- Submissions

Full Text
Evolutions in Mechanical Engineering
Additive Manufacturing of Scaffolds for Bio-implants
Asit Kumar Gain*
The University of New South Wales (UNSW), Australia
*Corresponding author:Asit Kumar gain, Research Fellow, School of Mechanical and Manufacturing Engineering, The University of New South Wales (UNSW), Sydney, NSW 2052, Australia, Email:a.gain@unsw.edu.au/akgain@gmail.com
Submission: October 12, 2018;Published: October 25, 2018

ISSN 2640-9690 Volume1 Issue5
Editorial
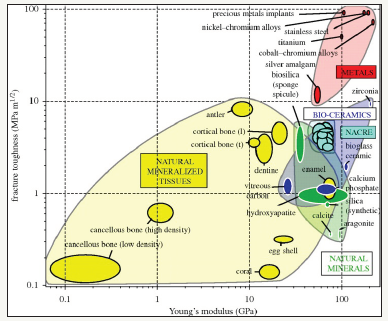
One of the critical challenges in artificial implant materials is the design of scaffolds and implants that mimic the physical and mechanical behavior of the human bones. Since the introduction of ceramic-based artificial bio-implants in 1960s, the possibility of the usages of metallic biomaterials, e.g., titanium and its alloys, stainless steel and cobalt-chromium alloys have been growing rapidly in biomedical applications [1,2]. In early stages, some physical properties (e.g. biocompatibility) and chemical reactivity (e.g. non-toxicity) are considered as the key selection criteria for application suitability. However, nowadays some additional physical and mechanical properties that are required to imitate the growth of cells and tissues as in the natural bones are taken as important factors [2-4]. This creates a new challenge of the artificial implant materials made of these metals and their alloy is to adjust the mechanical properties especially stiffness of this bio-implants. In general, the artificial implant materials have high stiffness than that of the natural bones as presented in Figure 1, leading to stressshielding - a major cause for bone resorption and premature failure of such implants [5]. According to Wegst et al. [5] investigation, the stiffness of widely used metallic implants i.e., stainless steel, Co-Cr, Ti alloys are about 180GPa, 210GPa and 110GPa, respectively while the stiffness of cortical bones is about 10-30GPa. Therefore, to minimize the stress-shielding effect at the bone-implant interface, the stiffness of the artificial implant materials to be adjusted as close to the human cortical bones. To match the material properties of artificial bio-implants, many studies have tried to develop porous scaffold materials which could be both bio-compatible and mech-compatible. However, the strength of scaffolds decreases significantly with the porosity, and may become very low than that of human bone when with the porosity is very high. This creates a new potential problem. Therefore, it has been challenging to develop both bio-compatible and mech-compatible scaffolds for bio-implants. Thus, a promising technique for manufacturing bone substitute scaffolds thus emerges: to match the hierarchical structure of the natural tissues because this manufacturing technique will tolerate to custom design property at various structural length scales so that the scaffolds can fulfil the biocompatible and mech-compatible requirements.
Figure 1:A material property chart showing fracture toughness plotted against Young’s modulus biomaterials [5].
Traditional manufacturing techniques for preparation of scaffold materials include liquid state processing (e.g., freeze casting, direct foaming, sol-gel, etc.), solid state processing (e.g., powder metallurgy, multi-pass extrusion and sintering of powders etc.) and vapour deposition [6,7]. Notwithstanding the advancements that have attained in scaffolds manufacturing, the control over scaffolds structure using these traditional methods is highly process dependent. Rapid prototype additive manufacturing technology has been considered as a feasible alternative for fabrication of desired scaffolds structure that mimic the natural bones.
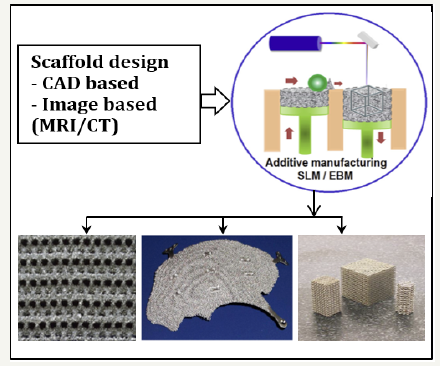
Additive manufacturing (AM) technique creates threedimensional (3D) components by successively adding thin layer of materials followed by a computer-aided design (CAD) model. After preparing the first layer, a new layer of material is deposited on the top and this process is repeated until manufacture the final product according to Figure 2 [8-10]. Heinl et al. [11] manufactured cellular type Ti foams using prototype AM technique at a vacuum pressure (10-4 to 10-5mbar) to avoid contamination due to oxygen or nitrogen. The manufactured interconnected porous structures with different volume percent of porosities (25%, 38%, and 60%) were well controlled the stiffness as close to the natural bones. Furthermore, Harrysson et al. [12] fabricated hip stem using AM technique that well match the physical and mechanical properties as close to natural bones. The low stiffness hip-stem mimics the stiffness of human bone to reduce stress shielding and bone remodeling.
Figure 2:Display of internal filling ratio in parts.
Overall, even though a lot of progressive results have been reported for AM technique of manufacturing scaffold materials, it is still in its early stages. Multidisciplinary research will be essential to minimize the challenges (e.g., excess melt pool temperature, undesirable microstructure, residual stress, surface roughness and local porosity) and completely utilized the AM technique for manufacturing the desired scaffolds in biomedical applications.
References
- Best SM, Porter AE, Thian ES, Huang J (2008) Bioceramics: Past, present and for the future, Journal of the European Ceramic Society 28(7): 1319- 1327.
- Gain AK, Zhang L, Quadir MZ (2016) Composites matching the properties of human cortical bones: The design of porous titanium-zirconia (Ti-ZrO2) nanocomposites using polymethyl methacrylate powders. Materials Science and Engineering: A 662: 258-267.
- Han MK, Hwang MJ, Yang MS, Yang HS, Song HJ, et al. (2014) Effect of zirconium content on the microstructure, physical properties and corrosion behavior of Ti alloys. Materials Science and Engineering: A 616: 268-274.
- Lee BT, Lee CW, Gain AK, Song HY (2007) Microstructures and material properties of fibrous HAp/Al2O3-ZrO2 composites fabricated by multipass extrusion process. Journal of the European Ceramic Society 27(1): 157-163.
- Wegst UGK, Schecter M, Donius AE, Hunger MP (2010) Biomaterials by freeze casting. Philos Trans A Math Phys Eng Sci 368(1917): 2099-2121.
- Paul RK, Gain AK, Lee BT, Jang HD (2006) Effect of addition of silicon on the microstructures and bending strength of continuous porous SiCSi 3N4 composites. Journal of the American Ceramic Society 89(6): 2057- 2062.
- Ryan G, Pandit A, Apatsidis DP (2006) Fabrication methods of porous metals for use in orthopaedic applications, Biomaterials 27(13): 2651- 7260.
- Parthasarathya J, Starly B, Ramana S (2011) A design for the additive manufacture of functionally graded porous structures with tailored mechanical properties for biomedical applications. Journal of Manufacturing Processing 13(2): 160-170.
- Taniguchi N, Fujibayashi S, Takemoto M, Sasaki K, Otsuki B, et al. (2016) Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater Sci Eng C Mater Biol Appl C 59: 690-701.
- Wang X, Xu S, Zhou S, Xu W, Leary M, et al. (2016) Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 83: 127-141.
- Heinl P, Rottmair A, Körner C, Singer RF (2007) Cellular titanium by selective electron beam melting. Advanced Engineering Materials 9(5): 360-364.
- Harrysson O, Cansi Zoglu O, Marcellin-Little DJ, Cormier DR, West HA (2008) Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Materials Science and Engineering C 28(3): 366-373.
© 2018 Asit Kumar Gain. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






