- Submissions

Full Text
Evolutions in Mechanical Engineering
Hydrogen Economy: Alternative Approaches
Vicente Fachina*
Project Manager in the Energy Industry, Brazil
*Corresponding author:Vicente Fachina, Project Manager in the Energy Industry, Brazil
Submission: August 09, 2018;Published: October 31, 2018

ISSN 2640-9690 Volume1 Issue5
Abstract
Besides traditional approaches for producing and storing hydrogen from cheap, surplus electricity or heat to use in energy conversion processes, this paper aims at simulating the feasibility of using water as energy carrier by two alternative approaches: hydrogen combustion from water splitting, and LENR-Low Energy Nuclear Reactions. The former can yield 106 J/kg-water magnitude energy intensity; the latter can yield 109 J/kg-water magnitude energy intensity.
Keywords: Hydrogen combustion; Hydrogen economy; LENR
Introduction
The original concept of the hydrogen economy [1] is based on cleanly and economically extracting hydrogen out of hydrogenrich compounds for chemical-energy conversion processes, either combustion or redox reactions. In this context, “cleanly” means not using fossil fuels for extracting hydrogen, and “economically” means competitive as to another alternative. Nevertheless, managing to get both conditions satisfied has been a challenge. It has been cheaper to extract hydrogen from reforming natural gas than by electrolysis from wind, solar power [2]. Also, depending on economic scenarios, it may be more expensive to use energy for extracting hydrogen than to directly dispatching it to final use [3].
Besides cleanliness and economics, a third issue of the traditional hydrogen economy is energy intensity. In comparison to fossil fuels or nuclear energy, the so-called renewable energy such as biomass, hydro, geothermal, wind or solar are low-energy intensity conversion processes, thus extracting large-scale hydrogen quantities from them requires lots of physical volume, thus with relevant energy footprints. Not a problem if large volumes in land or sea are available, without jeopardizing other economic sectors. Therefore, alternative approaches for energy-conversion processes must satisfy the three aforementioned conditions simultaneously: clean, economical and high energy-intensity carriers, in order to be competitive as to fossil fuels, nuclear fission, and renewable energy.
Traditional Hydrogen Economy
Traditional hydrogen economy encompasses extraction processes of hydrogen out of hydrogen-rich compounds for use in chemical-energy conversion processes, either combustion or redox reactions.
Usual hydrogen-producing industrial processes are:
a. Water electrolysis by surplus electricity;
b. Water thermolysis by heat sources or wastes;
c. Steam reforming of natural gas.
All these processes depend on water and exogenous energy supplies for water splitting or steam reforming. The yielded hydrogen has to be compressed and stored, which is energyintensive for the very high specific volume of hydrogen. Also, if energy comes from renewable sources, large energy footprints may be needed for both water splitting and hydrogen handling. Not a problem if there are enough land or sea areas for economical surplus electricity or heat sources and wastes, and without jeopardizing other economic sectors. On the steam reforming, natural gas is an economical energy carrier for use in fuel cells together with steam reformers; nonetheless, natural gas depends on fossil reserves.
On the other hand, the energy quantity carried by the yielded hydrogen may be no larger than the energy quantity needed for yielding hydrogen itself, which makes the whole chain unsustainable by energy accounting alone. Utilizing surplus electricity or heat sources and wastes elsewhere may be better than utilizing them for yielding hydrogen.
Alternative Hydrogen Economy
The traditional hydrogen economy is inherently limited for large scale use in space and time by two reasons: exogenous energy supplies, and energy-chain unsustainability eventually. The alternative hydrogen economy proposes to do away with such hindrances. The first process of an alternative hydrogen economy utilizes water as a chemical energy carrier. The energy comes from hydrogen combustion, the hydrogen itself stemming from water electrolysis. It is shown that such a process has 106 J/kg-water magnitude energy intensity, the same one as fossil fuels but high values, and it can work indeed once proper catalysts and efficient combustion technology are provided.
Secondly, there is a process that utilizes water as a nuclear energy carrier. The energy comes from hydrogen fusion inside a proper material lattice, and with resonant electric stimuli. It is shown that such a process has 109 J/kg-water magnitude energy intensity, and it can work indeed once proper material lattice, very high hydrogen concentration, and proper resonant electric stimuli are provided. An energy conversion process with such an energy intensity magnitude is a thousand times more energy intensive than fossil fuels. Nonetheless, these alternative hydrogen energy vectors do have setbacks: water as energy carrier implies either water withdrawal or consumption, and such technologic routes have not been settled down in conventional knowledge fields [4].
Hydrogen Combustion from Water Splitting
One amongst several methods for making water to split into hydrogen and oxygen is electrolysis. The electrolysis energy decreases with temperature, and it is zeroed on reaching the thermolysis temperature eventually. By simultaneously combining high temperature with resonant electric stimuli for splitting water [5], hydrogen combustion can sustain the water splitting, and yield surplus energy as well. Figure 1 shows a block diagram for the hydrogen combustion from water splitting.
Figure 1:H2 combustion from water splitting.

Equation (1) models the water splitting enthalpy (hH2O), with the k split factor over the formation enthalpy of water (h0 H2O) accounting for the effect of temperature.
Equation (2) models the heat of combustion (Q), which stems from the lower heating value of hydrogen and its mass. The hydrogen mass stems from the mass balance of the water splitting. Equation (3) models the rejected heat (Q0), which is carried away by steam and air.
Equation (4) models the conversion of the heat of combustion (Q) to gross electricity (WT), with the energy efficiency (η2) to depend on temperature and the conversion technology.
Equation (5) models the electrolysis energy.
Equation (6) models the water desalination and pumping for injection in the combustion system.
Equation (7) models the overall energy balance, with combustion heat (Q), gross electricity (WT), and rejected heat (Q0). Equation (8) models the electric balance, with gross electricity (WT), electrolysis energy (WE), water desalination and pumping (WP), and net electricity (W).
Energy conservation implies the following equation system:
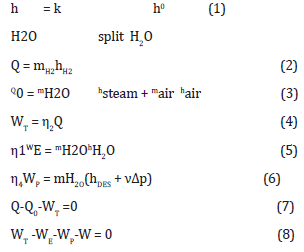
Figure 2 plots the main energy figures: gross heat (Q) from the H2 combustion, net heat (H from Figure 1), and net electricity (W).
Figure 2:Power map for H2 combustion from water splitting.
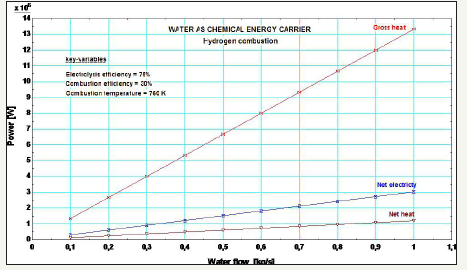
The net heat is defined as the exergy of the steam after the H2 combustion, with environment temperature and pressure as reference values.
The energy intensity is the sum of the net heat and electricity at 1kg/s water flow, which is 4.2MJ/Kg-water, about 3.5 times smaller than fossil fuels´.
The energy efficiency is calculated by the ratio between the net heat and electricity, and the gross heat, which yields 31.5%. This is equivalent to 0.46 CoP1, the Coefficient of Performance, which is the ratio between the net heat and electricity, and the difference between the gross heat and the net heat and electricity. The 0.46 CoP means the net output is 54% less than the internal consumptions and losses. The major consumption of the H2 combustion from water splitting stems from the water electrolysis, about 960 kJ/kgwater.
Hydrogen combustion from water splitting hinges upon electrolysis efficiency no lower than 75%, and combustion efficiency no lower than 30%. Proper electro catalysis can further increase efficiency above 75% [6,7]. Also, proper system design and heat-resistant materials can further increase combustion efficiency above 30% [8]. Now, if hydrogen from water electrolysis is to burn together with fossil fuels, energy intensity shall be higher than 4.2MJ/Kg-water. For instance, methane and hydrogen can yield 19MJ/Kg-methane energy intensity, against 15MJ/Kg-methane only, which reduces carbon footprint for fossil fuels.
LENR Processes
Traditional nuclear fusion energy has been under research and development since the 1950´s, with no commercial success thus far. There are some prospects that it may become scalable by the mid- 21st century. Traditional nuclear fusion energy means harnessing energy by making hydrogen atoms hit one another with enough energy in order to overcome the Coulomb barrier, that fusion happens, and energy, helium and neutrinos are released (stars achieve that by gravity).
Nevertheless, making hydrogen atoms hit one another requires lots of energy. In order to be viable, there has to be an appropriate quantity of net energy to deliver. A record value of 0.02-CoP, which is equivalent to 1.96% energy efficiency, was obtained in UK by 1997 with tokamak technology [9].
Figure 3:A LENR block diagram.
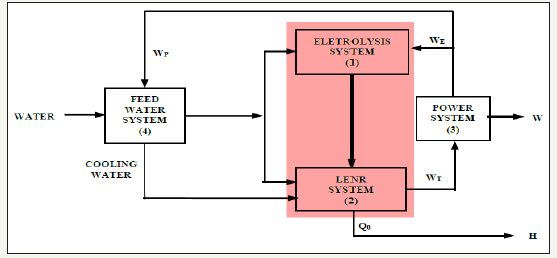
The LENR process (Low Energy Nuclear Reactions) has been under research and development since the 1990´s [4], with no commercial success thus far. The conditions of the LENR process are not those of the traditional nuclear fusion energy [4]. The fundamental difference is that the conditions of LENR aim to minimize the degrees of freedom of the H2 atoms, together with resonant electric stimuli, for breaking the Coulomb barrier and thus to make the H2 atoms hit one another. In order to achieve that, the H2 atoms have to be fed above 90% concentration levels into nanometric surface cracks of a proper material substrate (usually palladium, copper or nickel). When resonant electric stimuli are applied, LENR do happen, with the production of helium, neutrinos, and excess heat that is incompatible with chemical reactions whatsoever [10]. Figure 3 shows a LENR block diagram.
Equation (9) models the water splitting enthalpy (hH2O), with the k split factor over the formation enthalpy of water (h0 H2O) accounting for the effect temperature.
Equation (10) models the LENR available energy (Q), which stems from the LENR appropriate conditions being achieved (11). Equation (11) models the rejected heat, which is carried away by cooling water.
Equation (12) models the conversion of the available (Q) to gross electricity (WT), with an energy efficiency (η2) to depend on the conversion technology.
Equation (13) models the electric charges in water, which are the electrolysis energy and the electric resonant stimuli for triggering the LENR process.
Equation (14) models the water desalination and pumping in the feed water system, which supplies desalinated and cooling water for the LENR system.
Equation (15) models the overall energy balance, with the gross heat (Q), the gross electricity (WT), and the rejected heat (Q0) Equation (16) models the electric balance, with gross electricity (WT), electric charges in water (WE), water desalination and pumping (WP), and net electricity (W).
Energy conservation implies the following equation system:
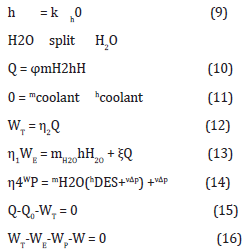
Figure 4:Power map for LENR processes.
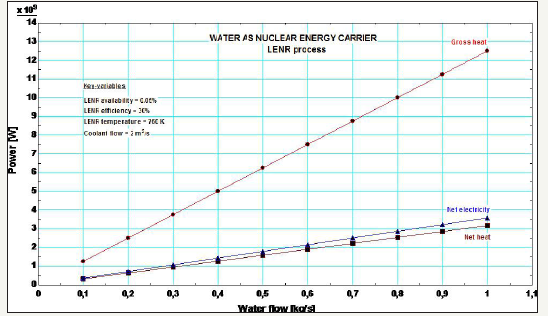
Figure 4 plots the main energy figures: gross heat (Q) from the LENR process, net heat (H from Figure 3), and net electricity (W).
The net heat is the exergy of the steam after the LENR process, with environment temperature and pressure as reference values. The energy intensity is the sum of the net heat and electricity at 1kg/s water flow, which is about 6.7GJ/Kg-water. The energy efficiency is calculated by the ratio between the net heat and electricity, and the gross heat, which yields 54%. This is equivalent to 1.17 CoP, the Coefficient of Performance, which is the ratio between the net heat and electricity, and the difference between the gross heat and the net heat and electricity. The 1.17 CoP means the net output is 17% more than the internal consumptions and losses. The LENR process hinges upon the energy availability factor (ϕ-factor from Equation (10)), which depends on appropriate materials, H2 concentration, and electric stimuli in order to trigger the LENR. Once the above conditions are satisfied with appropriate technology, the LENR process can work indeed.
Economics of an Alternative Hydrogen Economy
On the economic prospects of using water as energy carrier, there can be centralized and distributed modes. A centralized mode means a GW-magnitude energy supply chain, and a distributed mode means a kW or MW-magnitude energy supply chain. Both ones are modeled in Figure 5.
Figure 5:Economic model.
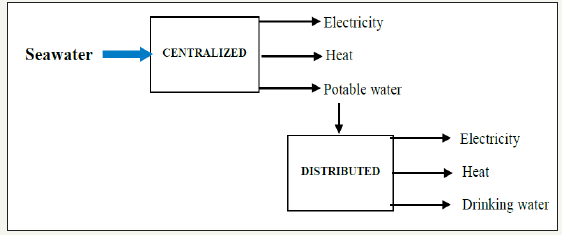
For their energy intensity being larger than fossil fuels´, LENR processes should be used in centralized modes to tap into seawater for yielding either electricity, heat or potable water to local modes, cities included. For instance, with 1kg/s water consumption and 2m3/s coolant water withdrawal, 6.7GW industry complexes can be feasible.
On distributed modes by LENR processes, with 3.6GJ/kg-water net electricity, 0.05kg/h water flow is needed for a 50kW-electric vehicle, which means up to 1,200 running hours with 60 liters of water, for instance. On the other hand, by utilizing hydrogen combustion from water splitting, which yields about 3MJ/kg-water net electricity, 60kg/h water flow is needed, which means up to one running hour with 60 liters of water. In this latter case, it is better to use hydrogen together with another fuel for higher energy intensity.
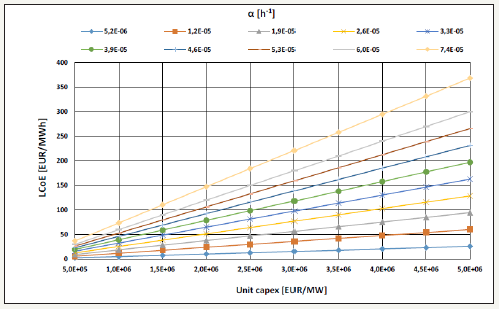
Figure 6 illustrates a linear model for calculating the Levelized Cost of Energy-LCoE [EUR/MWh] as a function of the unit capex [EUR/MW]. The α [h-1] parameter is an angular coefficient that depends on interest ratios, lifetimes, capacity factors, and opex-tocapex ratios. The ranges are 0-10%/yr interest ratios, 10-30 yearlifetimes, 1%-10% opex-to-capex ratios, and 25%-95% capacity factors. As a reference LCoE value, the 2020 European strike price for offshore wind power is around 100EUR/MWh [11,12]. Therefore, if the water-to-energy route happens to cost either nearby or below that target, that shall indicate desirable unit capex values up to 1.5 million EUR/MW, which would accommodate all variable ranges. Higher unit capex values are possible for limited parameter ranges.
Figure 6:Levelized cost of energy.
Conclusion
Traditional hydrogen economy with water electrolysis by surplus electricity, water thermolysis by heat sources or wastes, or steam reforming of natural gas depends on water and exogenous energy supplies. Utilizing surplus electricity or heat sources and wastes elsewhere may be better than utilizing them for yielding hydrogen.
On the alternative hydrogen economy, the first process is by utilizing water as a chemical energy carrier; nonetheless, the energy intensity is 4.2 MJ J/kg-water, at least three times smaller than fossil fuels´. A better way is to burn hydrogen together with fossil fuels for increasing the overall energy intensity, thus reducing carbon footprint.
The second process is by utilizing water as a nuclear energy carrier through LENR (Low Energy Nuclear Reactions) processes, with 6.7GJ/Kg-water energy intensity.
On the economics of the alternative hydrogen economy, it is desirable unit capex values up to 1.5 million EUR/MW for LCoE nearby or below 100EUR/MWh.
References
- Rifkin J (2003) The Hydrogen Economy. Penguin, USA
- Dincer I, Rosen M (2007) Exergy. Elsevier Science, USA.
- Bossel U (2006) Does a hydrogen economy make sense? Proceedings of the IEEE 94(10): 1826-1837.
- McKubre M (2017) CMNS-Past, present and future. Journal of Condensed Matter Nuclear Sciences 24(2017): 15-24.
- Meyer S (1989) Process and apparatus for the production of fuel gas and the enhanced release of thermal energy from such gas. Energy conversion device.
- Zhang LL, Nordlund D, Chen H, Fan L, Zhang B, et al. (2018) Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalitic water oxidation. Nature.
- Zhou H, Yu F, Sun J, He R, Chen S, et al. (2017) Highly active catalyst derived from a 3D foam of Fe(PO3)2/Ni2 P for extremely efficient water oxidation. Proceedings of the National Academy of Sciences 114(2): 5607-5611.
- Caton J (2012) The thermodynamic characteristics of high efficiency, internal-combustion engines. Energy Conversion and Management 58: 84-93.
- Wesson J (1999) The science of jet. Joint European Torus, JET Joint Undertaking, Abingdon, England.
- Pons S (1990) Journal of international patent publication. Energy conversion device.
- Beiting E (2017) Investigation of the nickel-hydrogen anomalous heat effect. Los Angeles, USA.
- Wind Europe.
© 2018 Vicente Fachina. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






