- Submissions

Full Text
Examines in Marine Biology & Oceanography
Long- and Short-Term Salinity Changes of Ocean
Gaspar Banfalvi*
Department of Molecular Biotechnology and Microbiology, Hungary
*Corresponding author: Gaspar Banfalvi, Department of Molecular Biotechnology and Microbiology, 1 Egyetem Square, Debrecen 4010, Hungary
Submission: June 15, 2023;Published: June 28, 2023

ISSN 2578-031X Volume6 Issue1
Abstract
Applying Raoult’s law to the ocean confirms the notion, that the stability of the osmotic concentration (Osm) of the inner environment of land vertebrates (0.3Osm) and the much higher salinity of present day’s ocean (1.09 Osm) reflects a long-term salination process, which continues irrespective of the recent short-term global melting of dilution period. Climatic advances and retreats have been deduced from the law of dilute solutions applied to the ocean. Salinity changes over geological ages provide evidence for a dynamic osmolyte system against a general geochemical balance. The long-term increase in ocean salinity is contrasted by short-term fluctuations with severe climatic consequences. Fluctuations in salinity give an explanation of wet and dry climatic periods and are major driving forces of biological evolution. The recent global warming causing the expansion of sea volume, the melting of sea ice and ice sheets, contributes to the short-term dilution effect temporarily outweighing the long-term effects of salinity increase contributed by the human pollution.
Keywords:Osmolyte systems; Raoult’s law; Salination process; Global warming; Glacial and interglacial periods; Climatic changes; Consequences of salination
Abbreviations:Osmotic Concentration (Osm); European Space Administration (ESA); Sea Surface Salinity (SSS)
Introduction
Edmund Halley, an astronomer observed in 1715 that the ocean was constantly receiving salt. However, he could not estimate the age of Earth using the “salt clock” method as an earlier salt concentration would have been needed to calculate the rate of accumulation [1]. Thomas Mellard Reade was the first to apply Halley’s suggestion of using the salt clock and estimated that it would have taken 25 million years to reach the present day salt concentrations in the ocean [2]. John Joly also used the rate of salt supply by rivers to estimate the age of Earth and determined that the ions would have accumulated in 99.4 million years [3]. This estimate for the age of earth was close to that calculated by Lord Kelvin (William Thomson) in 1864 and was widely accepted. Lord Kelvin estimated that how long it would take an earth-sized molten sphere to cool to today’s temperatures, and he obtained a maximum age near 100 million years. Ten years later Joly revised his equation and calculated the age of Earth to be between 80 and 150 million years old. The salt clock method soon fell out of favor as the salination process was not regarded as a constant process, moreover the initial and final salt concentration were not known. As reliable records that would have extended back to at least one million years were missing, geologists and palaeontologists have dealt mostly with the long term mean of salt concentration of ocean. The constancy was explained by the complex chemistry of seawater and by multistep equilibrium processes operating simultaneously giving the impression of a short-term balance. The idea of a general geochemical balance provided a model to make such constancy plausible [4,5]. Ocean salinity is still regarded stable for billions of years as a consequence of a chemical/tectonic system that removes as much salt as is deposited [6].
From a biologist point of view the constancy of seawater salinity is seriously doubted. There is a general concensus that the extracellular and intracellular concentrations of living organisms were identical and vertebrates remained in osmotic equilibrium with sea before they moved to land [7]. Although, we know neither the initial concentration nor the salinity of sea at the time of emergence of land vertebrates, the electrolyte concentration of today’s ocean should conform biological functions, unless major changes occured either in the electrolyte concentrations of living organisms or in the salinity of the sea. The osmotic concentration of seawater (1.09 Osm) is now more than three times higher than the isotonic concentration of land vertebrates (0.3 Osm). This osmotic concentration seems to be optimal for the biological function of informational macromolecules (DNA, RNA, proteins). The preservation of stable isotonic concentration of terrestrial vertebrates may indicate that the elevation of osmotic concentration of sea could have taken place [8]. The uniform osmolarity of blood in land vertebrates is regarded as a reflection of osmolarity of seawater at the time of their evolutionary emergence from their marine environment. The transition from aquatic to terrestrial life is thought to have occured during the Devonian Era (some 400 million years ago) [9]. Despite the evolutionary divergence and different means of osmotic regulation, terrestrial vertebrates (mammals, reptiles, snakes, lizards, turtles, birds) maintained nearly identical ion concentrations (0.3 Osm) in their blood. Exceptions are amphibians which have significantly lower (0.16-0.24 Osm) and variable ion concentrations and birds with slightly elevated (0.317 Osm) electrolite concentrations. These strikingly similar values point to the biological importance of the ion balance and to the evolutionary stability of the inner environment of land vertebrates.
The variable ion levels in fishes and amphibians in the aqueous environment may reflect the selective osmotic pressure for the early forms of tetrapods. In view of the uniform osmolarity it is reasonable to assume that the osmotic blood concentration (internal environment, Claude Bernard’s “milieu intérieur”) of terrestrial vertebrates is a relic of ancient stage, namely the osmotic concentration of the ocean (external environment) at the time of vertebrates migration to land [8]. This interpretation of osmolarity is consistent with the idea that terrestrial vertebarates arose from fishes in marine waters rather than from slightly saline inland seas or lakes [9]. Although, the salinity change of ocean has an impact on the water cycle and ocean circulation, but less attention has been paid to those factors that cause these changes. To give resonable answer to the salination of oceans the review focuses on the ocean as a semi-dilute solution, the salinity changes of ocean, the salinity of present day’s ocean, and the long-term salinity increase contrasted by the global warming periodes.
Ocean, The Oldest Dilute Solution
Laws of dilute solutions
The criteria of dilute solutions will be discussed later. For the sake of simplicity and better understanding, the ocean with its recent avarege of 1.09 Osm concentration is still regarded as a dilute solution of several salts. Osmotic Concentration (Osm) is used as there are different osmotically active particles in the ocean avoiding the Molar concentration (M) of individual components. Vapor above the sea (as moisture, water gas) is one of the most important gases in the atmosphere beside oxygen, nitrogen and carbon dioxide. Vapor causes to form humidity, fog, clouds, rain, snow, hail and sleet. Most of the global vapor (~85%) is present over the ocean.
Related to the vapor pressure and evaporation, the major principles of dilute solutions have been described by several laws (Henry’s, Dalton’s, Thomson’s, and Raoult’s law). Henry’s law is commonly used for calculating the solubility of a gas in a liquid. Dalton’s law of partial pressures states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture. Idealized models of evaporation are essentially governed by Raoult’s law and Dalton’s law. Thomson’s law states that the growth-rate of the cloud-droplets depends on the radius of the droplet. Here we deal neither with the solubility of gases nor the droplet formation, thus Dalton’s and Thomson’s laws will not be discussed. Moreover, to consider either Raoult’s or Henry’s law is mainly the question of convenience. William Henry’s law applies to the solute in a solution, while Raoult’s law applies to the solvent in a solution. The basic difference in the two laws is that in dilute solutions solutes are surrounded by unlike solvent molecules, while the solvent molecules are surrounded by their own kind of solvent molecules (Francois Raoult). This allows a further simplification due to the easier application of Raoult’s law and its use for quick estimations.
Raoult’s law (1886): p=p*x
states that the vapor pressure (p) of a component is proportional to its concentration, where: p is the vapor pressure over the solution in a sealed container, x is the mole fraction of the component (moles of solvent/total number of moles), p* is the equilibrium vapor pressure of the pure solvent. Raoult’s law explains how in an aqueous solution containing non-dissociating solutes the lowering of vapor pressure of the increasing salt concentration affects boiling point elevation (0.52 oC mol/l), freezing point depression (-1.86 oC mol/l) and the osmotic pressure approximated using the Morse equation [10,11]. Raoult’s law will be discussed here in relation to the salinity of ocean. Raoult’s law applies to dilute, ideal solutions. Although, the ocean is not an ideal solution (ideal solutions simply do not exist), Raoult’s law even under these less ideal conditions has a tremendous impact, but has never been applied to the climate of Earth.
Global aspects of Raoult’s law
Based on Raoult’s law, the antifreeze solutions lower the freezing
point of water, preventing them from freezing in the winter
and from boylover in the summer. The vapor pressure of solutions
is a fundamental property of vapor liquid equilibrium, making use
of it in other processes such as distillation, absorption, stripping,
flash separation, etc. Raoult’s law can be applied to the ocean provided
that multiple criteria of dilute solutions are met. The criteria of
dilute solutions are met when:
a) The concentration of the solution is at least 100 times higher
than the dissolved material. The molal water concentration
(1000g solution containing molar amounts of water in grams)
regarding its H2O content is 1000g/18g=55.5M. When ionic
substances are dissolved it is just to speak about gion/1000g
concentration rather then molal concentration (M).
b) The solute is non-volatile. The sea contains primarily non-volatile
salts, that have no tendency to form vapor over the surface.
The marine iodine cycle has significant impacts on air quality
and atmospheric chemistry. Relatively little data is available
to estimate the global surface iodide concentrations, and this
data has not been openly available in digital form.
c) The solvent evaporates. Those water molecules on the surface
of the sea that are moving fast enough may escape. Other water
molecules return to the solution. Evaporation takes place only
on the surface of the sea.
d) More water molecules brake away at higher temperature (without
boiling) from the surface layer.
e) Evaporation of a liquid takes place in a closed system similarly
to a closed container. The ocean with the atmosphere of the
Earth represents a semi-closed system. Neither the water of
the ocean nor the vapor of the atmosphere will disappear over
time.
f) Equilibrium will be reached in which the number of water particles
leaving the surface is balanced by the precipitation returning
to it. Global equilibrium cannot be reached, local saturation
causes precipitation. The vapor pressure above the sea is
the pressure exerted by the water vapor in the air.
Evaporation from water surfaces and evapotranspiration from Earth’s land surface and plants are the major sources of water vapor of global proportion. During evaporation water molecules at the surface of seawater gain enough energy to escape as vapor into the air above. The warmer the seawater, the easier the evaporation will be, generating higer vapor pressure. The temperature of the surface waters varies mainly with latitude. The temperature of the sea surface is high (27-30 °C) near the equator, often the maximum value occurs a few degrees of latitude north or south of the equator. The Persian Gulf (low latitude) can be as warm as 36 degrees Celsius (96.8 degrees Fahrenheit) and the Red Sea in some locations can even warm up to +42 °C/107.6 °F. Global warming of sea results in a general vapor pressure increase and an expansion of sea volume. The temperature of sea varies also with depth. The deep sea (>3000m) consists of horizontal layers of equal salt density between 0 and 3 oC (32-37.5 Fahrenheit) and is colder than 5 °C above 1000m depth. The surface temperature of polar seas at 3.5% salinity and high latitude can be as low as 2 °C (28.4 degrees Fahrenheit) and freezes at -1.94 oC (28.5 degrees Fahrenheit). At low latitude the seawater at depth is creating a thermocline, which is a layer of rapidly changing temperature.
Salinity and temperature changes cause local differences in vapor pressure, with no vapor pressure equilibrium on a global scale. The salinity distribution differs significantly from global temperature changes. High salinty was registered in the center of the ocean basins, subtropical regions, landlocked seas in arid regions, high rates of evaporations with clear skies, little or no rain, prevailing wind. The sea surface salinity is highest (over 3.7%) in the mid- and lower-Atlantic Ocean, in the Mediterranean Sea and Red Sea. Lower salt concentrations (3.2%) were found near the Arctic, Antarctic and the coastal regions of east Asia and western North America. With its lowest salinity (~1/3 that of seawater) the Caspian sea is a landlocked remnant of the ancient Parathetis Ocean some 50-60 million years ago that connected the Atlantic and the Pacific Oceans. The northern Caspian Sea bottom is dating back to Precambrian times, of at least about 540 million years ago. Until the geologically recent times (~10 million years ago) it was linked through the Sea of Azov, Black Sea, and the Mediterranean Sea, to the world ocean. After loosing connection with the ocean completely some 5 million years ago it turned into the the world’s largest isolated inland sea. Its salinity indicates its marine origin. Over 130 rivers provide inflow of fresh water, with the Volga River being the largest, explaining why the Caspian sea is now less salty than the ocean. It is believed that most desalinated period of the Caspain was some 2-5 million years ago [12].
Earlier the in situ collection of salinity data has been limited primarily to main shipping routes resulting in large gaps. At any geographic location the saturation vapor pressure (equilibrium) depending on temperature and salinity of seas or lakes is the pressure exerted by the water vapor molecules when the air is saturated with water. At the saturation vapor pressure precipitation begins in the form of rain, snow or ice. Vapor equilibrium is scarce in some low latitude places (e.g. plateau of the Atacama Desert in South America), much more water is lost by evapotranspiration than formed by the rare occasions of precipitation. Contrary to the evaporation at an alarming rate the elevated salt concentration of inner seas and lakes without sufficient water supply generates a low vapor pressure. This process known as „continental weathering” reduces the frequency of precipitate formation that often drops to nearly zero leading to the shrinking of these water bodies and to their hypersaline concentration (Dead Sea, 33.7%, Badwater Basin in Death Valley with high versus lower salinity areas, depending on “small springs versus. large springs”. Remnants of ancient lakes that still exist today are the Great Salt Lake or the Bonneville Salt Flats in Utah. Chemical pollution may have a similar effect on salination, e.g. the Aral Sea is heavily polluted and steadily shrinking. The rivers that fed the Aral Sea were diverted in the 1960s for irrigation purposes further aggravating the retreat of the Aral Sea. The human interference caused climate change, with hotter and drier summers and colder and longer winters in the Aral Sea region.
Possible Causes of Salinity Changes
Beside the average salt concentration of the modern ocean (3.49%) the salt content of the sea water may vary according to evaporation, depth, temperature, currents, but mostly with fresh water supply by rainfall, rivers and melting of ice and snow. A related question to be answered is whether the constant flux of ocean is recycling with little or no salinity change or a dynamic process progressing with an initially lower, but increasingly higher salt concentration. To come to conclusion the possible causes of desalination and salination processes will be discussed.
Salt extraction causing decrease in ocean salinity
Rising sea beds, the limited flow or loss of contact of inner shallow seas to the ocean may cause bottle-necking. Similarly, the assembling of continents (e.g. Pangea 250 million years ago) dest royed parts of the continents where once many marine organisms thrived. In inner seas with no feeding rivers, the rate of evaporation and precipitation determine the speed of salination. Complete evaporation of inner seas left large salt deposits often in the form of salt domes within restricted marine basins. The discovery that salt was accumulating in basins (Gulf of Mexico, Mediterranian Sea, Red Sea) [13] indicated that wast amounts of salt could have been deposited in ocean depth. The salt extraction from evaporite deposits could have an effect on the decrease of ocean salinity [14]. Large reservoirs of salt stored in brines in the deeply burried sediments fueled the speculation that salinity of the ocean could have changed through time [15-17]. Others thought that evaporite salt deposits could not have decreased the salinity more than a few parts per thousand [18,19].
Salinity changes due to the circulation of ocean
Salt is not only deposited, but salt gradient may develop in deep sea due to the inefficient irculation and high pressure of water. The lack of data regarding the distribution of salinity in deep sea can be explained by the crushing pressure exerted within the depths in the ocean. Nevertheless, it is known that once a cavity of water becomes salty enough, it sinks to the deep sea due to its higher density, drawing in water from surrounding areas, and initiating an ocean circulation loop called thermohaline overturning. This thermohaline cyle is known as the ocean’s conveyor belt that runs because cold water is denser than warm water and salt water is denser than fresh water. This conveyor belt is a global heat engine that carries warm, less dense surface water from the equator toward the poles to replace the cold and dense water that sinks to the ocean floor. One thermohaline cycle takes at least 1,000 years to complete in which all ocean water will flow around the globe. Global warming by melting the polar ice projects an immense amount of fresh water into the arctic see and as it is less dense, and stays at the surface near the polar region where it cools down. This will result in the sinking of the less dense but salty water and could slow down the thermohaline cycle causing climatic turbulances of global proportions.
Salinity increase caused by continental drift
The outporing of molten rock constantly increases and reshapes the surface of the sea floor (e.g. mid-atlantic ridge). The volcanic gases (CO2, H2S, HCl) and the soluble salts of the new crustal material of the erupting lava are dissolved in the ocean contributing to the salination process.
Salination caused by weathering and denudation
These processes carry away the surface of the soil, cracking stones and sand by the wind. Endogenic processes (volcanos, earthquakes and plate tectonic uplift) also expose the continental crust to the exogenic denudation processes of wheathering, erosion and mass wasting. Most of the material carried by the wind is deposited in the seas, the salt content of which contributes to the salination of the ocean. The increasing amount of sand of the spreading deserts carried away by the wind is accelerating the salination process. Erosion is accelerated by man.
Hydrological cycle - Salination effect of rivers
Beside the weathering of rocks and surface denudation of land there are other processes that contribute to the salinity of the ocean. Evaporation of ocean water and formation of sea ice decrease the volume, and elevate the salinity of the ocean. These „salinity raising” factors are are reduced but not completely counterbalanced by processes that decrease salinity, such as the melting snow and melting of ice and the nearly 80% of global precipitation of rain that occurs over the ocean. All fresh water bodies contain some salt (<0.1%). The salt of the fresh water is carrying constantly dilute salt to the ocean. The salination by rivers that was once part of the salt clock method [3] supports the notion of the continuous salinity intake of Ocean. Taking only the impact of the largest river, the Amazon is carrying about 5% of the Earth’s fresh water to the ocean, the salinity of which would have increased the salinity to an estimated 1 Osm during the last 10 million years. However, there are several balancing factors which influence the salinity of the ocean such as the anomalies in geochemical balance and the sea-to-sea variations in chemical composition.
Chemical pollution contributed by man
The steady-state ocean model gave the false impression of an inexhaustible compensational power. However, the last century has proved that global changes can be made within short period of time and reconfirming the quote of Heraclitus „Nothing endures but changes”. It is thus logical to assume that the chemical pollution will inrease the salinity of ocean. However, this could not be noticed, yet as the opposite dilution effect of global warming turned out to be much stronger than expected. Global warming from the end of the 19th century to 1975 was +0.4 °C, by 2000 +1 °C; and if this trend continues it will be +2 °C by 2028, and +3 °C by 2062 [20]. For the period of 1985–2025 an estimated lower greenhouse-gas-induced warming of 0.6-1.0 °C with the concomitant oceanic thermal expansion would generate a sea level rise of 4-8cm [21].
Dilution effects of global melting
Fluctuations in salinity between glacial and iterglacial periods
During the latest glacial maximum some 20 thousand years ago, the ice sheets over the continents had lowered the global sea level by approximately 130m compared to the level in 2000 [22]. In particular the northern hemispheric continents, Greenland, the polar regions, and the development of ice caps at the top of the mountains causing solvent deficiency of seas, leading to their increased salinity [23-26] and a lower (average 3.4 %) global salinity between ice-ages. This tendency also explains the gradual buildup of the Antarctic ice [27]. It is believed that the salinity decrease of the ocean during the current Phanerozoic eon [17,28] of about half billion years constitutes the multicellular period of abundant animal life, including the emergence of terrestrial plants, the development of complex plants, the evolution of fish, the emergence of terrestrial animals and the development of modern faunas.
Global warming after glacial periods resulted in the melting of ice in polar regions causing the solvent increase and the salinity decrease of the ocean. Wet, warm interglacial periods were more in favor of species living in land than for life in a warmer sea containing less dissolved oxygen. Assuming that the last ice age ended some 12,000 years ago, and the previous one lasted for 28,000 years, it is expected that without human intervention the present interglacial period could be longer than 12,000 [29-32] . By the end of the latest ice age the remaining ice was an estimated 5x107km3. For the future a further ≈4x107km3 loss of all polar and glacial ice has been predicted, which means nearly 80% loss of our fresh water reservoir [30]. Taking into account the many glacial advances and retreats that occurred in the past, there is no doubt that the concentration of the ocean was changing many times resulting in wet and dry climatic periods. The ice ages provide convincing evidence that the ocean concentration was changing over geologic ages. These salinity changes indicate that a general geochemical balance providing long-term constancy of ocean salinity never existed.
Dilution effect of global warming
One of the major causes of sea level rise is the rapid melting of Arctic sea ice and Greenland ice sheet. If we add to this the recent thermal expansion of seawater due to global warming which has a higher dilution effect than the melting itself, then the ongoing salinity decrease of the ocean could reach as much as 5-10%. As already mentioned, the projection of an immense amount of fresh water into the North Atlantic could be large and rapid enough to slow down, or at least temporarily disrupt, the thermohaline circulation. Convincing evidence has been provided that tropical ocean waters have become saltier over the past 50 years, while seas closer to Earth’s poles have become fresher [33-35]. These local salinity changes may slow down the thermohaline cycle. As the deep current carries more than 30 times the volume of all the rivers the slowdown is expected to be a protracted process. More data are necessary to prove convincingly the slowdown or disruption of the global conveyor belt provided by the programs of the European Space Administration (ESA), launching satellite and other projects recording the moisture of land and the Sea Surface Salinity (SSS). Similarly, the Aquarius/SAC-D mission developed by America’s space agency (NASA) and other Space Agencies such as the Argentine Comisión Nacional de Actividades Espaciales (CONAE) also measure global Sea Surface Salinity. The global data on sea surface salinty will refine the local changes in the open ocean ranging between 3.2 and 3.8%, but can be lower near the fresh water sources in Arctic regions or higher (4.2%) e.g. in the Red Sea. These measurements are expected to help to clarify several phenomena of the water cycle and ocean circulation representing the two major components of the climate system of Earth. The large-scale, relatively rapid oceanic changes suggest that the recent climate changes, including global warming, are likely to alter the fundamental planetary system that regulates evaporation and precipitation.
Climatic turbulances, insufficient mixing
It was generally believed that the ion balance and concentrations of seas during evolution were similar to those measured in ocean today [4]. This simplistic model of osmolar systems assumed that the observed chemical composition represented a steady-state ocean in which the amount of material introduced per unit time was compensated by an equal amount deposited as sediments [36]. This assumption that still holds, did not consider the fact, that there is no complete mixing of materials introduced into the ocean. Mixing takes place primarily by the conveyor belt (currents), but there are other turbulances involved, such as waves, ocean tides or volcanic activity. That the mixing in ocean is incomplete is indicated by the sea to sea and local variations in salinity providing a further evidence for global salinity imbalance of climatic consequences. Such climatic extremities occur at locations where: a) upwelling returns the deep water to surface e.g. Indian and Pacific Oceans. Upwelling could explain why major floods in eastern states of Australia have been doubled in the last 70 years relative to the frequency and volume of earlier floods, b) the surface ocean water absorbs radiation, heating up water that floats on top allowing to generate saturation vapor pressure with tropical, subtropical storms (e.g. North Atlantic cyclones, tornados). c) the global melting with a greater input of fresh water from glacier melting and runoff into the North Atlantic Sea with the potential of the conveyor belt be weakened.
To summarize the recent global climatic developments:
a) The dilution effect caused by global warming through the volumetric
expansion of ocean and by the continental glacial and
sea ice melting exceeds by far and is masking the damages of
the salinity increase brought about by the chemical pollution.
Local dilution effects show that already a small decrease in salt
concentration of the sea elevates vapor pressure, cloud and
precipitation formation in the atmosphere manifested in the
storm intensity. Similarly, water with less salt and lower heat
content warms up faster and generates a sudden high vapor
pressure that determines the occurrence and severity of local
precipitation and flooding of the region’s rivers especially in
late spring and early summer months.
b) The dilution effect is masking the chemical pollution. Pollution
is taking its measurable toll locally (e.g. Aral Sea), but in the
long run it will add up to the higher salinity of ocean, with all
of its harmful effects on global life.
These conclusions raise further questions, e.g. Is the recent dilution effect or the long-term concentration process more dangerous to life on Earth? The short answer is that the long-term salination is more harmful, explaind in the next chapter. The longer explanation would deserve another review.
Discussion
As far as the ong-term salination versus short-term fluctuations are concerned we do not have any record regarding the initial salt concentration of the sea at the time of emergence of life around 3.5 billion years ago.
Salinity of ancient ocean
There is even doubt how the primordial ocean came to be. Some researchers believe that comets containing large amounts of water delivered water especially at the end of the planet formation period when several satellite-sized bodies had formed that could have collided with the Earth or be captured. Such collision would also explain the formation of Moon by the tangental impact of a body the size of Mars [37]. The presence of volatile water trapped in lunar basalts would be equally difficult to explain as such an enormous impact could have caused a catastrophic heating event [38]. The collision theory was criticised by studies proving that comets contain 2 to 20 times more “heavy water” (D2O) relative to “normal” water (H2O), than is found in the ocean. A more acceptable explanation argues that water on Earth originated from the cloud of gas and dust that gave rise to the solar system and water molecules were trapped in the porous rock inside the hot planet and boiled out as steam and the water vapor wrapped the planet in a dense blanket. When the Earth cooled down water vapor condensed into clouds, further cooling precipitated the fresh water and and covered the entire planet to an unknown depth sometime between 4.3 billion and 3.8 billion years ago. The cooling of ancient sea from nearly boiling point could have lasted for millions of years and could have had a strong dilution effect. According to the plate tectonic theory, the Earth’s crust, the outer shell, broke into a number of rigid pieces, called tectonic plates, and the movement and collison started to form mountains and other features high enough to create continents above the sea level.
The salt of the molten rock and the gases of volcanic activity around the tectonic plates could have started the salination process several billion years ago. As there is no reliable evidence for extraterrestrial origin of water it is reasonable to assume that the volume of water did not change significantly over geological ages, only the state (water vapor, water, ice and snow) is changeing constantly. Correspondingly, the salinity of ocean depended initially on the salts and gases dissolved from the Earth’s crust and on the global temperature. Gases (HCl, CO2, SO2, HCN) released by cataclysmic volcanic eruptions induced acidification with an initial “acidic sea” and higher salt concentration as there was much more vapor in the hot atmosphere and less water that cooled down and covered the Earth.
Major, but less dramatic changes in temperature took place during glacial and interglacial periods with concomitant salinity oscillations. The long-term salination of ocean is regarded as a constant process, while the fluctuating dilutions suggest an initial low salt concentration of the ocean.
Salinity of ocean at the time of emergence of land vertebrates
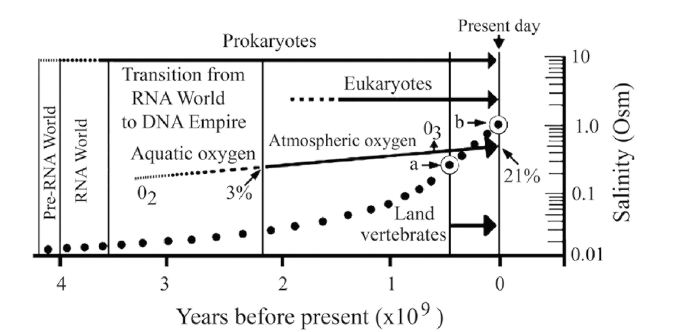
The maintainance of constant ion millieu (0.3 Osm) of land vertebrates versus the recent salinity of ocean (1.09 Osm) is taken as an evidence for a long-term salinity increase. Although, there were occasional desalination events, a long-term salination process is hypothesized, in which the osmotic concentration of the ocean increased in the past 400 million years from 0.3 Osm to 1.09 Osm. To judge the salinity change of the ocean by plotting salinity against geological time, only a rough estimation can be made (Figure 1).
Looking back at climatic changes, the general tendency of global warming can be charaterized by a long-term salination process, interrupted by intermittent fluctuations restricted to glacial concentration and interglacial dilution periods. The longterm salination suggests that the glacials and interglacials will be repeated at inceasingly higher salt concentration of ocean which is likely to result in gradually shortened and more frequently occuring ice ages. The widening gap between the increasing salinity of ocean and the melted ice suggests higher amplitudes of climatic oscillations, manifested in higher storm frequency and intensity [39,40].
Figure 1:Schematic representation of ocean salination. Three salt concentrations have been plotted against geological time. The initial salt concentration (zero time point) at the time when the world ocean cooled down to today’s temperature is unknown, but was probably much lower then the recent value. Its growth is indicated by the dotted curve. a) present day sea osmolarity after 4.5 billion years of global history (1.09 Osm), b) hypothetical salt concentration of Caspian Sea representing the Thetis Ocean (0.35 Osm) some 50-60 million years ago, and c) Devonian salt concentration of ocean after 4.1 billion years of global development, believed to correspond to the concentration of blood electrolytes of land vertebrates (0.3 Osm). By extrapolation the salinty increase to 5 Osm would take at least 500 million years. Assuming that salination follows a saturation tendency, the process can be characterized by a sigmoidal curve that may indicate an extended saturation and life on earth.
Combined effect of pollution and global warming
The Arctic ice is containing at least 15% sea ice and as a fresh water stream is carried by ocean currents also referred to as the meridional overturning or thermohaline circulation. Salinity changes have the potential to modify the layers of the ocean and affect the heat content. Salinity and temperature jointly determine the ocean’s density. Higher salinity and colder temperatures, result in increased ocean density and lower sea surface height. The density of seawater is only slightly affected by cooling near the freezing point. Rather salinization through sea-ice formation or evaporation is required to make water dense enough to sink into the ocean interior [17]. To the contrary warmer and fresher (diluted) sea waters, decrease the density and elevate the sea level. As a consequence of the increased evaporation of the diluted warmer seawater at low latitude, cloud formation, storm frequency and severity intensify. All these deductions lead to the conclusion that cloud formation on Earth is governed by Raoult’s law of dilute solutions. The lower the concentration of a solution and the higher its temperatute, the more its vapor pressure and the higher the evaporation of the solution and precipitation formation will be.
It is debated whether the temporary masking effect of global warming on the salination process will have an overall interglacial or glacial outcome. The global melting could initiate a warmer, wet period hall-marked by an overall higher water vapor pressure resulting in more cloud formation and precipitation. This tendency seems to be supported by the tendency of global warming and storm frequency and intensity at least in some regions e.g. Atlantic storms that have been doubled in the past 40 years. Computer simulations predict other unusual meteorologic activities and also favor the continuation of the ongoing interglacial period. Unusual climatic events are not restricted only to the north Atlantic region, recently more hurricanes hit the US South mainland than the average had been per decade in the previous 40 years. The increased storm frequency seems to favor the continuation, moreover to boost the antiglacial tendency.
Alternatively, one could argue that the recent dilution period is not uniform and more expressed in the polar regions and around the Greenland Ice sheet, where the temperature is close to the freezing point. Despite the dilution effect at this low temperature the few percentages of vapor pressure elevation may remain unnoticed and the storm intensity correspond to regular oscillations. Moreover, the sustained increase of evaporation and cloud formation could have a strong cooling effect on the atmosphere, initiating oscillation toward a glacial period.
It is assumed that the dilution period of global warming and polar melting, is outweighing the concentration effect of pollution, supporting the idea that the interglacial course will continue. Although, the consequences of air and water pollutions during the 20th century have shown that we were able to upset both the atmospheric balance and hydrologic cycle through the greenhouse effect resulting in global warming, glacial retreat, dilution of ocean by melting polar ice and loosing most of our fresh water reserves. Among these consequences it is worth mentioning that shift in the balance of the salinity was observed only locally primarily in polluted inland seas, lakes and rivers.
The application of Raoult’s law to the ocean is sending two important
warning messages:
a) Not only global warming but also salination of the ocean will
face serious consequences, not mentioning the combination of
the two of them.
b) The effect of the temperature on the volumetric increase of
ocean is an important factor to be considered in the dilution
process. The warmer the ocean, the more dissolved oxigen and
carbon dioxide will be released from it contributing to the greenhouse
effect. Warmer ocean also means higher vapor pressure,
favoring the processes of evaporation and cloud formation.
The short-term (tens of thousands of years dividing the
interglacial periods) dilution processes are contrasted by the
long-term salination of ocean (millions of years) with decreasing
vapor pressure and decreasing cloud formation. The higher
the salt concentration of the sea the lower the vapor pressure
in the atmosphere will be, with less cloud and precipitate
formation and with the likelyhood that less freshwater will be
contained in the global, mainly arctic ice reservoirs. To limit
pollution and to keep the ocean diluted is at least as important
as to prevent further global warming. Higher osmolality,
reduced oxygen content of warmer ocean and concomitant reduction
of fresh water will bring further unfavorable changes
for both aqueous and terrestrial life already experienced with
inner seas and lakes. The irresponsible contribution to the global
concentration process by polluting the atmosphere and the
hydrosphere will take its toll. The global warming is accounted
for by the masking effect of long-term salinity increase that
could remain hidden and unnoticed for another generation to
come, but when realized it might be just as late as experiencing
the effects of global warming [41-43].
Conclusion
Based on Raoult’s law applied to ocean as the ultimate dilute solution, the following short-term climatic scenario is envisaged: Global warming will probably melt most of the polar ice and snow caps of mountains exhausting most of our fresh water reserved as ice and snow. Antropogenic global warming in progress is likely to elevate further the temperature of sea, which in turn will significantly elevate the vapor pressure in the atmosphere and contribute to more cloud and precipitate formation especially at low altitude and in subtropical land regions. Similarly, the law of dilute solutions also helps to explain the extreme heat, drought and sudden precipitation waves becoming even more frequent. Due to the higher global temperature and dilution effect of melted snow and ice, the turnover of the hydrologic cycle is expected to speed up. From the mountains, which are less protected by forests and by snow caps, the rain will wash away more soil and vegetation causing massive landslides and floods. More importantly, the hydrologic cycle and denudation will carry more salt to the sea inceasing its salinity.
A further corollary of Raoult’s law is that the salination of ocean is the long-term (millions of years) period that will have an even more devastating effect than global warming. Global scale salina tion similarly to the concentration of inner seas and lakes will result in the decrease of vapor pressure, lower cloud but more intense precipitate formation, causing drastic loss of the recycling and renewable freshwater reservoir, spread of deserts. The gradual longterm increase of salinity raises the question how far the salination process can go on without threathening life on Earth. A further question concerning salinity is whether the increasing osmolarity of sea (e.g. 2-3Osm) associated with a declining vapor pressure in the atmosphere will produce enough precipitation to cool down the atmosphere and to produce enough polar ice and fresh water for the hydrological cycle to initiate a new cycle of ice age. Reduced wapor pressure without significant cloud formation, precipitation and without cooling effect will cause further global warming prone to generate a vicious cycle. The pessimistic forcast is that high salt concentration of the sea with increased heat capacity will further aggravate global warming. It is unreasonable to think that the temporary dilution period of ocean will be able to counteract the longterm salination (pollution) process. The frequency of interglacial periods in the future can be predicted. An unchanged salinity under the recent dilution effect could mean that the concentration effect has already started to take its toll, with most of the fresh water being lost without a chance to be regained in the future.
Acknowledgement
This work was supported by a grant of the Hungarian National Science and Research Foundation to G.B. (OTKA T42762) grant.
References
- Dalrymple BG (2004) Ancient earth, ancient skies. Stanford University Press, USA, pp. 1-39.
- Burchfield JD (1975) Lord kelvin and the age of the earth. Paleobiology, Geology, and Paleontology, Science History Publications, USA, pp. 1-278.
- Joly J (1899) An estimate the geological age of the earth. Transactions of the Royal Society of Dublin 2: 23-66.
- Rubey WW (1951) Geologic history of sea water: An attempt to state the problem. Geol Soc Am Bull 62: 1111-1147.
- Railsback LB, Anderson TF, Ackerly SC, Cisne JL (1989) Paleoceanographic modeling of temperature-salinity profiles from stable isotopic data. Paleoceanography 4(5): 585-591.
- Pinet P (2009) Invitation to oceanography. 8th (Edn.), Johns and Barlett Publishers, USA, pp. 1-598.
- McFarlan WN, Heiser JB (1979) Life in aater: Its influence on basic vertebrate functions. In Vertebrate Life. Macmillan, USA, pp. 221-284.
- Banfalvi G (1991) Evolution of osmolyte systems. Biochemical Education 19: 136-139.
- Gradstein FM, Ogg JG, Smith AG, Agterberg FP, Blocker W, et al. (2004) A geologic time scale. Cambridge University Press, USA.
- Pough FH (1979) Origin and radiation of amphibians in vertebrate life. Macmillan Publishers, USA, pp. 291-322.
- Mansoor M, Amiji MM, Beverly J, Sandmann BJ (2002) Applied physical pharmacy. McGraw-Hill Professional, UK, pp. 1-462.
- Aladin N, Plotnikov I (2008) The Caspian sea. Lake basin management initiative, pp. 1-29.
- Ewing WM, Worzel JL, Burk CA (1969) Regional aspects of deep sea drilling in the Gulf of Mexico, cast of the Bahama Platform, and the Bermuda Rise. In: Ewing WM, Worzel JL, Beall AO, Berggren WA, Bukry JD, (Eds.). Initial reports of deep sea drilling project. US Government Printing Office, Volume 1, USA, pp. 624-640.
- Southam JR, Hay WW (1981) Global sedimentary mass balance and sea level changes. In: The Sea, volume 7, Wiley-Interscience, USA, pp. 1617-1684.
- Zharkov MA (1974) Paleozoic Salt Formations Mira, Nedra, Mostov, Czechia.
- Zharkov MA (1981) History of pleozoic salt accumulation. 1st (Edn.), Springer Publishers, USA, pp. 1-316.
- Hay WW, Migdisov A, Balukhovsky AN, Wold CN, Flögel S, et al. (2006) Evaporites and the salinity of the ocean during the phanerozoic: Implications for climate, ocean circulation and life. Palaeogeography, Palaeoclimatology, Palaeoecology 240(1-2): 3-46.
- Holland HD (1974) Marinein evaporites and the composition of sea water during Phanerozoic. In: Studies in paleo-oceanography, volume 20, Society of economic paleonthologists and mineralogists, USA, pp. 187-192.
- Holland HD (1978) The chemistry of the atmosphere and oceans. John Wiley & Sons Inc, USA, pp. 1-369.
- García M (2004) Oil, population and global warming.
- Wigley TML, Raper SCB (1987) Thermal expansion of sea water associated with global warming. Nature 330: 127-131.
- Lambeck K, Chappel J (2001) Sea level change through the last glacial cycle. Science 292(5517): 679-686.
- Shackleton NJ, Kenneth JP (1975) Paleotemperature history of the cenozoic and the initiation of Antarctic glaciation: Oxygen and carbon isotope analysis on DSDP sites 277, 279 and 281. In: Initial reports of the deep sea drilling project, volume 29, U.S. Government Printing Office, USA, pp. 743-755.
- Shackleton NJ (1987) Oxygen isotopes, ice volume and sea level. Quat Sci Rew 6(3-4): 183-190.
- Duplessy JC, Labeyrie L, Juillet-Leclerc A, Maitre F, Duprat J, et al. (1991) Surface salinity reconstraction of the North Atlantic Ocean during the last glacial maximum. Oceanol Acta 14(4): 311-324.
- Duplessy JC, Bard E, Laberyrie L, Duprat J, Moyes J (1993) Oxygen isotope records and salinity changes in the northeastern Atlantic Ocean during the last 18,000 years. Paleoceanography 8(3): 341-350.
- Zachos JC, Stott LD, Lohmann KC (1994) Evolution of early cenozoic marine temperatures. Paleoceanography and Paleoclimatology 9(2): 353-387.
- Holser WT (1984) Gradual and abrupt shifts in ocean chemistry during Phanerozoic time. Holland HD and Trendall AF (Eds.), In patterns of change in earth evolution. Springer Publishers, UK, pp. 123-143.
- Augustin L, Barbante C, Barnes PRF, Barnola JM, Bigler M, et al. (2004) Eight glacial cycles from an Antarctic ice core. Nature 429(6992): 623-628.
- Berger A, Loutre MF (1996) Modelling the climate response to astronomical and CO2 Reports of the Academy of Sciences 323: 1-16.
- Loutre MF, Berger A (2000) Future climatic changes: Are we entering an exceptionally long interglacial? Climatic Change 46(1): 61-90.
- Berger A, Loutre MF (2002) An exceptionally long interglacial ahead? Science 297(5585): 1287-1288.
- Dickson B, Yashayaev I, Meincke J, Turell B, Dye S, et al. (2002) Rapid freshening of deep North Atlantic Ocean over the past four decades. Nature 416(6883): 832-836.
- Dickson RR, Curry R, Yashayaev I (2003) Recent changes in the North Atlantic. Phil Trans R Soc Lond A 361(1810): 1917-1934.
- Curry R, Mauritzen C (2005) Dilution of the Northern North Atlantic ocean in recent decades. Science 308(5729): 1772-1774.
- Sillen LG (1961) The physical chemistry of seawater. In: Oceanography, Sears. UAA, pp. 549-581.
- Cameron AGW, Ward WR (1976) The origin of the moon. Lunar and Planetary Science Conference, Houston, USA, pp. 120-122.
- Saal AE, Hauri EH, Cascio ML, Orman JA, Rutherford MC, et al. (2008) Volatile content of lunar volcanic glasses and the presence of water in the Moon's interior. Nature 454(7201): 192-195.
- Bouhifd MA, Jephcoat AP, Kelley SP (2008) Argon solubility drop in silicate melts at high pressures: A review of recent experiments. Chem Geol 256(3-4): 252-258.
- Goldfarb JL, Suuberg EM (2008) Raoult's law and its application to sublimation vapor pressures of mixtures of polycyclic aromatic hydrocarbons. Environ Engin Sci 25(10): 1429-1438.
- International Union of Pure and Applied Chemistry (IUPAC) (1994) "Triple point". Compendium of Chemical Terminology Internet edition.
- Mysen BO (1979) Nickel partitioning between olive and silcate melt: Henry’s law revisited. Am Mineral 64: 1107-1114.
- Smithonian Tables in Handbook of Chemistry and Physics, 63th (edn.), (1982) Weast RC and Astle MJ (Eds.), CRC Press, USA, Table E1.
© 2023 Gaspar Banfalvi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






