- Submissions

Full Text
Evolutions in Mechanical Engineering
Heat-Resistant Coating of Molybdenum and Tungsten Compounds with Silicon and Carbon on The Surface of Layered Nb/(Si-B) Composite
VP Korzhov*
Institute of Solid-State Physics, Russia
*Corresponding author:VP Korzhov, Institute of Solid-State Physics, Russian Academy of Sciences, Russia
Submission: February 27, 2019;Published: March 21, 2019

ISSN 2640-9690 Volume2 Issue4
Abstract
Using diffusion welding under pressure of multilayer packages with a powder coating of molybdenum, tungsten and silicon, heat resistant multilayer composites ~35×40×2-3mm based on niobium with hardening niobium compounds with carbon and boron and heat-resistant coatings on the surface were obtained. Carbon in the Nb-alloy and coating fell from the CO-atmosphere in the chamber for welding and graphite gaskets located between the package and the punches.
Keywords: Niobium, Layered composite, Heat resistance, Heat-resistant coating, Molybdenum silicide, Tungsten silicide, Solid-phase sintering
Introduction
Any material and a metal material in particular, intended to be operated at high temperatures, is subject to gaseous corrosion when interacting with the environment. Only noble materials have the natural ability to resist gas corrosion, called heat resistance. But there can be no talk of their widespread use in industry. Therefore, protective coatings and alloying are used to protect the surface from corrosion.
The classic way to the development of heat-resistant materials was doping Fe-, Nialloys, including steels, with chromium. The positive effect lies in the formation of (Fe, Cr)2O3 chromium oxide on the surface of steel, for example. Chromium alloyed steels are successfully used for operation at temperatures up to 1100 °C. The surface of steel with chromium becomes even more heat-resistant, if it is doped insignificant, up to 1%, the amount of yttrium. The unique properties of that combine strength at high temperatures, heat resistance, long-term strength under static and variable loads are alloys based on Ni-Al system. However, the low melting point limits their operating temperature slightly above 1100 °C.
Refractory metals, their alloys, composite materials, which are developed on the basis of them, and, in particular, layered composites have high heat-resistant properties at temperatures above 1150 °С [1]. But they are unstable against gas corrosion. For a chemically active metal, such as niobium and other refractory metals, the problem of its protection against gas corrosion cannot be solved by doping. Therefore, in the late 50s and early 60s of the last centuries, it became clear that research on the development of protective coatings would be more promising [2]. Among them were coatings of niobium aluminide, zinc, a mixture of alumina powders and thorium dioxide, molybdenum silicide, zirconium boride, etc. Interesting were also attempts to create a two-layer coatings and bimetals Nb-alloy/ Chromium [3].
Coating methods depended on the shape, size and purpose of the coated parts. Claddings, deposition from the gas phase, immersion into the melt, diffusion saturation, and fusion were used. For the latter method, a plasma torch was effectively used. It was noted [4] the promise of plasma coatings in space technology. They were particularly resistant to low environmental pressure and high temperatures, that is, under conditions similar to the return of a spacecraft to Earth. Protection of refractory materials against gas corrosion at high temperatures of 1100-1600 °C, as well as adjacent issues of the fragility of the protective layer and the strength of its adhesion to the base metal, have been and remain the most urgent in the problem of heat resistance. Its solution will greatly expand the scale of the use of refractory metals and highheat- resistant alloys [2] based on them. In the meantime, in order to protect against gas corrosion, it is necessary to use more inert gases, where possible, as a working medium [5].
In this work, for heat-resistant layered composites based on niobium, strengthened by its compounds with silicon and boron [6], it is proposed to use heat-resistant silicide coatings of molybdenum and tungsten. The first experiments on the development of a solidphase method for the production of these coatings in a single process with the formation of a composite layered structure are presented.
Assembly Procedure and Diffusion Welding of Coated Packages
The initial multilayer packages for the production of heat resistant composites were assembled from Nb-foils 60 microns thick, with a unilateral suspension (Si-B)-coating. Suspensions are concentrated suspensions of fine powders in organic liquids. As it polyvinyl butyral was used in its work. Coatings based on it dried in the air. For the (Si-B)-coating, a powder mixture of silicon and boron in the ratio Si/B = 3 was used, and for a coating of Molybdenum, Tungsten and Silicon-a powder mixture of composition 44% Mo- 44%W-12 wt. %Si, corresponding to one of the phases Novotny.
Packages were assembled from U-shaped elements in which Nb-foils had one-sided (Si-B)-coatings. One of the coatings turned out to be outside the element, the other - inside it (Figure 1). With such an assembly sequence, as shown in the figure, the assembled package had one Nb-surface without coating, and another surface with (Si-B)-coating, which was covered with a separate niobium foil. The final operation was the application of (Mo-W-Si)-coatings on both surfaces of the packet see Figure 1.
Figure 1:Assembly scheme of a multilayer package of U-shaped elements made of Nb-foils with a single-sided (Si-B)-coating. Right is Nb/(Si-B)- package with outer (Mo-W-Si)-coatings, ready for diffusion welding..
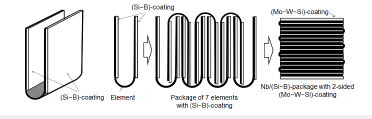
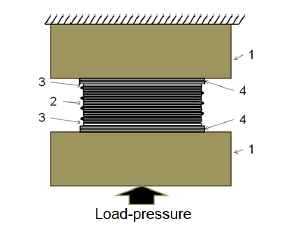
Thus, in the assembled state, the package had 15 layers of niobium (7×2++1=15) alternating with 14 layers of silicon with boron, the content of which in the package was 15,5wt.%, and the outer layers of molybdenum, tungsten and silicon (7,8 wt.%). Next, the package was subjected to diffusion welding under pressure (Figure 2), during which the composite layered structure was formed, and a heat-resistant coating was formed on its surface. Carbon penetrated into the coating due to gaskets 4 of thermally split graphite [7], located between the package and the punches see Figure 2.
Figure 2:Diffusion welding of Nb/(Si-B)-package with outer (Mo-W-Si)-coatings: 1-fixed and movable punches, 2-pack of Nbelements with (Si-B)-coatings, 3-(Mo-W-Si)- coatings, 4-thermally split graphite gaskets.
Microstructure
In Figure 3 shows the coating formed on the surface of the composite after diffusion welding at 1400 °C and a pressure of 7,4MPa for 1 hour. The coating thickness is ~60 microns. It looks quite dense, without transverse cracks and is in good contact with the composite-substrate. Between the coating and the volume of the composite with a layered structure, an intermediate layer ~10 microns thick is visible.
Figure 3:A fragment of the microstructure of the composite with a protective coating.
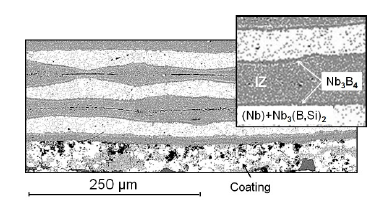
The layered structure of the composite consists of light layers that inherit Nb-foil and gray layers of the interaction zone (IZ) of Nb-foils with (Si-B)-coatings. According to X-ray spectral analysis, the light layers had the composition of the eutectic region of the Nb-B state diagram Nb65,0(B33,2si1,8)35,0 and the structure of the (Nb)- solid solution (dominant phase) and the Nb3(B, Si)2 intermetallic compound (solid solution of Si in boride Nb3B2) in the form of small inclusions of light gray color. These layers represent the viscousplastic component of the composite. The layers of the interaction zone are identified as intermetallic Nb3(B, Si)4=Nb2,92(B2,35SiS1,73)4,08 (solid solution of silicon in boride Nb3B4) and should perform a strengthening function in the composite. Between layers with (Nb)- matrix and layers of diffusional IZ a significantly thinner (about 3 μm) layer is again boride Nb3B4, but with very low silicon content- Nb3,01(B3,96Si0,03)3,99.
Three phases were found in the coating composition (Figure 4):
Figure 4:The microstructure of the coating on the surface of the composite: 1-9 and 11-numbers of the spectra.
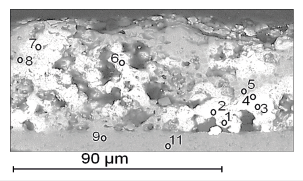
A. Carbide-silicide of molybdenum and tungsten with the composition (Mo30.4W15.7)46.1(Si27.7C26.2)53.9, which can be represented as equiatomic intermetallic compound (Mo0.61W0.31)0.92(Si0.55C0.53)1.08= (Mo, W) (Si, C);
B. Compound with the composition Mo37.8W2.3Nb0.6)40.7(-Si34.8C24.5)59.3, which at this stage of diffusion welding contains up to ~3 at.% W and Nb and can be identified as a silicide (Mo, Me)(Si, C)2= (Mo1.14Me0.08)1.22(Si1.04C0.74)1.78 or molybdenum carbide (Mo,Me) (C,Si)= (Mo0.76Me0.06)0.82(C0.48Si0.70)1.18, where Me is W and Nb;
C. Unused tungsten (light phase), which is still quite a lot.
The layer of the composite adjacent to the coating is niobium carbide with Mo, W and Si dissolved in it. Its average composition (NB41.5Me1.4)42.9(C51.3Si5.8)57.1 is close to the monocarbide (Nb,Me) (C,Si)1-x= (Nb0.83Me0.03)0.86(C1.03Si0.11)1.14, where Me is Mo and W.
Conclusion
A. During diffusion welding, 1400 °C for 1 hour, none of the Novotnaya phases of the stoichiometric compositions (Mo,W)5Si3C or (Mo,W)5Si2 not yet formed most likely due to low temperature and short time of the welding. However, already obtained compounds with molybdenum and tungsten are heat resistant. The experiment showed that the interaction between the refractory elements Mo and W with carbon and silicon is quite active.
B. This pilot experiment highlighted another possibility of the solid-phase technology developed by us, which consists in obtaining a heat-resistant coating from refractory compounds of molybdenum and tungsten compounds on the surface of the Nb/ (Si-B) composite at the same time as obtaining its heat-resistant layered structure.
Acknowledgment
The authors are grateful to the head of the laboratory of materials science, Corresponding Member of the RAS M.I. Karpov, as well as the laboratory team for the help and useful work discussions. This work was supported by the Russian Foundation for Basic Research. Grant 17-03-00687A.
References
- Savitsky EM, Burkhanov GS, Povarova KB (1986) Refractory metals and alloys. Metallurgy, p. 352.
- Savitsky EM, Burkhanov GS (1971) Metal science of alloys of refractory and rare metals. Science, p.354.
- Borisenko AI (1961) Protection of niobium from high-temperature gas corrosion. Editor in charge Doctor of Chemical Sciences A.A. Allen/Moscow, Leningrad: Publishing House of the Academy of Sciences of the USSR [Leningrad Branch], p. 41.
- Jaffee RI (1966) In «Refractory Metals and Alloys», v. III, Applied aspects Publishers, p. 9.
- Maksimovich GG, Shatinsky VF, Lyutyi EM, Goykhman MS, Rybakov SV (1982) High-temperature performance of refractory metals and alloys in aggressive media. Kiev Naukova Dumka, Uakraine, p. 224.
- Korzhov VP, Kiyko VM (2017) Structure of Mo-Si-B and Nb-Si-B Layered Composites. Izvestiya RAN. Series physical 81(11): 1513-1521.
- Sorokina NE, Avdeev VV, Tikhomirov MA, Lutfullin AS, Saidaminov MI (2010) Intercalated graphite-based composite nanomaterials. A manual for students in the specialty Composite nanomaterials. M Moscow State University, M.V. Lomonosov, Russia, p. 50.
© 2019 VP Korzhov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






