- Submissions

Full Text
Examines in Marine Biology & Oceanography
Alcanivorax borkumensis and Bioremediation: The Actual Perspective of a Vintage Bacteria
Sabrina Patania1, Alessia Lunetta2, Simone Cappello2* and Maria Genovese2
1PhD School of “Applied Biology and Experimental Medicine”, Faculty of Sciences, University of Messina, Italy
2Institute for Biological Resources and Marine Biotechnologies, Section of Messina, National Research Council (CNR-IRBIM), Italy
*Corresponding author: Simone Cappello, Institute for Biological Resources and Marine Biotechnologies, Section of Messina, National Research Council (CNR-IRBIM), Spianata S. Raineri 86, 98122 Messina, Italy
Submission: January 31, 2024;Published: February 16, 2024

ISSN 2578-031X Volume6 Issue4
Opinion
Approximately twenty-six years after its discovery, Alcanivorax borkumensis and the hydrocarbonclastic bacteria present themselves as one of the most efficient microbiological tools offered by nature to be used in the environmental recovery processes (bioremediation) of areas contaminated by oil and its derivatives. However, the careful study of the physiological and metabolic properties of this bacterium and the development of modern technologies for environmental recovery are of primary importance for achieving increasingly better performance (maximum degradation in the shortest possible time).
Twenty-six years ago, in 1998 Yakimov et al. reported the isolation and characterization from a sediment collected in the island of Borkum (Germany), of a new bacterium strain named Alcanivorax borkumensis, an unusual marine microorganism able to grow using as only carbon and energy sources a highly restricted spectrum of substrates, predominantly alkanes [1]. This discovery of this bacterium is the beginning of an important advancement in microbiological research and at the same time, in the technology for the development of strategies for bio-recovery of environments polluted by hydrocarbons (bioremediation). The isolation of Alcanivorax correspond to the identification a one eco-physiologically unusual group of marine hydrocarbon-degrading bacteria named Obligate Hydrocarbonoclastic Bacteria (OHCB) comprising, overtime, different bacterial genera, such as Cycloclasticus, Oleiphius, Oleispira, Thalassolituus and clearly Alcanivorax. Within a short period of time, was massive the isolation of bacteria related to Alcanivorax, or detection of its 16S rRNA gene sequences, from samples taken from surface water, shallow and deep sea water bodies, sediments, hydrothermal vents and mud volcanoes, ridge flank crustal fluids and grey whale carcass, in corals, sponges and aquaculture-poisoning dinoflagellates. Sequences of Alcanivorax like bacteria have also been detected in a few terrestrial environments that share relevant properties (salinity, presence of hydrocarbons) with marine ecosystems.
This ubiquity of A. borkumensis presumably results from its capacity to grow on many saturated petroleum fraction constituents and on biogenic hydrocarbons: Straight-chain and branched alkanes, isoprenoids and long sidechain alkyl compounds [2,3]. At the same time, the presence of A. borkumensis associated with marine invertebrates seems to reflect a special ecological niche containing readily accessible hydrocarbons produced by the animal partners. Interestingly, although bacteria and Alcanivorax-related 16S rRNA gene sequences has been retrieved from microbial communities inhabiting cold polar areas, the organism itself has so far only been isolated from more temperate lower latitudes [2,4,5]. Normally, this bacterium is found in low numbers in unpolluted environments, but it quickly becomes the dominant microbe in oil-polluted open ocean and coastal waters, where it may comprise up to 80–90% of the oil-degrading microbial community [6-8]. Several field studies on bacterial community dynamics and hydrocarbon degradation in coastal systems have demonstrated the pivotal role of A. borkumensis in oil-spill bioremediation [9]. The type of strain of genus Alcanivorax is North Sea isolate A. borkumensis SK2 [1]. Since its isolation, different species have thus far been described, including A. jadensis and A. venustensis [10,11], A. dieselolei [12], A. balearicus [13], A. hongdengensis [14] and A. pacificus [15] that were isolated from marine environments. Recently been isolated other species as A. marinus [16], A. xenomutans [17], A. gelatiniphagus [18], A. mobilis [19], A. nanhaiticus [20], A. indicus [21], A. profundi [22], A. sediminis [23], A. profundimaris [24], A. limicola [25], A. quisquiliarum [26] and A. xiamenensis [2].
A. borkumensis [27] is classified within the order Oceanospirillales of the class of the Gammaproteobacteria. Recent phylotaxogenomic analysis [16S rDNA sequencing, DNA–DNA Hybridization (dDDH), Average Nucleotide Identity (ANI), Average Amino Acid Identity (AAI), Percentage of Conserved Proteins (POCP) and comparative genomic studies [20,28] showed that Alcanivorax species formed three clades named Isoalcanivorax gen. nov. and Alloalcanivorax gen. nov., respectively, along with the emended description of the genus Alcanivorax sensu stricto (Table 1). The peculiar physiological and metabolic characteristics of Alcanivorax group are, absolutely recognized, the basis of the application of environmental recovery techniques based on the use of microorganisms. Bioremediation is considered as one of the most important eco-friendly and cost-effective technologies for marine ecological restoration, which leads to a complete decomposition of complex petroleum hydrocarbons of spilled oil into non-toxic compound [29,30]. Bioremediation, including biostimulation and bioaugmentation, has proven to be an effective method for cleaning up residual oil in a variety of environments [31] and has been proposed as the only viable management option that can be implemented on a large scale in marine environments [29,31].
Table 1:Schematic representation of taxonomic distribution of bacteria related to Alcanivoracacae family.

In marine environment, hydrocarbons biodegradation can be limited by many factors (e.g. nutrients, pH, temperature, oxygen and contaminant presence, Figure 1). Biostimulation involves the modification of the environment to stimulate existing bacteria capable of bioremediation. This can be done by addition of various forms of limiting nutrients and electron acceptors, which are otherwise available in quantities low enough to constrain microbial activity. As indicated [31] as the addition of nutrients, oxygen or other electron donors and acceptors to the coordinated site in order to increase the population or activity of naturally occurring microorganisms available for bioremediation. The primary advantage of biostimulation is that bioremediation will be undertaken by already present native microorganisms that are well-suited to the subsurface environment and are well distributed spatially within the subsurface (Figure 2).
Figure 1:Fate of an oil spill. Oil fate is dependent on many natural processes as the oil begins to reach the surface. These processes include spreading, evaporation, and photo-oxidation on the surface of the water. Emulsification and dispersion take place below the surface, which can assist with microbial degradation of the oil. Oil can also sediment to the sea floor. A, seawater / water column.
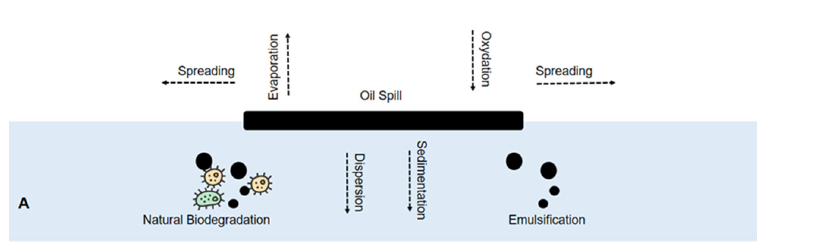
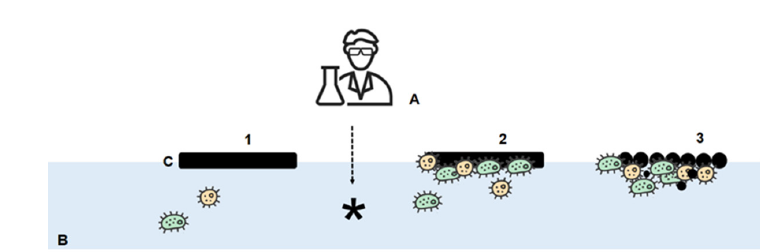
Figure 2:Schematic description of bioremediation (biostimulation) process in marine environment. A: Specialist that according operative protocols alter the natural condition (insertion of nutrients, surfactants, enhancement of microbial growth) to favour the development of natural microbial population with biodegradative capability; B: seawater (water column); C: crude oil. 1: stimulation of natural bacteria; 2: adhesion of bacterial inoculated to crude oil and initial proliferation; 3: growth of bacterial and increment of biodegradation process.
Bioaugmentation involves the introduction of microorganisms isolated from the contaminated site, from a historical site or carefully selected and genetically modified to support the remediation of petroleum hydrocarbon contaminated sites based on the assumption and/or confirmation that indigenous organisms within the impacted site cannot biodegrade petroleum hydrocarbon. Successful bioaugmentation treatments depend on the use of inocula consisting of microbial strains or microbial consortia that have been well adapted to the site to be decontaminated. Foreign microorganisms (those in inocula) have been applied successfully but their efficiency depends on ability to compete with indigenous microorganisms, predators and various abiotic factors. Factors affecting proliferation of microorganisms used for bioaugmentation including the chemical structure and concentration of pollutants, the availability of the contaminant to the microorganisms, the size and nature of the microbial population and the physical environment should be taken into consideration when screening for microorganisms to be applied (Figure 3).
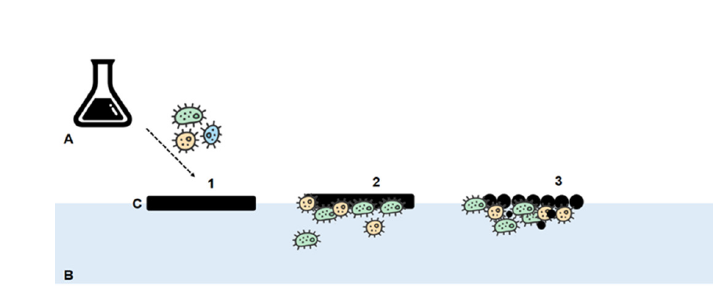
Figure 3:Schematic description of bioremediation (bioaugmentation) in marine environment. A: Flask with microbial culture of hydrocarbon degrading bacteria and/or hydrocarbonoclastic bacteria; B: seawater (water column); C: crude oil. 1: inoculum of bacteria cultivated in laboratory; 2: adhesion of bacterial inoculated to crude oil and initial proliferation; 3: growth of bacterial and increment of biodegradation process.
Furthermore, recent studies [32-34] have proven how Alcanivorax bacteria is important polypropylene degraders in mesopelagic environments. Alcanivorax strain isolated from plastic marine debris [34] exhibited rapid growth in the presence of weathered low density PE (LDPE) as the sole carbon source; in the same time bacteria related to Alcanivorax borkumensis were indicated to be enriched most abundantly on liquid polypropylene and on its structurally similar branched alkane, pristane. Conceptually, the evolution of the application of this bacteria (or bacteria belonging to the same family) can pass through a synergy with different chemical-physical-engineering strategies and technologies. In fact, the application of particular tools (e.g. bioreactor, systems with activated carbon filters...) can favour an increase in biodegradative capability, which therefore allow the normal limits imposed by the natural physiology of these bacteria to be overcome. However, the search for new genera and increasingly performing bacteria, through bi-prospecting that aims at the study of atypical environmental matrices and which could be innovative sources of microorganisms must always be a present point.
References
- Yakimov MM, Golyshin PN, Lang S, Moore ER, Abraham WR, et al. (1998) Alcanivorax borkumensis nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int J Syst Bacteriol 48(2): 339-348.
- Sabirova JS, Ferrer M, Regenhardt D, Timmis KN, Golyshin PN (2006) Proteomic insights into metabolic adaptations in Alcanivorax borkumensis induced by alkane utilization. J Bacter 188(11): 3763-3773.
- Schneiker S, Dos Santos VAM, Bartels D, Bekel T, Brecht M, et al. (2006) Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat biotec 24(8): 997-1004.
- Yakimov MM, Timmis KN, Golyshin PN (2007) Obligate oil-degrading marine bacteria. Curr Op Biotec 18(3): 257-266.
- Cappello S, Corsi I, Patania S, Bergami E, Azzaro M, et al. (2022) Characterization of five psychrotolerant Alcanivorax spp. strains isolated from Antarctica. Microorg 11(1): 58.
- Kasai Y, Kishira H, Sasaki T, Syutsubo K, Watanabe K, et al. (2002) Predominant growth of Alcanivorax strains in oil‐contaminated and nutrient‐supplemented sea water. Envir Microb 4(3): 141-147.
- Cappello S, Caruso G, Zampino D, Monticelli LS, Maimone G, et al. (2007a) Microbial community dynamics during assays of harbour oil spill bioremediation: A microscale simulation study. J App Microb 102(1): 184-194.
- Cappello S, Denaro R, Genovese M, Giuliano L, Yakimov MM (2007b) Predominant growth of Alcanivorax during experiments on “oil spill bioremediation” in mesocosms. Microb Res 162(2): 185-190.
- Mapelli F, Scoma A, Michoud G, Aulenta F, Boon N, et al. (2017) Biotechnologies for marine oil spill cleanup: Indissoluble ties with microorganisms. Tren Biotec 35(9): 860-870.
- Bruns A, Berthe-Corti L (1999) Fundibacter jadensis gen. nov. sp. nov., a New slightly halophilic bacterium isolated from intertidal sediment. Int J of Syst Bact 49(2): 441-448.
- Fernández-Martínez J, Pujalte MJ, García-Martínez J, Mata M, Garay E, et al. (2023) Description of Alcanivorax venustensis s nov. and reclassification of Fundibacter jadensis DSM 1 21 78T (Bruns and Berthe-Corti 1999) as Alcanivorax jadensis comb. nov., members of the emended genus Alcanivorax. Int J Syst Evol Microbiol 53(1): 331-338.
- Liu C, Shao Z (2005) Alcanivorax dieselolei nov., a novel alkane-degrading bacterium isolated from sea water and deep-sea sediment. Int J Syst Evol Microbiol 55(3): 1181-1186.
- Rivas R, Garcia-Fraile P, Peix A, Mateos PF, Martinez-Molina E, et al. (2007) Alcanivorax balearicus nov., isolated from Lake Martel. Int J Syst Evol Microbiol 57(6): 1331-1335.
- Wu Y, Lai Q, Zhou Z, Qiao N, Liu C, et al. (2009) Alcanivorax hongdengensis nov., an alkane-degrading bacterium isolated from surface seawater of the straits of Malacca and Singapore, producing a lipopeptide as its biosurfactant. Int J Syst Evol Microbiol 59(6): 1474-1479.
- Lai Q, Wang J, Gu L, Zheng T, Shao Z (2013) Alcanivorax marinus nov., isolated from deep-sea water. Int J Syst Evol Microbiol 63(12): 4428-4432.
- Lai Q, Fu X, Wang J, Dong C, Wang L, et al. (2023) Alcanivorax xiamenensis nov., a novel marine hydrocarbonoclastic bacterium isolated from surface seawater. Int J Syst Evol Microbiol 73(3).
- Rahul K, Sasikala C, Tushar L, Debadrita R, Ramana CV (2014) Alcanivorax xenomutans nov., a hydrocarbonoclastic bacterium isolated from a shrimp cultivation pond. Int J Syst Evol Microbiol 64(Pt_10): 3553-3558.
- Kwon KK, Oh JH, Yang SH, Seo HS, Lee JH (2015) Alcanivorax gelatiniphagus nov., a marine bacterium isolated from tidal flat sediments enriched with crude oil. Int J Syst Evol Microbiol 65(7): 2204-2208.
- Yang S, Li M, Lai Q, Li G, Shao Z (2018) Alcanivorax mobilis nov., a new hydrocarbon-degrading bacterium isolated from deep-sea sediment. Int J Syst Evol Microbiol 68(5): 1639-1643.
- Lai Q, Zhou Z, Li G, Li G, Shao Z (2016) Alcanivorax nanhaiticus nov., isolated from deep sea sediment. Int J Syst Evol Microbiol 66(9): 3651-3655.
- Song L, Liu H, Cai S, Huang Y, Dai X, et al. (2018) Alcanivorax indicus nov., isolated from seawater. Int J Syst Evol Microbiol 68(12): 3785-3789.
- Liu J, Ren Q, Zhang Y, Li Y, Tian X, et al. (2019) Alcanivorax profundi nov., isolated from deep seawater of the Mariana Trench. Int J Syst Evol Microbiol 69(2): 371-376.
- Liao X, Lai Q, Yang J, Dong C, Li D, et al. (2020) Alcanivorax sediminis nov., isolated from deep-sea sediment of the Pacific Ocean. Int J Syst Evol Microbiol 70(7): 4280-4284.
- Dong C, Lai Q, Liu X, Gu L, Zhang Y, et al. (2021) Alcanivorax profundimaris nov., a novel marine hydrocarbonoclastic bacterium isolated from seawater and deep-sea sediment. Curr Microbiol 78(3): 1053-1060.
- Zhu L, Wang Y, Ding Y, Luo K, Yang B, et al. (2022) Alcanivorax limicola nov., isolated from a soda alkali-saline soil. Arch Microbiol 204(1): 106.
- An MM, Shen L, Liang RN, Lu YJ, Zhao GZ (2023) Alcanivorax quisquiliarum nov., isolated from anaerobic fermentation liquid of food waste by high-throughput cultivation. Int J Syst Evol Microbiol 73(4): 005764.
- Rai A, Suresh G, Ria B, Biswas R, Sreya PK, et al. (2023) Phylogenomic analysis of the genus Alcanivorax: proposal for division of this genus into the emended genus Alcanivorax and two novel genera Alloalcanivorax nov. and Isoalcanivorax gen. nov. Int J Syst Evol Microbiol 73(1): 005672.
- Hassanshahian M, Tebyanian H, Cappello S (2012) Isolation and characterization of two crude oil-degrading yeast strains, Yarrowia lipolytica PG-20 and PG-32, from the Persian Gulf. Mar Pol Bull 64(7): 1386-1391.
- Xue J, Yu Y, Bai Y, Wang L, Wu Y (2015) Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: A review. Curr Microb 71(2): 220-228.
- Van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microb Mol Bio Rev 67(4): 503-549.
- Adams GO, Fufeyin PT, Okoro SE, Ehinomen I (2015) Bioremediation, Biostimulation and bioaugmention: A review. International Journal of Environmental Bioremediation & Biodegradation 3(1): 28-39.
- Delacuvellerie A, Cyriaque V, Gobert S, Benali S, Wattiez R (2019) The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J Hazar Mat 380:120899.
- Zadjelovic V, Cassola GC, Obrador-Viel T, Lester D, Eley T, et al. (2022) A mechanistic understanding of polyethylene biodegradation by the marine bacterium Alcanivorax. J Hazar Mat 436.
- Koike H, Miyamoto K, Teramoto M (2023) Alcanivorax bacteria as important polypropylene degraders in mesopelagic environments. App Env Microb 89(12): e0136523.
© 2024 Simone Cappello. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






