- Submissions

Full Text
Examines in Marine Biology & Oceanography
From Atlantic to Greece. The Case of Nasal Mite in Mediterranean Monk Seal
Natalia Athinaiou1, Joanne Sarantopoulou1, Anastasia Komnenou2, Elias Papadopoulos2, Athanasios Exadactylos1 and Georgios A Gkafas1*
1Hydrobiology-Ichthyology Laboratory, Department of Ichthyology and Aquatic Environment, School of Agricultural Sciences, University of Thessaly, Greece
2School of Veterinary Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Greece
*Corresponding author: Georgios A Gkafas, Hydrobiology-Ichthyology Laboratory, Department of Ichthyology and Aquatic Environment, School of Agricultural Sciences, University of Thessaly, Fytokou str., 38446, Volos, Greece
Submission: October 09, 2023;Published: December 18, 2023

ISSN 2578-031X Volume6 Issue3
Abstract
Marine mammal dispersion and heterogeneity is worldwide and depends on the geographical area. Some species live isolated, while others can be found all over the oceans. This isolation can possibly be the case of different kinds of diseases among them as in each geographical area different pathogens prevail. Pinnipeds seem to be the main host of nasal mites, while gray seals, especially in Atlantic waters, seem to develop vulnerability to the species Halarachne halichoeri. A Mediterranean monk seal was found dead in the Pagasetic Gulf in Greece by professional fishermen. Anatomy in the cardiorespiratory system revealed the presence of parasitic individuals in both lungs. Internal Transcribed Spacer 1 (ITS1) and 5.8S ribosomal RNA gene sequencing results of the examined parasites showed 91.8% similarity to the species Halarachne halichoeri as the overlap reaches 95%. This species lives in the respiratory system of flippers mainly in the Baltic Sea and the European Atlantic coast. It has not been observed in the Mediterranean to date and it is very likely that this also applies to the parasite under study. Although the species found in the Mediterranean seal’s respiratory system has not been identified, speculation about its origin and the pathology it causes has been linked to Halarachne halichoeri. The most probable scenarios are the entry of the seal from the Atlantic Ocean to the Mediterranean, the entry of another seal species, host of Halarachne halichoeri, from the Atlantic to the Mediterranean and its contact with the person under study, as well as the passage of the parasite to the Mediterranean, as in the larval phase it may live outside its host, but these scenarios are assumed, since to date there are no recordings.
Keywords:Pinnipeds; Monachus monachus; Mediterranean sea; Nasal mite; Internal Transcribed Spacer 1 (ITS1); 5.8Sr-RNA; Halarachne
Introduction
Marine mammals, consist out of 129 species presenting an heterogenous distribution all over the oceans, while they can also be found in some fresh water environments. An average geographic range that applies to all these species, does reach 52 million km2 [1]. Species site fidelity, can differ with respect to the geographical region. It has been proven, that along the coasts of North and South America, Africa, Asia, and Australia, the abundance of species is much higher, due to upwelling systems, which enrich the fish communities as a consequence of high levels of nutrients [2]. Although this is true, climate change is affecting the primary productivity more and more over the years, fact that long term complicates this natural phenomenon. Marine mammals though, are able to locate new high productivity areas as they develop [3]. In addition, three are the geographical barriers that seem to have influenced marine mammal biogeography. The tropical waters of the equator [4], the Isthmus of Panama which prevented the gene flow between the Atlantic and the Pacific [5] and the Bering Strait, which created a new northern connection between the North Atlantic and the North Pacific ocean [6]. Interestingly, the distribution patterns in marine mammal species differ intensely when referring to higher taxa. Pinnipeds, are most likely found in the poles, when Mysticetes are commonly found around the 30° S latitude areas and Odontocetes near tropical waters. Taxonomic speaking, the closer to the species, the higher the distribution [1]..
Different species can be found in different geographical areas. A typical example is the two types of monk seals. The Hawaiian monk seal (Neomonachus schauinslandi) which lives in the Hawaiian archipelago and the Mediterranean monk seal (Monachus monachus), which can be found mostly in the Mediterranean sea [7]. The grey seal (Halichoerus grypus), is found in the North Atlantic with two major populations around the coast of Canada and the United Kingdom [8]. Furthermore, the Baikal seal (Pusa sibirica), is endemic to the Lake Baikal in Siberia, the Australian sea lion (Neophoca cinerea), is found only in Australia, the Galapagos fur seal (Arctocephalus galapagoensis) and the Galapagos sea lion (Zalophus wollebaeki), endemic to the Galápagos Islands, the New Zealand dolphin (Cephalorhynchus hectori), endemic to New Zealand and the vaquita (Phocoena sinus), endemic to the northern Gulf of California [1,9,10]. Ringed seals Pusa hispida and white whales Delphinapterus leucas, are Arctic species and can only be found close to Svalbard and Norway [11].
This isolation of species and subspecies, can possibly result in different kinds of diseases among them, as in each geographical area different pathogens prevail. In this case, individuals from different species who naturally do not get in contact with each other would not share the same pathogens. However, it is possible, especially for seals, to come in direct contact with other species, which can be a result of either direct transmission, or via an intermediate host and therefore they might become infected by other pathogens and carry them on to their own population [12]. Different kinds of migrations, would allow the direct or indirect contact of different species and therefore the transmission of pathogens.
?It has been documented that parasites are common in marine mammals [13] and regulate their populations playing a very important role in an ecological and evolutionary scale [14,15]. On the other hand, different host species inhabit relative different geographical areas, share common parasites. Lehnert et al. [13] in a study conducted in New Zealand and Australian waters, showed that the parasite Bolbosoma capitatum can be detected in false killer whales (Pseudorca crassidens) in Prime’s Beach and St. Vincent Gulf of Adelaide (South Australia) and in Augusta (Western Australia) as well as in long-finned pilot whales (Globicephala melas) in the Macquarie Harbour in Tasmania. Additionally, the parasite Corynosoma bullosum, can be found in leopard seals (Hydrurga leptonyx) around Heard Island southwest of Australia and in southern elephant seals (Mirounga leonina) close to Campbell and Macquarie Island south of New Zealand. Walker [16] described the larval cestode Phyllobothrium delphini? in Dall’s porpoises (Phocoenoides dalli) in the northwestern North Pacific Ocean, while another study conducted at the same period from Mazzariol et al. [17], shows the existence of the same parasite in the dorsal muscles and in the blubber of the dorsocaudal regions in different dolphin species like the Striped Dolphin (Stenella coeruleoalba), stranded in the Adriatic Sea coastline. In addition, in 1993 Moser and Rhinehart first recorded an infected beaked whale calf (Mesoplodon sp.) by an adult nematode Halocercus Pseudaliidae in Monterey Bay, California (USA), when the first sight of the same parasite in the lungs of two stranded killer whales (Orcinus orca) in Norway and Germany was described by Reckendorf et al. [18]. Furthermore, Koitsanou et al. [19] showed the presence of Pseudoterranova bulbosa in the stomach of a female Mediterranean monk seal (Monachus monachus), with this being the first and only sight of the particular parasite in the Mediterranean. Instead, Pseudoterranova spp, are usually found in Arctic and Sub- Arctic regions [20], with main hosts pennipeds like the northern elephant seal (Mirounga angustirostris), the harbor seal (Phoca vitulina) and the sea otter (Enhydra lutris) [21].
Out of the marine mammal class, pinnipeds are the ones who constitute a very common host for nasal mites. Mites of the family Halarachnidae, are mainly inhabiting the respiratory tract of marine mammals [22]. Two are the genera that infect marine mammals, Halarachne [23] and Orthohalarachne (Newell 1947). The genus Orthohalarachne is typically found in otariids and odobenids, while the genus Halarachne is typically found in phocids [24] and especially seals [25]. However, the extent of the particular mite transmition between the different hosts and the affinity for specific host species are not totally understood yet. For instance, the infection of sea otters can highly occur due to their contact with Pacific harbor seals [26,27].
It has been argued that in North Atlantic European waters, the nasal mite Halarachne halichoeri affects Grey seals, infecting their respiratory system and creating different kinds of related diseases [23]. The same mite has recently been found in gray seals, constituting the first observation in Polish waters [28]. Halarachnid mites have been detected during necropsies the TMMC (The Marine Mammal Center) in Sausalito California carried out, in Pacific harbor seals, California sea lions, northern elephant seals, northern fur seals, and Guadalupe fur seals. TMMC additionally found the presence of other Halarachne species in other pennipeds. Halarachne miroungae was found in the northern elephant seals, when mites from the species Orthohalarachne attenuata were found in northern fur seals, California sea lions and Guadalupe fur seals [22].
This study reports the first record of Halarachne halichoeri nasal mite found in Mediterranean monk seal. The aim of the study was to document the molecular systematics of the parasite and discuss host-parasite interactions. Possible scenarios of the entrance of the parasite in the Mediterranean Sea are described with respect to either direct transmission, or via an intermediate host. On the other hand, potential effects of climate change are argued to shape species distribution, and therefore the level of parasite-host interactions.
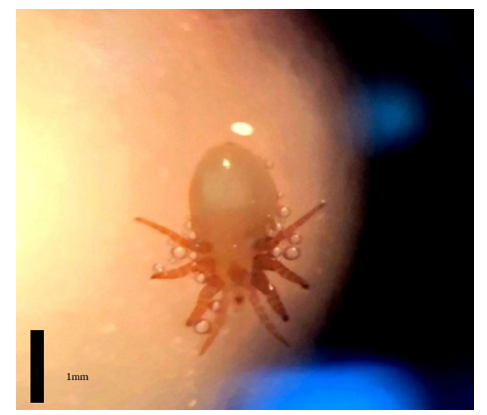
In November 2019, a young female Mediterranean monk seal was found dead in Pagasitikos gulf of Thessaly Greece. It was transferred by fishermen to shore and from there to the Hydrobiology and Ichthyology Laboratory of the University of Thessaly, Department of Ichthyology and Aquatic Environment, where an anatomy of the individual was conducted. The purpose of this research was the identification of a phthiraptera parasite species, found in the respiratory system of this individual, using molecular methods (Figure 1).
Figure 1:One of the parasitic phthiraptera that was found in the lungs of the Mediterranean monk seal (credits: Laboratory of Ichthyology and Hydrobiology, Dept. of Ichthyology and Aquatic Environment, University of Thessaly). Black line approximately 1mm.
Materials and Methods
Sample procedure
Both lungs were used for parasite infestation. Each lung was weighed to the closest milligram. The lung was opened starting from the main bronchus of the upper lobe which is connected to the trachea, and then the duct of each bronchioles and alveoli were followed through to the end of the bottom lobe. Parasites were collected, and then stored in saline buffer. The buffer helps to maintain a constant pH and it is an isotonic and not-toxic solution [29]. After cleaning with the isotonic buffer, the parasites from each lung were preserved in 70% alcohol. After the gross examination, lungs were washed out on a 0.2mm sifter and any parasites obtained were collected. All parasites were examined under a stereoscope for morphological identification. Further to that, 10% of the total number of parasites were prepared and screened in a microscope to ensure the consistency of species identification. A Petri dish with divided areas was used for the parasite counting. Parasites of each lung were combined for the total individual lung-parasite burden. Parasites were stored in 70% ethanol for potential back up analysis and for further molecular analysis.
Molecular methods
A modified DNA extraction protocol was conducted according to the phenol/chloroform procedure [29]. For the mite species identification the genomic area of Internal Transcribed Spacer 1 (ITS1) and 5.8S ribosomal RNA gene was amplified using the primers as described in Nadler et al. [21]. PCR reactions were performed in 25 mL reaction mixtures containing ~10ng template DNA, 5mL of 10×PCR buffer (Invitrogen), 2.5mM MgCl 2 (Invitrogen, Waltham, MA, USA), 0.2μL of 10mM each deoxyribonucleotide triphosphate (dNTPs) (Invitrogen), 0.3μL of each 10 mM primer (Operon- Invitrogen), and 1 unit of Taq polymerase (Invitrogen). A PTC-200 thermocycler (MJ Research, Waltham, MA, USA) was used, and PCR amplification was applied under the following cycling conditions: An initial denaturation at 95 °C for 10 min followed by 35 cycles. Each cycle included the following steps: a denaturation at 95 °C for 30s, an annealing at 53 °C for 30s, and an extension at 72 °C for 1 min. A final extension at 72 °C for 10 min was applied.
The PCR amplification products were separated in 1.5% (wt/vol) agarose gels using 1X Tris Acetate EDTA (TAE) and photographed on a UV transilluminator. PCR amplification products were purified by using the NucleoSpin Extract Kit (Macherey Nagel, Duren, Germany) in order to remove secondary metabolites prior to sequencing. All sequences were determined on an ABI PRISM® 3700 DNA Analyzer (Applied Biosystems). Each fragment used was sequenced in both directions to maximize the accuracy of the sequencing.
Phylogenetic analysis
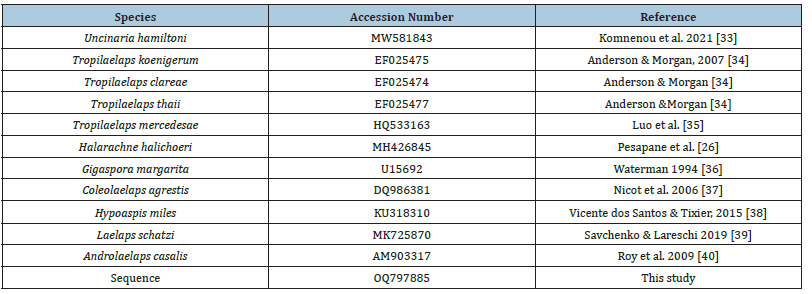
Sequence alignment was carried out with AliView software v.1.27 [30]. Subsequently, MEGA software v.11.0.8 [31] was used for the analysis of the sequences, implementing the Bootstrap method and the Kimura 2-parameter model [32]. The phylogenetic tree was created using MEGA software, based on the Maximum Composite Likelihood model, with 1,000,000 repetitions and bootstrap was set to 500,000. For the above procedure, sequences from eleven different species were used (Table 1). Uncinaria hamiltoni, which was used as an outgroup species, Tropilaelaps koenigerum, T. clareae, T. thaii, T. mercedesae, Halarachne halichoeri, Gigaspora margarita, Coleolaelaps agrestis, Hypoaspis miles, Laelaps schatzi, Androlaelaps casalis as well as the sequence obtained by the molecular analysis of this study, which for the present procedure was named “Sequence”.
Table 1:Accession number of the species used for the construction of the phylogenetic tree.
Result
During organ dissection, nine individuals of parasites were found on the right lung and three in the left one. Each one of the, was 2mm in length and mm in width. In the phylogenetic tree (Figure 2) the phylogenetic relations of the ITS1-5.8SrDNA revealed through molecular analysis are presented, as well as the bootstrap values which show the frequency of occurrence of a species in the cladogram when creating the phylogenetic tree according to the total sample. The closer the species are in the diagram, the closer they are genetically. The sequence “Sequence” appears to be genetically very close to the sequence of the species Halarachne halichoeri, with a bootstrap value of 0.95, unlike the other sequences. The species of the genus Tropilaelaps showing a bootstrap value of 0.71, is genetically the farthest relative to the “Sequence” sequence. Following, the species Gigaspora margarita with a bootstrap value of 0.63, then Coleolaelaps agrestis with a value of 0.62 and the most distant genetic species in relation to that of the research are Hypoaspis miles, Laelaps schatzi, Androlelaps casalia and Uncinaria hamiltoni.
Figure 2:Phylogenetic tree. The number 0.4 corresponds to the size scale and the numbers included in the phylogenetic tree are the bootstrap values.

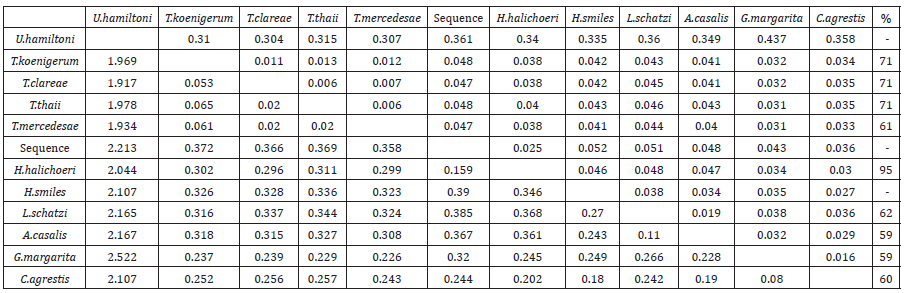
In addition, NCBI blast (access on March 2023) showed that the parasite isolated from the respiratory system of the seal and submitted in molecular analysis, reveals a 91.8% similarity with the species Halarachne halichoeri, while the overlap of these two species is 95% (Table 2). The model Kimura 2- parameter, displayed the genetic distances of the species that arose through it sequencing, revealing that the species Halarachne halichoeri is the closest genetically with the parasite found in the seal’s lung relative to the rest, with an index of 0.1585, which was also the smallest index of the species that interests us in relation to the rest of the species (Table 2). Studying the phylogenetic tree and through the presented bootstrap values, a polyphyletic relationship between the under research species and the species Halarachne halichoeri, as well as the species Tropilaelaps koenigerum, Tropilaelaps clareae, Tropilaelaps thaii and Tropilaelaps mercedesae is revealed, with a bootstrap value of 71%.
Table 2:Genetic distances using the Kimura 2-parameter model. Numbers below the diagonal represent the genetic distance and numbers above the diagonal represent the standard deviation (standard error) and overlap percentages of the species used for the phylogenetic tree creation in relation to the species of the research, according to the database ncbi (GenBank® -www.ncbi.nlm.nih.gov/genbank/).
Discussion
The present study reveals a close evolutionary relationship between the specimen species and Halarachne halichoeri, fact that indicates a possibility of this parasite being present in Mediterranean waters. Halarachne halichoeri belongs to the class Phthiraptera, to the genus Halarachne of the family Halarachnidae which includes exclusively parasites infecting the respiratory system of all species of mammals [24]. While most phthiraptera are ectoparasitic, individuals of the family Halarachnidae consist mostly of endoparasites. Their evolution over the years seems to be parallel to the one of carnivorous animals, but eventually acquired a symbiotic relationship with pinnipeds, which are their final hosts [33]. A fact that makes them distinguish from their relatives is that through evolution, they managed to adapt to the environment of a semi-aquatic host [34], although the information concerning the shift of ectoparasites to endoparasites using aquatic and semiaquatic organisms as hosts, are limited (Moon et al. 2019).
As it has already been mentioned, the respiratory system of pinnipeds seems to be the main host of parasites of the genus Halarachne [33]. It is mainly observed in the nasal passage of otters [24,35] as well as in the body of the gray seal Halichoerus grypus [28]. It was first detected by Dr O’Brien Bellingham in 1837, in the respiratory system of a gray seal found dead onshore of Dublin. However, the first official record was made ten years later by George James Allman [33]. The species Halarachne halichoeri causes many different forms of respiratory diseases [36,37], damaging the mucous tissue of the respiratory system or by reducing the respiratory capacity of the host [23,35,36,38]. They are transmitted between individuals either through coughing, or through close nasal or physical contact [39]. In general the effect of Halarachne halichoeri on the health of pinnipeds should be studied more extensively in order to have more accurate information regarding the damage it can create in the respiratory system [23]. However, it is known to be a quite selective parasite in terms of the organ it will parasitize, which will include the upper and lower respiratory system [40].
In the larval development stage, they begin as free hexapods, which are mainly responsible for the spread of infections. In this form they are capable of surviving out of their host in an aquatic environment, until they sense a high concentration of carbon dioxide exhaled from the hosts and penetrate from its nostrils into the respiratory system [39]. Adults appear in an eight-legged form [23], located in the nasopharyngeal and pulmonary mucosa, the only environment they are able to survive [25,35]. Studies have shown that the species Halarachne halichoeri parasitized the seal Halichoerus grypus until the end of the 19th century in German waters. The seal species though extinct from the area, due to overexploitation of natural resources by human activities and with it, the parasite extinct as well. Although, from 2014, along with the appearance of grey the seals Halichoerus grypus and Phoca vitulina in the Baltic Sea, the parasite also reappeared at the same time as its main host, also indicating its transmission from one seal species to another [33].
Research carried out on the north-west coast of Spain between 1999 and 2009 by Alonso F et al. [23], recorded the first sight of Halarachne halichoeri in the area. In addition, it confirmed the hypothesis that gray seals are their main hosts. Research shows that this species has been identified in European waters in coastal areas of Spain in the Atlantic ocean, in Great Britain, in Holland and in Germany [18,23] and usually in gray seals. Other pinniped species that it has been identified, are Cystophora cristata, Enhydralutris, Halichoerus grypus, Mirounga leonine, Phoca largha, Phoca vitulina, Pygoscelis papua, although it seems like a random event, most likely due to favorable conditions [28]. The first confirmed record of it in the Baltic Sea however, was in 2018 [28].
Based on current studies and scientific records so far, the existence of the species Halarachne halichoeri in Mediterranean waters is almost impossible. However, molecular evidence in the present study reveal that the species Halarachne halichoeri and the one extracted from the respiratory system of the Mediterranean monk seal are 91.8% identical, with this value being the highest in comparison to the other species it was compared to. As shown in the researches above, a parasitic species genetically close to the Halarachne halichoeri could have been transmitted to the Mediterranean Monk seal of the present study through another individual seal, or through random hosts. The fact that this parasite and probably neither the one under study, has not been detected in the Mediterranean before, may mean the invasion of some random host from the Strait of Gibraltar and its subsequent transmission to the Mediterranean Monk seal, but this can only be based on assumptions. According the above, the following cases are mentioned.
A possible explanation may be the entry of a Mediterranean seal into the Mediterranean from the Atlantic Ocean, through the Strait of Gibraltar. While it is mentioned as a Mediterranean species, according to NCBI (GenBank® -www.ncbi.nlm.nih.gov/genbank/), Mediterranean seal individuals are also observed in the coasts of Portugal, the Madeira Islands and the Azores, parts of the Atlantic Ocean. This explanation can include two assumptions. Populations of Mediterranean seals living in the Atlantic can enter the Mediterranean Sea for search of better living conditions and food. The diet of the Mediterranean seal mainly includes cephalopods such as octopuses and squids, teleosts and crustaceans [41]. It is likely that the conditions in Mediterranean waters do not demand such competition between species with similar dietary preferences. Moreover, the only predators, other than humans all kinds of seals have, are sharks and killer whales. Another possible scenario is that this particular seal came in close contact with another seal individual of another species that probably hosted parasites like the ones of this particular study. The original host seal in this case may have passed through the Atlantic ocean in the western Mediterranean and transmitted the parasite to the Monk seal. Accordingly, this chain could have a greater extent, starting with pinnipeds in the Baltic Sea and ending in the Mediterranean. Another conceivable explanation for this phenomenon could concern the larvae of Halarachne halichoeri, which as already mentioned, has the ability to live out their aquatic hosts [39]. Therefore, the hypothesis that this parasite entered the Mediterranean sea either due to intense currents, or by artificial mediums like ships, should be taken under consideration. So, except from the possible explanation of finding such a parasite in a Mediterranean Monk seals’ body, a new parasitic alien species emerges in the Mediterranean, since to this day there is no official record concerning it.
Learmonth et al. [42], argue about how the global climate change can affect the distribution of fish and marine mammals and therefore the parasites they host. Changes in temperature, in ocean circulation and climate patterns, are some of the factors that can force marine animals to change their environment, fact that can lead to the spread of different diseases. A similar transmition of Halarachne halichoeri from one species to another, was noticed during necropsies performed on southern sea otters (Enhydra lutra nereis) from Californian waters submitted to the Marine Wildlife Veterinary Care and Research Center’s Sea Otter Necropsy Program between 2012 and 2017. Scientists observed Halarachne halichoeri mites, a species typically associated with harbor seals (Phoca vitiluna), parasitized a 25.6% of the sea otters the necropsy was performed on, indicating the first documentation of Halarachne halichoeri mite exchange between southern sea otters and harbor seals [26].
Conclusion
Concluding, since transmitions of this parasite between individuals from different species have been recorded in the past, it is not hard to believe that this will keep happening, until the in-host or out-host environment won’t allow it anymore. The above cases in the future could be directly related to cases of host switching for various parasites. For many years now, due to the increase in maritime traffic, many marine species are introduced to new environments and naturally forced to harmonize with native species [43-45], with which they do not share a common evolutionary history, neither behavior or ecology [46-48]. A parasite transmission from one species to another is also an expected phenomenon, which results from the unexpected but inevitable species contact [49-59] and could be most of the times disrupt the ecosystem, although adaptation procedures seem to be the solution in every evolution related case after all [60-68].
References
- Pompa S, Ehrlich PR, Ceballos G (2011) Global distribution and conservation of marine mammals. PNAS 108(33): 13600-13605.
- Pauly D, Christensen V (1995) Primary production required to sustain global fisheries. Nature 374(6519).
- Harwood J (2001) Marine mammals and their environment in the twenty-first century. J Mammal 82(3): 630-640.
- Davies JL (1963) The antitropical factor in Cetacean Speciation. Evolution 17(1): 107-116.
- O’Dea A, Lessios HA, Coates AG, Eytan RI, Restrepo-Moreno SA, et al. (2016) Formation of the isthmus of Panama. Sci Adv 2(8): e1600883.
- Marincovich L, Gladenkov AY (2001) New evidence for the age of Bering Strait. Quat Sci Rev 20(1-3): 329-335.
- Littnan C, Karamanlidis AA, Dendrinos P (2018) Monk seals: Monachus monachus, Neomonachus schauinslandi, and N. tropicalis. In: Würsig B, Thewissen JGM, Kovacs KM (Eds.), Encyclopedia of Marine Mammals. 3rd (Edn.), Academic Press, USA, pp. 618-622.
- Hall AJ, Russell DJF (2018) Gray seal Halichoerus grypus. In: Würsig B, et al. (Eds.), Encyclopedia of marine mammals. Academic Press, USA, pp. 420-422.
- Rojas-Bracho L, Reeves RR, Aramillo-Legorreta A (2006) Conservation of the vaquita Phocoena sinus. Mamm Rev 36(3): 179-216.
- NOAA (2023) National Oceanic and Atmospheric Administration.
- Hamilton C, Lydersen C, Fedak M, Carla F, Hindell M, et al. (2019) Bearded seal pup ontogeny. Mar Ecol Progr Ser 627: 179-194.
- Pool R, Chandradeva N, Gkafas GA, Raga JA, Fernández M, et al. (2020) Transmission and predictors of burden of lungworms of the Striped Dolphin (Stenella coeruleoalba) in the Western Mediterranean. J Wild Dis 56(1): 186-191.
- Lehnert K, Poulin R, Presswell B (2019) Checklist of marine mammal parasites in New Zealand and Australian waters. J Helminthol 93(6): 649-676.
- Raga JA, Balbuena JA, Aznar FJ, Fernandez M (1997) The impact of parasites on marine mammals: A review. Parasitologia 39(4): 293-296.
- Aznar FJ, Balbuena JA, Fernández M, Raga JA (2001) Living together: The parasites of marine mammals. In: Evans PGH, Raga JA (Eds.), Marine mammals: Biology and conservation. Springer Publishers, USA, pp. 385-423.
- Walker WA (2006) Geographical variation of the parasite, Phyllobothrium delphini (cestoda), in Ball’s porpoise, Phocoenoides dalli, in the Northern North Pacific, Bering Sea, and Sea of Okhotsk. Mar Mamm Sci 17(2): 264-275.
- Mazzariol S, Centelleghe C, Petrella A, Marcer F, Beverelli M, et al. (2021) Atypical toxoplasmosis in a mediterranean monk seal (Monachus monachus) Pup. J Comp Pathol 184: 65-71.
- Reckendorf A, Ludes-Wehrmeister E, Wohlsein P, Tiedemann R, Siebert U, et al. (2018) First record of Halocercus sp. (Pseudaliidae) lungworm infections in two stranded neonatal orcas (Orcinus orca). Parasitology 145(12): 1553-1557.
- Koitsanou E, Sarantopoulou J, Komnenou A, Exadactylos A, Dendrinos P, et al. (2022) First report of the parasitic nematode Pseudoterranova spp. found in mediterranean monk seal (Monachus monachus) in Greece: Conservation implications. Conservation 2(1): 1.
- Paggi L, Nascetti G, Cianchi R, Orecchia P, Mattiucci S, et al. (1991) Genetic evidence for three species within Pseudoterranova decipiens (nematoda, ascaridida, ascaridoidea) in the North Atlantic and Norwegian and Barents Seas. Int J Parasitol 21(2): 195-212.
- Nadler SA, Hoberg EP, Hudspeth DS, Rickard LG (2000) Relationships of Nematodirus species and Nematodirus battus isolates (Nematoda: Trichostrongyloidea) based on nuclear ribosomal DNA sequences. J Parasitol 86(3): 588-601.
- Pesapane R, Archibald W, Norris T, Fontaine C, Halaska B, et al. (2021) Nasopulmonary mites (Halarachnidae) of coastal Californian pinnipeds: Identity, prevalence, and molecular characterization. Int J Parasitol Parasites Wildl 16: 113-119.
- Alonso-Farré, JM, D’Silva JID, Gestal C (2012) Naso-pharyngeal mites Halarachne halichoeri (Allman, 1847) in Grey seals stranded on the NW Spanish Atlantic Coast. Vet Parasitol 183(3-4): 317-322.
- Furman DP, Dailey MD (1980) The Genus Halarachne (Acari: Halarachnidae), with the Description of a New Species from the Hawaiian Monk Seal. J Med Entomol 17(4): 352-359.
- Fay FH, Furman DP (1982) Nasal mites (Acari: Halarachnidae) in the spotted seal, Phoca largha pallas, and other pinnipeds of Alaskan waters. J Wildl Dis 18(1): 63-68.
- Pesapane R, Dodd E, Javeed N, Miller M, Foley J (2018) Molecular characterization and prevalence of Halarachne halichoeri in threatened southern sea otters (Enhydra lutris nereis). Int J Parasitol Parasites Wildl 7(3): 386-390.
- Shockling CED, Miller MA, Batac F, Dodd E, Smith W, et al. (2019) Pathology and epidemiology of nasopulmonary acariasis (Halarachne sp.) in southern sea otters (Enhydra lutris nereis). Int J Parasitol Parasites Wildl 9: 60-67.
- Rolbiecki L, Izdebska JN, Bidziński K, Jankowska-Jarek M (2018) Nasopharyngeal mites Halarachne halichoeri (Allman, 1847) parasitizing the gray seal Halichoerus grypus (Fabricius, 1791) in the Baltic Sea with notes on other parasitic Halarachnidae associated with marine mammals. Oceanol Hydrobiol Stud 47(4): 398-404.
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual. 2nd (edn.), volume 3, Cold Spring Harbor Laboratory Press, USA.
- Larsson A (2014) AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30(22): 3276-3278.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10): 2731-2739.
- Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2): 111-120.
- Komnenou A, Gkafas GA, Kofidou E, Sarantopoulou J, Exadactylos A, et al. (2021) First report of Uncinaria hamiltoni in Orphan Eastern Mediterranean monk seal pups in Greece and its clinical significance. Pathogens 10(12): 1581.
- Anderson DL, Morgan MJ (2007) Genetic and morphological variation of beeparasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species. Exp Appl Acarol 43(1): 1-24.
- Luo QH, Zhou T, Wang Q, Dai PL, Wu YY, et al. (2011) Identification of Tropilaelaps mites (Acari, Laelapidae) infesting Apis mellifera in China. Apidologie, Chinese Academy of Agricultural Sciences, Institute of Apicultural Research, China 42: 485-498.
- Waterman LD (1994) Sequence analysis of ribosomal DNA amplification products and investigations on protoplast isolation from vesicular-arbuscular mycorrhizal fungi of the genus Gigaspora. PhD Thesis, University of the West Indies, Department of Biology, Bridgetown, Barbados, West Indies, pp. 1-2.
- Nicot A, Niogret J, Stordeur E (2007) Combination of morphological characters and ITS-sequence to characterize a new species of Macrocheles (Acari: Macrochelidae). Zootaxa 1386: 19-29.
- Santos VV, Tixier MS (2017) Which molecular markers for assessing which taxonomic level? The case study of the mite family Phytoseiidae (Acari: Mesostigmata). Cladistics 33(3): 251-267.
- Savchenko E, Lareschi M (2019) A new species of Laelaps (Koch, 1836) (Mesostigmata: Laelapidae) parasitic of the sigmodontine rodent Oligoryzomys flavescens. Waterhouse, 1837 (Rodentia: Cricetidae): Molecular and morphological characterization. Acta Trop 199: 105146.
- Roy L, Dowling AP, Chauve CM, Buronfosse T (2009) Delimiting species boundaries within Dermanyssus Duges, 1834 (Acari: Dermanyssidae) using a total evidence approach. Mol Phylogen Evol 50(3): 446-470.
- Reckendorf A, Wohlsein P, Lakemeyer J, Stokholm I, Vietinghoff V, et al. (2019) There and back again – The return of the nasal mite Halarachne halichoeri to seals in German waters. Int J Parasitol Parasites and Wildl 9: 112-118.
- Furman DP (1979) Specificity, adaptation and parallel evolution in the endoparasitic mesostigmata of mammals. In: Rodriguez JG (Ed.), Recent Advances in Acarology. Volume 2, Academic Press, USA, pp. 329-337.
- Geraci JR, St. Aubin DJ (1987) Effects of parasites on marine mammals. Int J Parasitol 17(2): 407-414.
- Baker JR (1987) Causes of mortality and morbidity in wild juvenile and adult grey seals (Halichoerus grypus). Brit Vet J 143(3): 203-220.
- Baker JR, Jepson PD, Simpson VR, Kuiken T (1998) Causes of mortality and non-fatal conditions among grey seals (Halichoerus grypus) found dead on the coasts of England, Wales and the Isle of Man. Vet Rec 142(22): 595-601.
- Measures LN (2018) Helminths and parasitic arthropods. In: Gulland FMD, Dierauf LA, Whitman KL (Eds.), CRC Handbook of Marine Mammal Medicine. CRC Press, USA, pp. 471-498.
- Furman DP, Smith AW (1973) In vitro development of two species of Orthohalarachne (Acarina: Halarachnidae) and adaptations of the life cycle for endoparasitism in mammals. J Med Entomol 10(4): 415-416.
- McFarlane RA, Norman RJB, Jones HI (2009) Diseases and parasites of antarctic and sub-antarctic seals. In: Kerry KR, Riddle M (Eds.), Health of Antarctic wildlife: A challenge for science and policy. Springer Publishers, USA, pp. 57-93.
- Gilmartin WG, Forcada J (2009) Monk seals: Monachus monachus, M. tropicalis, and schauinslandi. In: Perrin WF, Würsig B, Thewissen JGM (Eds.), Encyclopedia of marine mammals. 2nd (edn.), Academic Press, USA, pp. 741-744.
- Learmonth JA, MacLeod CD, Vazquez MBS, Pierce GJ, Crick HQP, et al. (2006) Potential effects of climate change on marine mammals. Oceanogr Mar Biol 44: 431-464.
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of earth’s ecosystems. Science 277(5325): 494-499.
- Lowe S, Browne M, Boudjelas S, Poorter M (2000) 100 of the World’s Worst Invasive Alien Species A selection from the global invasive species database. Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), Hollands Printing Ltd, New Zealand, pp. 1-11.
- Arena PC, Steedman C, Warwick C (2012) Amphibian and reptile pet markets in the eu: An investigation and assessment. pp. 1-53.
- Huxel GR (1999) Rapid displacement of native species by invasive species: Effects of hybridization. Biol Conserv 89(2): 143-152.
- Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. PNAS 98(10): 5446-5451.
- Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17(4): 170-176.
- Lodge DM (1993) Biological invasions: Lessons for ecology. Trends Ecol Evol 8(4): 133-137.
- Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84(5): 468-478.
- Hudson P, Greenman J (1998) Competition mediated by parasites: Biological and theoretical progress. Trends Ecol Evol 13(10): 387-390.
- Holway DA, Suarez AV (1999) Animal behavior: An essential component of invasion biology. Trends Ecol Evol 14(8): 328-330.
- Tompkins DM, Sainsbury AW, Nettleton P, Buxton D, Gurnell J (2002) Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc R Soc B: Biol 269(1490): 529-533.
- Clay K (2003) Parasites lost. Nature 421(6923): 585-586.
- MacNeil C, Dick JTA, Hatcher MJ, Terry RS, Smith JE, et al. (2003) Parasite-mediated predation between native and invasive amphipods. Proc R Soc B: Biol Sci 270(1521): 1309-1314.
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421(6923): 628-630.
- Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ 2(4): 183-190.
- Smith KF, Sax DF, Lafferty KD (2006) Evidence for the role of infectious disease in species extinction and endangerment. Conserv Biol 20(5): 1349-1357.
- Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE (2008) The spread of invasive species and infectious disease as drivers of ecosystem change. Front Ecol Environ 6(5): 238-246.
- Moser M, Rhinehart H (1993) The lungworm, Halocercus spp. (Nematoda: Pseudaliidae) in Cetaceans from California. J Wildl Dis 29(3): 507-508.
© 2023 Georgios A Gkafas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






