analysis, it can be concluded that all colloids, where spectra show a peak near 400nm contain spherical nanoparticles, Table 1. It is obvious that in all solutions that have a peak above 500nm the presence of triangles nano-prisms was observed. The higher the value of the peak wavelength, the greater portion of triangular prisms in the colloid. If there is a significant peak at ~500nm, rods may be synthesized, but this peak must be perceived in combination with other peaks, especially with a peak at higher wavelengths. The more the maxima are shifted to higher wavelengths, the sharper the triangles [4]. There are many works dealing with the effect of nanoparticle shape on toxicity and with the main mechanisms of AgNPs toxicity on living cells [5]. Nanoparticles can have a toxic effect on the cell through three mechanisms: oxidative stress, DNA damage, and reproductive disorders.
Figure 1:UV-VIS spectra and TEM micrographs of colloids. Sample: Ag1 to Ag6 corresponds to g)-l) microphotographs.
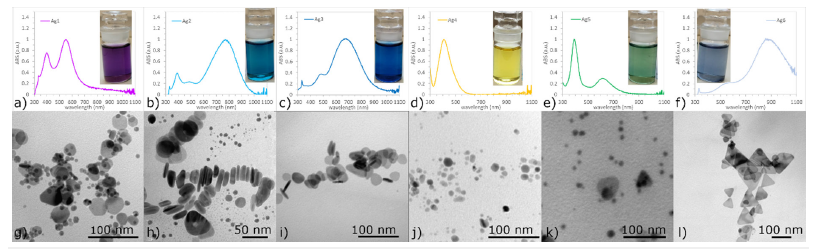
Table 1:Comparison of peaks wavelength and AgNPs shapes.
The degree of antibiofilm/toxic effect of prepared AgNPs on Ch. kessleri is sown in Figure 2. After 14 days of cultivation, just a very low, negligible inhibition zones were observed for colloid samples. Inhibition zones were max 9mm in diameter, which can be considered as a negligible toxic effect. On the other hand, AgNO3 control showed a 12mm inhibition zone, which proved that Ag+ ions have a good toxic effect. No inhibition zones were observed at the agar well diffusion method performed on E. coli and S. aureus (data not shown).
Figure 2:The anti-biofilm effect of AgNPs colloids and AgNO3 solution.

With proper AgNPs stabilization [6] there is a low probability of Ag+ ions release from AgNPs. Among the most common stabilization agents belong polymers, such as polyvinylpyrrolidone, polyethylene glycol, thiol-capping agents, etc. Surface coatings of nanoparticles are used mainly to stabilize them and prevent them from aggregating, it also reduces their toxicity by preventing them from interacting with biological molecules. The choice of surface coating depends on the specific application and the desired properties of the nanoparticles. TSC is mainly a stabilization agent, Gontijo LAP et al. [7] report that the silver colloidal particles possessed a negative charge due to the adsorbed citrate ions, a repulsive force worked along particles and prevented aggregation [7] (electrostatic repulsion), Figure 3. The latest research shows that citrate ions alone can stabilize the silver nanoplates by stabilizing formed silver nuclei through preferential binding to the {111} plains [8]. But its stabilization effect must be supported by another ligand. The combination of citrate and PVP helps to stabilize and control the formation of nanoparticles shape. PVP provides covalent organic ligands and allows steric repulsion to prevent aggregation, moreover, it can protect AgNPs surfaces from oxidation and instability [9].
Figure 3:Scheme of AgNPs synthesis and stabilization by TSC and PVP.
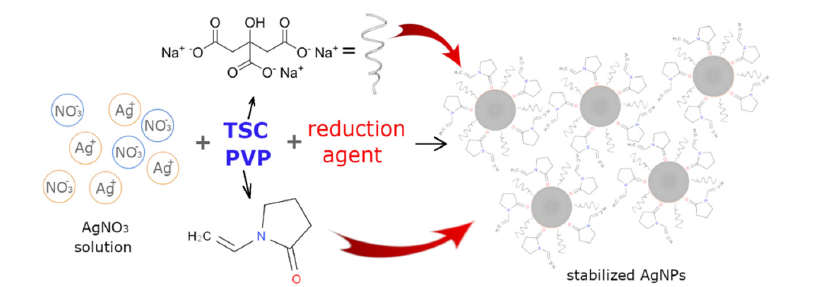
PVP has a higher affinity for the AgNPs surface than TSC. Citrate is generally thought to be weakly bound to the surface. In contrast, PVP coordinates with the AgNPs surface through van der Waals interactions and direct bonding interactions with the Ag d-band [10]. Due to direct bonding interactions, PVP is more complicated to displace than citrate. Studies have also shown that AgNPs exhibit dose-dependent toxicity, increasing their toxicity with increasing concentrations. The reason why our AgNPs are not highly toxic is that they have low solubility in water because of the good coating/stabilizing effect (PVP and TSC). The exact mechanisms underlying the toxicity of AgNPs are still not fully understood. The concentration and stabilization of AgNPs surface are key factors but it was found that the shape of AgNPs is also one of the important factors. Some shapes are more toxic than others. Spherical AgNPs have been shown to have lower toxicity compared to other shapes. The reason is that spherical AgNPs have a low surface area-tovolume ratio, which reduces their ability to interact with biological molecules and cells. Rod-shaped AgNPs have been found to be more toxic than spherical ones. Rod shape of nanoparticles allows them to penetrate cells more easily, leading to increased cellular damage. Triangular AgNPs have been found to be the most toxic shape. This is thought to be because the sharp edges and corners of the triangles can cause physical damage to cells and tissues. Also, the release of silver ions from triangular corners is more likely than from the surface of spherical nanoparticles.
Conclusion
We successfully prepared differently shaped silver nanoparticles by changing the concentration of the main reducing agent. The shape of nanoparticles influenced the color of the colloidal solution. It was proved that despite the toxicity of silver nanoparticles the use of PVP and TSC as stabilizing/capping agents secured the nontoxicity of synthesized AgNPs regardless of their shape. The proper coating increases the application possibility of nanoparticles.
References
- Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B 107: 668-677.
- Hutter E, Fendler JH (2004) Exploitation of localized surface plasmon resonance. Advanced Materials 16: 1685-1706.
- Huang X, Li L, Liu T, Hao N, Liu H, et al. (2011) The shape effect of mesoporous silica nanoparticles on biodistribution, clearance and biocompatibility in vivo. ACS Nano 5(7): 5390-5399.
- Raza M, Kanwal Z, Rauf A, Sabri A, Riaz S, et al. (2016) Size and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 6(4): 74.
- Stensberg MC, Wei Q, McLamore ES, Porterfield DM, Wei A, et al. (2011) Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 6(5): 879-898.
- Majeed S, Aripin FHB, Shoeb NSB, Danish M, Ibrahim MNM, et al. (2019) Bioengineered silver nanoparticles capped with bovine serum albumin and its anticancer and apoptotic activity against breast, bone and intestinal colon cancer cell lines. Mat Sci Eng C 102: 254-263.
- Gontijo LAP, Raphael E, Ferrari D, Ferrari JL, Lyon J, et al. (2020) PH effect on the synthesis of different size silver nanoparticles evaluated by DLS and their size-dependent antimicrobial activity. Rev Matéria 25(4): e12845.
- Zhang Q, Li N, Goebl J, Lu Z, Yin Y (2011) Systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? J Am Chem Soc 133(46): 18931-18939.
- Miesen TJ, Engstrom AM, Frost DC (2020) A hybrid lipid membrane coating “shape-locks” silver nanoparticles to prevent surface oxidation and silver ion dissolution. RSC Adv 10(27): 15677-15693.
- Jacobson KH, Gunsolus IL, Kuech TR, Troiano JM, Melby ES, et al. (2015) Lipopolysaccharide density and structure govern the extent and distance of nanoparticle interaction with actual and model bacterial outer membranes. Environmental Science & Technology 49(17): 10642-10650.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






