- Submissions

Full Text
Evolutions in Mechanical Engineering
Design and Manufacture of an Electrolyser for its Application in Obtaining Hydrogen as Fuel
Derick Zeceña and Jorge Ivan Cifuentes*
School of Mechanical Engineering, Universidad de San Carlos de Guatemala, Guatemala
*Corresponding author: Jorge Ivan Cifuentes, School of Mechanical Engineering, Faculty of Engineering Universidad de San Carlos de Guatemala 01012, Guatemala
Submission: March 11, 2020;Published: July 07, 2020

ISSN 2640-9690 Volume3 Issue2
Abstract
This paper shows the most important aspects of the operation of an alkaline electrolyser which was sized to adapt to the fuel demand conditions of an internal combustion engine under idle speed and considering a stoichiometric mixture. In the experimental part, the hydrogen flow rate generated by the electrolyzer was determined under nominal conditions, using a flowmeter based on the principle of gas collection in water. With the built electrolyser, a capacity factor of 2.62ml W-1 min-1 and a flow rate of 3.29lpm were obtained which was sufficient for the satisfactory operation of the engine. With the development of this project was proved that an ordinary internal combustion engine can operate using only hydrogen as fuel, without the need for any modification in its factory design.
Keywords: Hydrogen; Electrolyser; ICE; Alkaline electrolysis
Nomenclature
Ɛ: Efficiency; HHO: Oxyhydrogen; lpm: Liter per minute: STP: Standard conditions
Introduction
The internal combustion engines are machines that transform the chemical energy contained in the fuel into mechanical energy, which can be used in endless applications, however, these machines feed on non-renewable resources such as fossil fuels, mainly Petroleum. It is common knowledge that the combustion of fossil fuels generates various polluting gases that increasingly aggravate the current environmental problems. This is the main reason why the automotive industry is in search of new alternatives that may gradually allow fossil fuel replacement [1-8].
Over time, the use of hydrogen as a fuel for internal combustion engines has been considered, because the chemical energy of hydrogen is transformed cleanly, since the only by-product is water. Hydrogen is considered one of the most promising energy carriers of the future, because it is the most abundant element in the universe, totaling more than 70%, it also has high efficiency and can be used for transport, heat and generators.
One of the most important processes for hydrogen generation is electrolysis, which consists of breaking the water molecules into hydrogen and oxygen, using electricity. The use of electricity as an energy source for hydrogen production is clean and safe. Therefore, it is relevant to study the potential of hydrogen generation through electrolysis and analyze the general aspects for its use as a fuel in internal combustion engines, which seeks to evaluate an alternative that in the future, can support resolution of the current environmental problem.
Materials and Methods
Electrolyser construction
The electrolyser built was an alkaline electrolyser with a configuration of 7 plates per cell (+ n n n n n -) and with seven stacks or cells, with a total of 43 plates, 4 positive, 4 negative and 35 neutral. For the electrodes, 304mm stainless steel of 0.9mm thickness was used. The joints were made with 1/16” thick rubber, with 42 joints. The front and rear covers are made of 15mm thick high density polyethylene sheet. Each cover has a lower hole for the electrolyte inlet and an upper hole for the gas outlet. The front cover has two extra holes in which two couplings joined by a segment of vinyl hose that will serve as a liquid level meter inside the cell will be placed (Figure 1).
Figure 1: Seven stacks alkaline electrolyser.

Theoretical flow calculation
According to the Faraday’s Law and using the ideal gases equation and the coulomb definition we have that the theoretical gas flow can be calculated as follows:
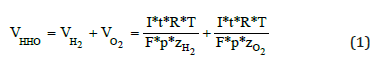
Where: V = Gas volume (l) R = Universal constant of ideal gases (latm mol-1 K-1)) I = Electric current (A) T = Temperature (K) F = Faraday’s Constant (A s mol-1) p = Pressure (atm) t =Time (s) z = Number of electrons in excess (2 for hydrogen and 4 for oxygen)
Assuming that the electrolyser works under standard conditions (STP) at 1A and for 1 minute we have:
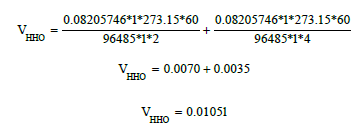
This corresponds to 0.0105lpm A-1 o 1.5944A lpm-1 per water compartment.
The measurement of the current flowing through the cell was performed using a digital hook multimeter, maintaining a stable reading of 109A, therefore there is a stacking current of 15.57A which is less than 26.05A which is the limit to maintain an efficient process. The theoretical flow can be calculated using the value obtained in the deduction of equation (1) and the values obtained in the measurements [9-15].

Real flow estimation
The real gas flow of the electrolyser was measured using a flowmeter that is based on the principle of gas collection in water. Ten tests were carried out of which the average time it took for the electrolyser to generate 200ml of gas was obtained Table 1.
Table 1: Time detain the generation of 200ml of hydrogen.
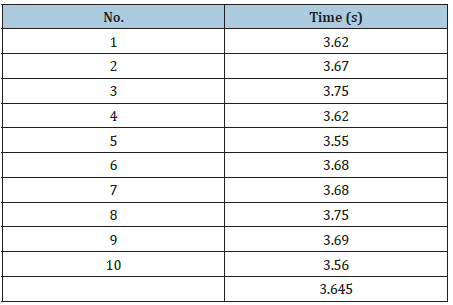
With the average time the real flow of the electrolyser was determined.
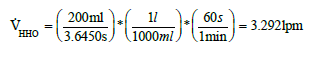
Results and Discussion
The operating efficiency of the electrolyser can be calculated using the following expression:
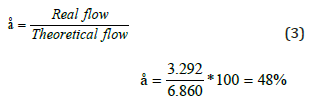
Capacity factor calculation
The capacity factor can be calculated whit the following expression:
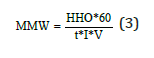
Where: MMW = Milliliters per minute per Watt HHO = Gas production volume (ml) I = Current (A) V = Voltage (V) t = Time (s)
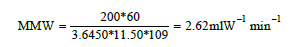
Calculation of the cost for the energy used for the generation
We start by calculating the real power consumed by the cell: P = I*V (4) Where: P = Power (W) I = Current (A) V = Voltage (V)
P = 109*11.50 = 1253.50W
Energy charge 0.260$/kWh
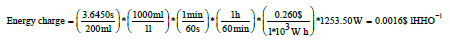
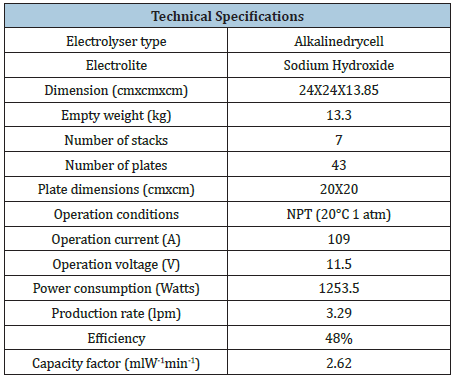
Electrolyser’s technical specifications
The Electrolyser’s technical specifications are showed in the Table 2.
Table 2: Electrolyser’s technical specifications.
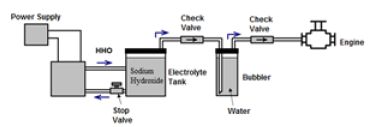
During the first tests in the experimental part with the engine running, a backfire occurred which caused the destruction of the electrolyte tank. This demonstrates the importance of having an intermediate element that does not allow the flame to reach the tank. That’s why a bubbler was placed between the electrolyte tank and the engine (Figure 2).
Figure 2: Final gas generation system configuration.
Conclusion
One of the biggest challenges for this kind of project is the obtaining of the desired hydrogen production rate using the least amount of electrical energy, for which a dry cell alkaline electrolyzer was selected, because it is the configuration that allows having the lowest energy losses.
There are many variables involved in the proper functioning of an electrolyser and it is impossible to modify any of them without affecting the others, those that were considered critical for the realization of this project were the active area, current density and voltage per plate.
The real production rate was lower than the theoretical one, mainly because in the moment when the gas generation starts, the pressure exerted by it causes part of the electrolyte to return to the tank, which reduces the active area. It should be noted that during the experimental part was possible to demonstrate that it is possible for an internal combustion engine without any modification to work using only hydrogen as fuel [16-26].
Acknowledgements
Research department at School of Mechanical Engineering, University of San Carlos of Guatemala.
References
- Akash Nirmal Kumar J, Gowtham N, Senthil S (2019) Design and fabrication of hydrogen powered ic engine. Int J Appl Eng Res 4(5): 423-426.
- Bessarabov D, Wang H, Li H (2019) PEM electrolysis for hydrogen production: principles and applications. Taylor & Francis, Abingdon, UK.
- College of the Desert (2019) Module 3: Hydrogen use in internal combustion engines. Hydrogen Fuel Cell Engines and Related Technologies, pp. 1-29.
- Corbo P, Migliardini F, Veneri O (2019) Hydrogen fuel cells for road vehicles.
- Felder R, Rousseau R (2019) Elementary principles of chemical processes.
- Gandia L, Arzamedi G, Dieguez P, Luis MG (2019) The name of the book.
- Genovese J, Harg K, Paster M, Turner J (2019) Hydrogen production cost estimate using water electrolysis: Independent review.
- Gillingham K (2019) Hydrogen internal combustion engine vehicles: a prudent intermediate step or a step in the wrong direction? Department of Management Science & Engineering, Global Climate and Energy Project, Precourt Institute for Energy Efficiency, Standford, USA.
- Godula-Jopek A, Stolten D (2019) Hydrogen production: by electrolysis.
- Göllei A (2019) Measuring and optimisation of HHO dry cell for energy efficiency.
- Harper GDJ (2019) Fuel cell projects for the evil genius.
- Kleperis J, Linkov V (2019) Electrolysis.
- MacCarley C (1978) Electronic fuel injection techniques for hydrogen fueled internal combustion engines.
- Molkov V (2019) Fundamentals of hydrogen safety engineering I.
- Molkov V (2019) Fundamentals of hydrogen safety engineering II.
- Revankar S, Majumdar P (2019) Fuel cells: principles, design, and analysis. 2019
- Sherif S, Goswami D, Stefanakos E, Steinfeld (2019) A Handbook of hydrogen energy.
- Stolten D, Emonts B (2019) Hydrogen science and engineering: materials, processes, systems and technology.
- Verhelst S, Wallner T (2019) Hydrogen-fueled internal combustion engines.
- Teed Litherland P (2019) The chemistry and manufacture of hydrogen.
- Weeks S (2019) Review of the prospects for using hydrogen as a fuel source in internal combustion engines.
- Wisema G (2019) Brown’s gas: Book 2.
- Yan X, Hino R (2019) Nuclear hydrogen production handbook.
- Zeng K, Zhang D (2019) Recent progress in alkaline water electrolysis for hydrogen production and applications.
- Zhang J, Li J, Li Y, Zhao Y (2019) Hydrogen generation, storage, and utilization.
- Zoulias E, Varkaraki E, Lymberopoulos N, Christodoulos C, Karagiorgis G (2019) A review on water electrolysis.
© 2020 Jorge Ivan Cifuentes. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






