- Submissions

Full Text
Examines in Marine Biology & Oceanography
Scuba Divers Behavior Shapes Fish Wariness and Boldness
Jenny Tynyakov1,2,3, Elena Kheifets1, Nadav Shashar1*, Assaf Pertzelan1,2 and Jonathan Belmaker3
1Eilat Campus, Department of Life Sciences, Ben Gurion University, Israel
2Interuniversity Institute for Marine Sciences at Eilat, Israel
3School of Zoology, George S. Wise Faculty of Life Sciences and The Steinhardt Museum of Natural History, Israel
*Corresponding author: Nadav Shashar, Eilat Campus, Department of Life Sciences, Ben Gurion University, Israel
Submission: January 24, 2023;Published: February 09, 2023

ISSN 2578-031X Volume5 Issue4
Abstract
Responses of Chromis viridis, a coral dwelling damselfish, to divers in sites differing by pressure of diving and by type of diving activity (recreational versus scientific) were examined. We measured variation in hiding time and the distance from a refuge of the fish as they were exposed to divers. Populations of C. viridis were tolerant to divers at locations of high regular exposure. Overall, younger fish were more tolerant to divers than older fish. However, where both recreational and scientific diving occurred, fish remained wary, displaying a behavior comparable to the site in which diving is banned. These results show a dynamic response of fishes to human visitation. We suggest that diving clubs and authorities should invest in divers training. Last, we warn that scientific procedures, even if targeted towards a specific fish species, may cause behavioral changes across the fish community..
Keywords: Coral reef fish; Diving; Human visitation; Tolerance; Wariness
Introduction
Human activities influence wildlife on the land [1] and underwater [2,3]. Since animals can rarely predict human intentions, even non-consumptive human activities can influence animal behavior [4]. For many species of prey, the most effective method of predator avoidance is hiding in a refuge [5]. However, hiding in a refuge comes at costs such as reduced foraging [6], less opportunities for mate searching [7,8], which in turn may reduce fitness [9]. Animals living in the absence of harvesting but with frequent human disturbance often show less fear or high tolerance of humans [10,11]. In many marine touristic sites, fish must co-op with insensitive diving activities [12-16]. In protected areas large and mobile fish species are more tolerant compared to areas with harvesting behaviour [11,17-19]. However, highly mobile reef fish groups often leave areas with intensive diving [17,20-22]. Yet, studies found that territorial damselfishes hide more and miss feeding opportunities or fail in nest defense in the presence of divers [16,23]. Here we examined how activities of sites attached to reef fish change among sites that vary in diving intensity and diver’s behavior.
Methods
The Blue green damselfish Chromis viridis is a common coral-dwelling planktivore in indo-pacific coral reefs and is found in shoals of juveniles and adults around branching corals that serve as a refuge [24-26]. We examined four sites that differ in the number and type of diving activity. The Oil jetty (29°31’29.8”N 34°56’03.5”E) was closed to recreational divers and experienced under 20 scientific dives per year. The Eilat Coral Natural Reserve (NR) (29°30’34.9”N 34°55’21.4”E) and the Caves (29°29’51.9”N 34°54’40.7”E) each had over 25,000 recreational SCUBA dives per year [27]. The Inter University Institute for marine sciences (IUI) also had over 25000 recreational dives per year and 926 scientific dives, including collection and manipulation of non chromis fish species (2015). We tested 6-10 fish shoals per site (34 total), each composed of three fish size classes small (<2.4cm, 57% of all fishes, 80±8 individuals per shoal), medium (2.4≤x<4.9cm, 23% of the fish, 32±3 individuals), and large (≥4.9cm, 20% of the school, 28±3 individuals). Using videotaping we examined hiding time and the distance to the refuge at start of escape, when exposed to a passing diver stimulus [28,29]. All experiments were video recorded starting 5 min prior to the disturbance and until 15 min after. Videos were analyzed using the Behavioral Observation Research Interactive Software (BORIS) [30]. Our response variable included hiding time and distance to the refuge. We used two ways ANOVA with Tukey post hoc tests to determine differences among sites and size classes.
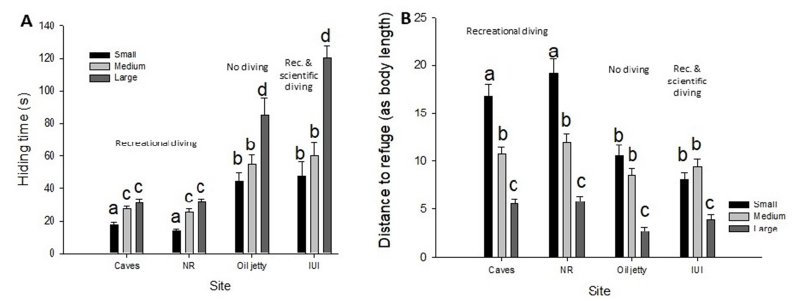
Figure 1:A. Smaller C. viridis hide less than older ones. Bar chart of time spent in the coral after a diver’s passage stimulus for C. viridis of different size categories (Small, Medium and Large) by sites: Hiding lowest at sites where only recreation diving occurred. B. Smaller C. viridis keep longer relative distances from their refuge as compared to larger fish. Bar chart of distance from refuge of C. viridis of different size categories by site. Different letters above columns represent statistically significant differences (p<0.05) and error bars represent standard error (±SE).
Results
Overall, 493 fish belonging to 34 shoals distributed over the 4 sites were examined. We found differences in fish behavior between sites associated with different human exposure levels and between fish sizes categories. Hiding time varied significantly between sites and was low for highly dived sites and high for non-visited areas as well as where scientific activities took place (F3, 481=45.37, P<0.001) (Figure 1). Hiding duration for small individuals was significantly shorter compared to large individuals (F11, 481=24.13, P <0.001) (Figure 1A). Small individuals kept longer distances from their refuge compared to large individuals among all the sites) distances calculated in body length per fish) (F11, 481=33.44, P<0.001) (Figure 1B). These translate to a distance of 28 cm on average for small fish forging and 22cm for large fish.
Discussion
We examined the effects of diving activities on fish tolerance behavior. We found that Blue green damselfish can habituate to human presence and become tolerant to diver’s disturbance. This is particularly important since these fishes are known to have very small home ranges [31] and are loyal to specific coral bummie [32], thus presenting a truly site attached coral reef fish. The fish displayed tolerance to divers, manifested as shorter hiding times and longer distance to a refuge at sites where recreational diving is common (Figure 1). However, even when fish species are not targeted to fishing, their wariness stays high [33]. In our case, where scientific fish handling and collection occurred fish remained as warry as in not visited areas. Hence, promoting non-harvesting diver behavior, even for scientific diving, is pivotal for reducing the impact of divers on coral reef fishes. Small individuals showed riskier behavior, meaning hid less (Figure 1A) and kept larger distances from the coral refuge (Figure 1B) compared to larger fish. This held both in absolute (cm) distances, and in the relative value of body lengths (which is presumably relevant to fish). The above differences in fish behavior may result from different costs of hiding or different perceived risks for small versus large fish [3,34-36]. Small fish have higher metabolic cost of hiding [37] and are considered as less risk aversive [38,39]. This can be because small individuals have “less to lose” and hence are capable of taking higher risks [40,41].
Conclusion
Anthropogenic effects on coral reefs are often examined using community and population level indices such as biomass, diversity and/or coral coverage. Here we show a fish behavioral effect which is depended on human behavior. From a reef management perspective, this study emphasis the importance of training and briefing visitors to avoid any manipulation of the reef. On the other hand, scientists should use caution in their research, as their activities may effect a wide range of species and not only the one they target.
References
- Balmford A, Beresford J, Green J, Naidoo R, Walpole M, et al. (2009) A global perspective on trends in nature-based tourism. Plos Biology 7(6): 1000144.
- Huveneers C, Watanabe YY, Payne NL, Semmens JM (2018) Interacting with wildlife tourism increases activity of white sharks. Conservation Physiology 6(1).
- Samia DS, Bessa E, Blumstein DT, Nunes JA, Azzurro E, et al. (2019) A meta-analysis of fish behavioural reaction to underwater human presence. Fish And Fisheries 20(5): 817-829.
- Peckarsky BL, Abrams PA, Bolnick DI, Dill LM, Grabowski JH, et al. (2008) Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator-prey interactions. Ecology 89(9): 2416-2425.
- Sih A, Petranka JW, Kats LB (1988) The dynamics of prey refuge use: a model and tests with sunfish and salamander larvae. The American Naturalist 132(4): 463-483.
- Koivula K, Rytkonen S, Orell M (1995) Hunger-dependency of hiding behavior after a predator attack on dominant and subordinate willow tits. Ardea 83: 397-404.
- Sih A, Krupa J, Travers S (1990) An experimental study on the effects of predation risk and feeding regime on the mating behavior of the water strider. The American Naturalist 135(2): 284-290.
- Crowley PH, Travers SE, Linton MC, Cohn SL, Sih A, et al. (1991) Mate density, predation risk, and the seasonal sequence of mate choices: A dynamic game. The American Naturalist 137(4): 567-596.
- Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48(1): 25-34.
- Mccleery RA (2009) Changes in fox squirrel anti-predator behaviors across the urban-rural gradient. Landscape Ecology 24: 483-493.
- Benevides LJ, Pinto TK, Nunes JDACC, Sampaio CLS (2018) Fish escape behavior as a monitoring tool in the largest Brazilian multiple-use marine protected area. Ocean & Coastal Management 152: 154-162.
- Davis D, Tisdell C (1995) Recreational scuba-diving and carrying capacity in marine protected areas. Ocean and Coastal Management 26(1): 19-40.
- Harriott VJ, Davis D, Banks SA (1997) Recreational diving and its impact in marine protected areas in eastern Australia. Ambi 26(3): 173-179.
- Hawkins JP, Roberts CM (1997) Proceedings of the 8th international coral reef symposium.
- Zakai D, Chadwick FNE (2002) Impacts of intensive recreational diving on reef corals at Eilat, Northern red sea. Biological Conservation 105(2): 179-187.
- Benevides LJ, Cardozo-FGC, Ferreira CEL, Pereira PHC, Pinto TK, et al. (2019) Fear-induced behavioural modifications in damselfishes can be diver-triggered. Journal of Experimental Marine Biology and Ecology 514-515: 34-40.
- Gotanda KM, Turgeon K, Kramer DL (2009) Body size and reserve protection affect flight initiation distance in parrotfishes. Behavioral Ecology and Sociobiology 63: 1563-1572.
- Januchowski FA, Cinner JE, Graham NAJ (2014) Fishery benefits from behavioural modification of fishes in periodically harvested fisheries closures. Aquatic Conservation Marine and Freshwater Ecosystems 24(6): 777-790.
- Goetze JS, Januchowski FA, Claudet J, Langlois TJ, Wilson SK, et al. (2017) Fish wariness is a more sensitive indicator to changes in fishing pressure than abundance, length or biomass. Ecological Applications 27(4): 1178-1189.
- Kulbicki M (1998) How the acquired behaviour of commercial reef fishes may influence the results obtained from visual censuses. Journal of Experimental Marine Biology and Ecology 222(1-2): 11-30.
- Dickens LC, Goatley CHR, Tanner JK, Bellwood DR (2011) Quantifying relative diver effects in underwater visual censuses. Plos One 18965.
- Titus BM, Daly M, Exton DA (2015) Do reef fish habituate to diver presence? evidence from two reef sites with contrasting historical levels of scuba intensity in the bay islands, Honduras. Plos One 10(3): 0119645.
- Giglio VJ, Blumstein DT, Motta FS, Pereira FGH (2022) Diver presence increases egg predation on a nesting damselfish. Journal of Experimental Marine Biology and Ecology 549: 151694.
- Allen GR (1991) Damselfishes of the world.
- Ben-To, Abelson A, Polak O, Kiflawi M (2008) Habitat selection and the colonization of new territories by Chromis viridis. Journal of Fish Biology 73(4): 1005-1018.
- Lecchini D, Shima J, Banaigs B, Galzin R (2005) Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia 143(2): 326-334.
- Tynyakov J, Rousseau M, Chen M, Figus O, Belhassen Y, et al. (2017) Artificial reefs as a means of spreading diving pressure in a coral reef environment. Ocean and Coastal Management 149: 159-164.
- Grant JWA, Noakes DLG (1987) Escape behaviour and use of cover by young-of-the-year brook trout, salvelinus fontinalis. Canadian Journal of Fisheries and Aquatic Sciences 44(8): 1390-1396.
- Frid A, Dill L (2002) Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology 6(1): 11.
- Friard O, Gamba M (2016) Boris: A Free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7(11): 1325-1330.
- Streit RP, Hemingson CR, Cumming GS, Bellwood DR (2021) How flexible are habitat specialists? short-term space use in obligate coral-dwelling damselfishes. Reviews in Fish Biology and Fisheries 31: 381-398.
- Wilson SK, Burgess SC, Cheal AJ, Emslie M, Fisher R, et al. (2008) Habitat utilization by coral reef fish: Implications for specialists vs. generalists in a changing environment. J Anim Ecol 77(2): 220-208.
- Tran DSC, Langel KA, Thomas MJ, Blumstein DT (2016) Spearfishing-induced behavioral changes of an unharvested species inside and outside a marine protected area. Current Zoology 62(1): 39-44.
- Dill LM, Gillett JF (1991) The economic logic of barnacle Balanus Glandula (darwin) hiding behavior. Journal of Experimental Marine Biology and Ecology 153(1): 115-127.
- Blumstein DT, Pelletier D (2005) Yellow-bellied marmot hiding time is sensitive to variation in costs. Canadian Journal of Zoology 83: 363-367.
- Chan Y, Lo S, Quan A, Blumstein DT (2019) Ontogenetic shifts in perceptions of safety along structural complexity gradients in a territorial damselfish. Current Zoology 65(2): 183-188.
- Krause J, Loader SP, Mcdermott J, Ruxton GD (1998) Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proceedings of The Royal Society of London B: Biological Sciences 265(1413) 2373-2379.
- Januchowski-HFA, Graham NAJ, Feary DA, Morove T, Cinner JE (2011) Fear of fishers: Human predation explains behavioral changes in coral reef fishes. Plos One 6(8): 22761.
- Catano LB, Rojas MC, Malossi RJ, Peters JR, Heithaus MR, et al. (2016) Reefscapes of fear: Predation risk and reef hetero-geneity interact to shape herbivore foraging behaviour. Journal Of Animal Ecology 85(1): 146-156.
- Clark CW (1994) Antipredator behavior and the asset-protection principle. Behavioral Ecology 5(2): 159-170.
- Wootton RJ (1994) Energy allocation in the three spine stickleback. The Evolutionary Biology of the Three spine Stickleback 114-143.
© 2023 Nadav Shashar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






