- Submissions

Full Text
Examines in Marine Biology & Oceanography
Sea Surface Temperature and Marine Heatwaves Impacts on Marine Macroalgae
Cláudia S Karez*, Maria J Willemes, Rodrigo T Carvalho and Leonardo T Salgado
Instituto de Pesquisa Jardim Botânico do Rio de Janeiro, Brasil
*Corresponding author: Cláudia S Karez, Instituto de Pesquisa Jardim Botânico do Rio de Janeiro, Rio de Janeiro, Brasil
Submission: April 08, 2024;Published: April 29, 2024

ISSN 2578-031X Volume6 Issue5
Abstract
Ecological impacts associated with ocean warming and marine heatwaves have been extensively reported recently, as shifts in species distribution, changes in structures of communities and local extinctions, alien species spreading as well as economic impacts on seafood industries. Marine heatwaves, particularly, exert major negative impacts on seaweed (macroalgae) communities and their ecological functions and services. The most widespread impact in macroalgae biodiversity is the decrease of kelp and fucoid communities and associate organisms in almost the whole world. However, the changes in the macroalgae distribution and structure in coastal ecosystems are probably the most concerning impact which have not yet been widely studied in these highly biodiverse ecosystems.
Keywords: Algae; Coastal and marine ecosystems; Ocean warming; Extreme temperature events
Introduction
In general, mean Sea Surface Temperature (SST) changes are critical to the increasing trend in Marine Heatwaves (MHWs) duration and intensity. MHWs is foreseen to increase everywhere, and the increase is expected to be higher in the tropics due to smaller variation in SST, both seasonally and annually [1,2]. Ocean Warming (OW or gradual increase in SST) threatens the coastal marine environment and impacts their functioning worldwide. Deleterious impacts across several biological processes and taxa are foreseen, in particular, in the most sensitivity ecosystems/regions (coral reefs, Artic biota, seagrasses, kelp habitats and mangrove/salt-marshes) and ecosystem services (fisheries, aquaculture and coastal protection) [3,4].
Ecological impacts associated with OW and MHWs have been reported recently, including shifts in species distribution, changes in structures of communities and local extinctions, as well as economic impacts on seafood industries [5,6]. Furthermore, climate change can increase the spread of thermophilic Invasive Alien Species (IAS), Caulerpa cylindracea, after the decrease in native ones, as canopy-formers, in many Mediterranean rocky reefs [7]. Tropical coral reefs and temperate kelp forests are some of the most threatened habitats by OW and MHWs due to the sensitivity of the dominant foundation species (coral and kelps/fucoids) to thermal stress [8]. However, resilience and future functioning of these systems also depends on the biological interactions with other groups, including fleshy and coralline algae. The tolerance to OW and MHWs by some functional groups, like coralline algae, suggests that they may play ecological roles in a OW by increasing the resilience of coralline reef structures, becoming the most important foundation taxa. In the tropics, this could mean that coralline algae take up space formerly occupied by corals and thus play a larger role in existing reef structures [8].
Considering marine macroalgae communities, temperature may represent a major factor influencing their biology [9], which is manifested mainly by different temperature tolerance limits. Although they may be well adapted to their normal temperature ranges, during periods of temperature anomalies, these communities are exposed to sufficiently high or low temperatures that can result in disruptive stress, in the form of cellular and subcellular damage, reducing photosynthetic performance [10]. These ecological responses at population, community and ecosystem levels should be understood in order to make sound management and restauration options for degraded ecosystems.
Key Factors in Macroalgae Distribution
Macroalgae, as photosynthetic organisms, have a very broad latitudinal distribution, from 77° S to 80° N [11] and grow on both hard and soft bottoms. The depth distribution of macroalgal ranges is from upper intertidal to 268m [12]. It is remarkable that some calcifying Rhodophyta can tolerate long periods of darkness and remain photosynthetic active [13], which is possible by the energy accumulation during the periods of light and photosynthesis [14].
The macroalgae diversity follows latitudinal distribution gradients, which can be, or not related to environmental conditions, depending on their group. In the phylum red algae Rhodophyta, a strongest relationship with the environment is observed and an increased diversity from a north to south gradient, while in the brown algae Ochrophyta, diversity has a less striking gradient and increases towards north, and in the green algae Chlorophyta, there is a little latitudinal variation in their diversity [15]. Distributional limits of multiple macroalgal species suggest that temperature extremes may limit diversity. However, biological interactions (e.g., competition, grazing) may exert more control on biodiversity and biomass in the tropics, while environmental variables (e.g., temperature, nutrients) show more control in the temperate regions.
Amongst the ocean regions, the South Atlantic Ocean displays the highest percentage of macroalgal DNA (17% of the total across all basins), while the lowest genetic diversity is in the Mediterranean Sea and the Indian Ocean (8% each) [16]. There is a stronger influence of environmental conditions on macroalgal diversity across some ocean locations but not in others which suggests the need for biogeographical analyses [15]. In the Great Barriers Reef (GBR), for example, the prediction of environmental factors regarding the increase of macroalgae cover towards high latitudes, suggests that temperature may be the main factor for the latitude decline [17]. Macroalgae cover has also been reported to increase with latitude in the Caribbean, the Red Sea, the Hawai’ian Islands [18-20]. It was typically attributed to declining SST and to increasing chlorophyll and nutrient concentrations [17].
Impact of OW and MHWs on Macroalgae Biodiversity
MHWs are widely known to exert major negative impacts on seaweed (macroalgae) communities and the ecological functions and services they provide [20] more than average SST gradual change in macroalgae. MHWs are particularly harmful for species located at the equatorial edge [21], because the temperatures rise above thermal tolerances, causing cell damage [22]. Therefore, it is expected that cold-water species will be replaced by more warm-water species. This is by far the most widespread impact in macroalgae biodiversity which affects kelp communities and associate organisms in almost the whole world with exception of climate refuges which are the result of the combination of local conditions such as thermal buffering and wave exposition [22]. After loss or decrease of foundation species or in systems with additional seawater stressors (such as eutrophication), primary foundation species may be replaced by fast-growing algae, such as small turf-forming and filamentous seaweed [23].
A review on MHWs impacts on macroalgae reported the decline in abundance of canopy-forming kelps and fucoids after MHWs [24]. Kelps and fucoids are ecologically important large brown seaweeds abundant in temperate reef ecosystems. Loss of kelp is driven by temperature facilitated the domination of turfs and loss of understory algae. In addition, a displacement of tropical herbivorous affected the macroalgae canopy cover in reefs [25]. Over the last decade, kelp forests are being increasingly replaced by turfs, changing the reef seascape from a complex forest to a structurally simple low-lying alga representing a degradation of ecosystems and loss of biodiversity. A widespread disappearance of kelp forests has been reported along Atlantic Canada, Europe and Australia [26].
The algal flora of southern Australia includes 30-40% of the world’s species, where 50% are estimated to be endemic [27]. Among the observed impacts in this region, there is a decline of the surface-canopy of the giant kelp Macrocystis pyrifera. The distribution of other large macroalgae may also have been affected. Herbarium records suggest that the distribution of the three habitat-forming species, Ecklonia radiata, Phyllospora comosa and Durvillaea potatorum, have shifted southwards over recent decades [28]. Massive declines of large habitat-forming algae around the urban areas have been recorded, such as for P. comosa around Sydney and E. radiata around Adelaide [29]. These changes may be directly or indirectly driven by climate and local anthropogenic stressors.
Atmospheric heatwaves during low tide may threaten the predominant species, Bifurcaria bifurcata, Cystoseira tamariscifolia and Codium tomentosum, in the NW Iberian Peninsula probably preventing range shifts in response to increasing seawater temperature of these canopy forming macroalgae [30]. The kelp biomass off Nova Scotia has declined by 85-99%, resulting in a catastrophic phase shift to rocky reefs dominated by opportunistic turf-forming and the invasive algae along the central Atlantic coast [31]. In the early extreme event during1982/83 El Niño, Galapagos archipelago experienced declines of fucoids Sargassum sp. and Blossevillea galapagensis followed by colonization of the invasive turfs, Giffordia mitchelliae [32].
Macroalgae diversity decreased and biomass increased during the 1997/98 El Niño at the Pacific Mexican coast, whereas diversity increased, biomass decreased and the assemblage structure was altered following the El Niño mediated MHW [33]. During the El Niño period, the assemblages comprised species associated with tropical waters while in non-ENSO years, species had more temperate affinities. The canopy-forming seaweeds Sargassum spp. decreased in cover overall by 52% in Brazilian southwestern Atlantic warm temperate where the cover of filamentous turfs increased (24% over the last 27 years) along human-impacted coasts [34]. The decline of Sargassum spp. could only be because of warming waters exacerbated by increased competition with turfs that have also benefited from the urban development. Although kelps and fucoids are the most affected group of algae by OW and MHWs, in temperate as well as in tropical coastal reefs, other drastic impacts occurred in last decades. These include the expansion of macroalgae in coral reefs (coral-algal competition), in rhodolith beds as well as the range expansion of alien macroalgae in some regions (Table 1).
Table 1:Main impacts of OW and MHWs on the macroalgae-dominated habitats reported in the literature.
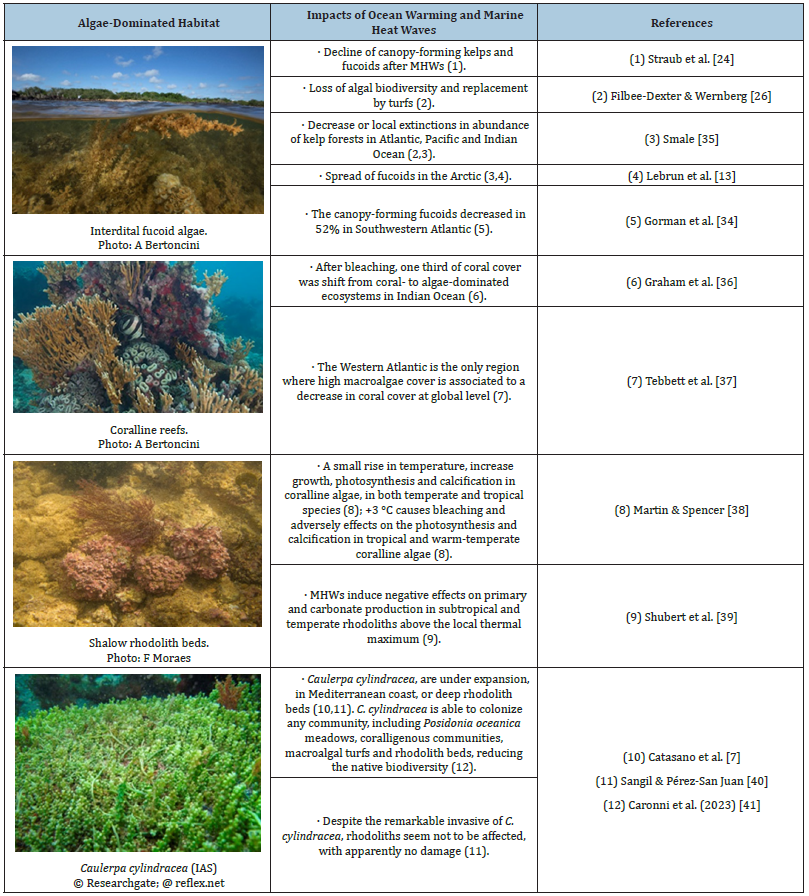
In the western Indian Ocean, MHWs in 1998 and 2016 both caused more than 70% decline of branching corals, habitat loss, and reduced abundance and richness of reef fish. Two-thirds of reefs had recovered to pre-1998 coral abundances by 2011; the remaining third underwent a regime shift from coral- to algaedominated ecosystems [36] and a shift in ecosystem services. At global level, only the western Atlantic coral reefs presented a decrease in coral cover with relatively high macroalgal cover, during all bleaching events from 1998 to 2017 [37]. In the case of Indo-West Pacific reefs, resilience mechanisms may be present, especially when compared to the western Atlantic, in which the predominance of Crustose Coralline Algae (CCA) and short algal turfs may be beneficial, to facilitate the coral recruitment.
Conclusion
Experimental research on marine climate change impacts has intensified dramatically in recent decades, in particular, studies focused on MHWs [21]. Although the number of studies describing impacts of MHWs has increased recently, they highlighted the need of baseline data regarding macroalgae distributions and performance [24]. However, field studies to monitor specific characteristics of MHWs that affect the vulnerability and resilience of macroalgae species are also needed considering increasingly important climatic perturbations. Laboratory investigations under controlled conditions have been used to determine cause-effect relationships for single stressors (in this case, MHWs), in isolation, from those of other stressors in the marine environment. These are particularly meaningful to experiment adaptation and resistance mechanisms in key species. Therefore, a major challenge will be to study the extreme temperature events in coastal sites with cooccurring potential stressors including altered current patterns, increasing herbivory, high particulate matter in seawater and eutrophication that are restructuring entire ecosystems.
References
- Frölicher TL, Fischer EM, Gruber N (2018) Marine heatwaves under global warming. Nature 560(7718): 360-364.
- Jacox MG, Alexander MA, Amaya D, Becker E, Bograd SJ, et al. (2022) Global seasonal forecasts of marine heatwaves. Nature 604(7906): 486-490.
- Gattuso JP, Magnan AK, Bopp L, Cheung WW, Duarte CM, et al. (2018) Ocean solutions to address climate change and its effects on marine ecosystems. Front mar sci 5: 410554.
- Wernberg T, Thomsen MS, Baum J, Bishop MJ, Bruno JF, et al. (2024) Impacts of climate change on marine foundation species. Ann Rev Mar Sci 16: 247-282.
- Free CM, Cabral RB, Froehlich HE, Battista W, Ojea E, et al. (2022) Expanding ocean food production under climate change. Nature 605: 490-496.
- Montie S, Thomsen MS (2023) Long-term community shifts driven by local extinction of an iconic foundation species following an extreme marine heatwave. Ecol Evol 13(10): e10584.
- Cantasano N, Di Martino V, Pellicone G (2024) The Invasion of Caulerpa cylindracea Sonder 1845 in the Calabria Coastal Seas. Coasts 4(1): 34-48.
- Krieger EC, Taise A, Nelson WA, Grand J, Ru EL, et al. (2023) Tolerance of coralline algae to ocean warming and marine heatwaves. PLOS Climate 2(1): e0000092.
- Davison IR, Pearson GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32(2): 197-211.
- Harley CD, Anderson KM, Demes KW, Jorve JP, Kordas, RL, et al. (2012) Effects of climate change on global seaweed communities. J Phycol 48(5): 1064-1078.
- Wiencke C, Amsler CD (2012) Seaweeds and their communities in polar regions. In: Seaweed biology: Novel insights into ecophysiology, ecology and utilization Berlin. Springer Publishers, UK, pp. 265-291.
- Littler MM, Littler DS (1994) Tropical reefs as complex habitats for diverse macroalgae. In: Lobban CS, Harrison PJ (Eds.), Seaweed Ecology and Physiology. Cambridge. University Press, USA, pp. 72-75.
- Lebrun A, Comeau S, Gazeau F, Gattuso JP (2022) Impact of climate change on Arctic macroalgal communities. Glob Planet Change 219: 103980.
- McCoy SJ, Kamenos NA (2015) Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J Phycol 51(1): 6-24.
- Keith SA, Kerswell AP, Connolly SR (2014) Global diversity of marine macroalgae: Environmental conditions explain less variation in the tropics. Glob Ecol Biogeogr 23(5): 517-529.
- Ortega A, Geraldi NR, Alam I, Kamau AA, Acinas SG, et al. (2019) Important contribution of macroalgae to oceanic carbon sequestration. Nat Geosci 12(9): 748-754.
- Fabricius KE, Crossman K, Jonker M, Mongin M, Thompson A (2023) Macroalgal cover on coral reefs: Spatial and environmental predictors, and decadal trends in the great barrier reef. PLoS ONE 18(1): e0279699.
- Díaz-Pulido G, Garzon-Ferreira J (2022) Seasonality in algal assemblages on upwelling-influenced coral reefs in the Colombian Caribbean. Bot Mar 45(3): 4-2.
- Ateweberhan M, Bruggemann J, Breeman A (2006) Effects of extreme seasonality on community structure and functional group dynamics of coral reef algae in the southern Red Sea (Eritrea). Coral Reefs 25: 91-406.
- Vroom PS, Braun CL (2010) Benthic composition of a healthy subtropical reef: Baseline species-level cover, with an emphasis on algae, in the Northwestern Hawaiian Islands. Plos One 5(3): e9733.
- Smith KE, Burrows MT, Hobday AJ, King NG, Moore PJ, et al. (2023) Biological impacts of marine heatwaves. Ann Rev Mar Sci 15: 119-145.
- Hanley ME, Firth LB, Foggo A (2024) Victim of changes? Marine macroalgae in a changing world. Ann Bot 133(1): 1-16.
- Wernberg T, Thomsen MS, Baum J, Bishop MJ, Bruno JF, et al. (2024) Impacts of climate change on marine foundation species. Ann Rev Mar Sci 16: 247-282.
- Straub SC, Wernberg T, Thomsen MS, Moore PJ, Burrows MT, et al. (2019) Resistance, extinction, and everything in between – The diverse responses of seaweeds to marine heatwaves. Front Mar Sci 6: 763.
- Smale DA, Wernberg T, Oliver EC, Thomsen M, Harvey BP, et al. (2019) Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat Clim Chang 9(4): 306-312.
- Filbee-Dexter K, Wernberg T (2018) Rise of turfs: A new battlefront for globally declining kelp forests. Bioscience 68(2): 64-76.
- Phillips JA (2001) Marine macroalgal biodiversity hotspots: Why is there high species richness and endemism in southern Australian marine benthic flora? Biodivers Conserv 10(9): 1555-1577.
- Millar AJK (2007) The Flindersian and peronian provinces. Algae of Australia: Introduction. Australian Biological Resources Study, CSIRO Publishing, UK, pp. 554-559.
- Connell SD, Russell BD, Turner DJ, Shepherd SA, Kildea T, et al. (2008) Recovering a lost baseline: Missing kelp forests from a metropolitan coast. Mar Ecol Prog Ser 360: 63-722.
- Román M, Román S, Vázquez E, Troncoso J, Olabarria C (2020) Heatwaves during low tide are critical for the physiological performance of intertidal macroalgae under global warming scenarios. Sci Rep 10(1): 21408.
- Filbee-Dexter K, Feehan CJ, Scheibling RE (2016) Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Mar Ecol Prog Ser 543: 141-152.
- Laurie WA (1990) Effects of the 1982-83 El Niño-Southern Oscillation event on Marine Iguana (Amblyrhynchus cristatus Bell, 1825) populations on Galá In: Elsevier Oceanography Series, Elsevier Publishers, Netherlands, 52: 361-380.
- Carballo L, Olabarria C, Osuna TG (2002) Analysis of four macroalgal assemblages along the Pacific Mexican Coast during and after the 1997-98 El Niñ Ecosystems 5: 749-760.
- Gorman D, Horta P, Flores AA, Turra A, Berchez FADS, et al. (2020) Decadal losses of canopy‐forming algae along the warm temperate coastline of Brazil. Glob Change Biol 26(3): 1446-1457.
- Smale DA (2020) Impacts of ocean warming on kelp forest ecosystems. New Phytol 225(4): 1447-1454.
- Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518(7537): 94-97.
- Tebbett SB, Connolly SR, Bellwood DR (2023) Benthic composition changes on coral reefs at global scales. Nat Ecol Evol 7(1): 71-81.
- Schubert N, Santos R, Silva J (2021) Living in a fluctuating environment increases tolerance to marine heatwaves in the free-living coralline alga Phymatolithon lusitanicum. Front Mar Sci 8: 791422.
- Martin S, Hall-Spencer JM (2017) Effects of ocean warming and acidification on rhodolith/maërl beds. In: Rhodolith/maerl beds: A global perspective, Springer Publishers, USA, pp. 55-85.
- Sangil C, Pérez-San JA (2020) Spread of Caulerpa cylindracea impacts: The colonization of Atlantic intertidal communities. Reg Stud Mar Sci 34: 100989.
- Caronni S, Bracchi V, Atzori F, Citterio S, Cadoni N, et al. (2023) Caulerpa cylindracea spread on deep rhodolith beds can be influenced by the morphostructural composition of the bed. Diversity 15(3): 349.
© 2024 Cláudia S Karez. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






