- Submissions

Full Text
Examines in Marine Biology & Oceanography
Valorisation of Seaweed Waste from Agar Processing Industry
Sushri SB1, Elavarasan K2*, Safeena MP1, Devi HM2 and Tejpal CS2
1Department of Fish Processing Technology, Kerala University of Fisheries and Ocean Studies, Panangad, Kochi-682506, Kerala, India
2ICAR-Central Institute of Fisheries Technology, Willingdon Island, Kochi, Kerala, India
*Corresponding author: Elavarasan K, ICAR-Central Institute of Fisheries Technology, Willingdon Island, Kochi, Kerala, India
Submission: September 19, 2022;Published: February 08, 2023

ISSN 2578-031X Volume5 Issue3
Abstract
The present era is witnessing a rise in research and development activities, focused on the conversion of waste, biomass, and various residues into energy, fuels, and other useful materials. Environmental distress with the recent economic crunch, and political insecurity has urged for industrial innovation, aiding new job opportunities, enhanced competitiveness, and improved economic growth. Large quantities of both liquid and solid waste are produced every year by the agar processing industries. These waste materials contain biodegradable organic and inorganic matter, and disposal of such waste eventually creates serious environmental problems. The waste loads of the processing plant can be reduced significantly through the use of new or modified processing methods or in-plant treatment. Many opportunities exist for better utilization of agar processing industrial wastes. This review has included the effective utilization of agar wastage and switched to the preparation of fertilizer, bio-fillers, feed, protein hydrolysis, flavor enhancer and other valorized food products.
Keywords: Agar wastage; Valorization; By-product development; Waste utilization
Introduction
Figure 1:Contribution of different seaweeds to global seaweed production (%). Source: FAO, 2016.
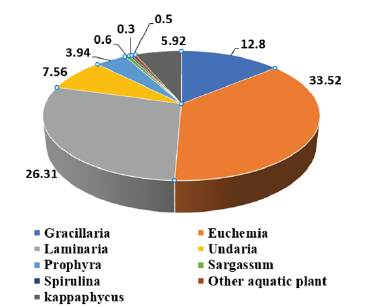
Utilization of any resource at its full scale is the need of present global situation considering the ever-growing population. The utilization at its maximum level is possible when zero waste is achieved in any of the processes. Seaweed is a type of aquatic vegetation that consists of an enormous amount of natural substances of highly beneficial to human life. Seaweed is being explored as the basic material for product development with specified functions and used in various industries, including food and medicines. The well-known industrial products derived from seaweed are agar, carrageenan and alginates. In 2018, the estimated world seaweed production was 30.4 million tons whereas the culture and wild collection contribute 29.4 million tonnes and 1.1 million tonnes, respectively. According to Food and Agriculture Organization [1], Gracilaria spp. one of the major raw materials for agar production contributes about 12.8% (3.9mmt) of global seaweed production. Other species include kappaphycus (5.92%), Euchemia (33.55%), Laminaria (26.31%), Undaria (7.56%), Porphyra (3.94%), Sargassum (0.6%), Spirulina (0.3%) and other aquatic plants (0.5%) (Figure 1). The leading producers of seaweeds are China, Japan, Norway, Chile, Indonesia and Philippines etc. According to FAO [2], in more than 50 countries, seaweed farming is practiced. It has gained a growth rate of 2.8% in the last decade (from 6 to 8.8%). Globally, around 37 species contribute to seaweed production including both wild and farming.
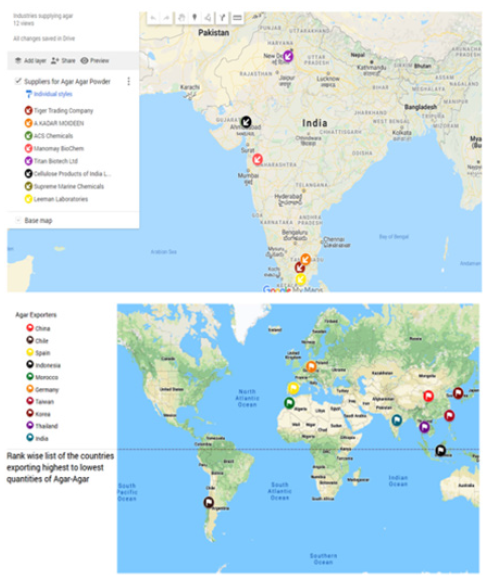
The global seaweed industry is worth more than USD 6 billion per annum. In terms of volumes, it accounts for 12 million tonnes/ year and out of which 85% (10.2 million tonnes) has been used for human consumption. Seaweed derived extracts like carrageenan, agar and alginates make up almost 40% of the world hydrocolloid market in terms of food [2]. The major agar producing countries are China, Chile, Spain, Indonesia and Morocco etc. In 2015, India secured 10th rank in agar production (Figure 2). The total production of agar-agar in the World (export-wise) is 15,406 tonnes and had a value of USD 247,006. From that, China exported the largest quantity of agar-agar i.e., 5846 tons in the year 2016 with a valued of 86,024 USD whereas India contributed 166 tonnes of agar-agar with a valued of USD 2909 [2]. Food grade agar production significantly increased (300 tonnes) during 2014-2015 and contributed twothirds of total agar production whereas bacteriological grade agar decreased (80 tonnes) in the same year [3]. In India, 100 tonnes of bacteriological grade and food grade agar are produced annually using 1000 tons of geophytes. The cost of one ton of raw material varies from Rs 42,000-45,000 tons in India [4]. Agar production practices generate large quantities of raw material as refuse which is rich in organic and inorganic matters such as proteins, fibers and minerals like iodine, phosphorus and other trace elements etc. Considering the growth of the human population and resource depletion, there is a necessity for effective/maximum utilization of seaweed processing by-products to benefit both humans and other life on the earth. In this review, an attempt has been made to review the literature and identify the gaps between recent research/ technology and the utilization of seaweed waste from the agar industry. This would help the academician, research scholar and industries to take off the agar industrial waste towards utilization.
Figure 2:World top 10 exporter of agar agar.
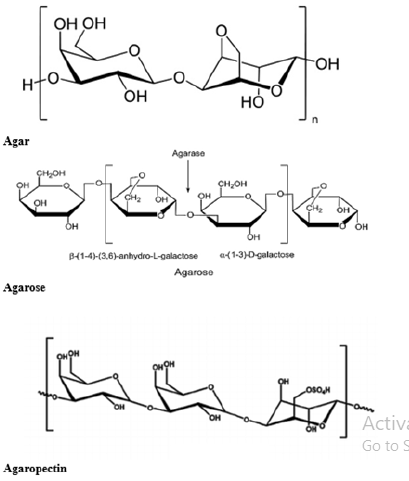
Chemistry of agar and its uses: Agar is composed of two polysaccharides i.e., agarose which is a linear polymer of anaerobioses and present at the level of 70% in agar preparation (Figure 3). Another polysaccharide is agaropectin composed of D-glucuronic acid, ester of sulphate and pyruvic acid and contributes around 30% on the wet composition of agar. Agarose built up, on the basis of an alternative unit like 1, 3 linked ᵦ-D-glucopyranose units and 1, 4 linked 3, 6 anhydro- ᾳ-L-glucopyranose (Figure 4) [12]. Agar is used predominantly for its stabilizing and gelling characteristics. It has the unique ability to hold a massive amount of moisture. It is mainly employed as a stabilizer in, piping gels, pie fillings, cookies, icings, cream shells etc. Agar is also used in forensic medicine, prosthetic dentistry pharmaceutical, photographic stripping films, electrophoresis, cosmetics, papers, and lotions as well as biodegradable thin films for various application [13].
Raw materials like Gelidiella acerosa, Gracilaria edulis, G. verrucosa and species of Gelidium amansi, Pterocladia capillacea and Ahenfeltia plicata are mostly used for the production of agar in industries. Globally, the production of Gracilaria species is comparatively higher than Gelidium species. Gracilaria acerosa yielding food grade agar with a gel strength of 600g.cm2 whereas for Gracilaria edulis, it is 120-150g.cm2 [5,6]. Seaweed (Gracilaria sp.) by-product after agar extraction contained 27.84% of protein, 0.6% of fat, 20.65% of fibre, 9.07% of ash and 41.83% of carbohydrate on a dry weight basis [7]. G. gracilis contained moisture (19.04%), protein (10.86%), ash (6.78%), fat (0.18%) and carbohydrates (63.13%) on a dry weight basis [8]. The proximate composition of Gellidium sp. contributed 13.23% protein, 1.16% lipid, 5.5% fiber, 26.45% ash and 53.66% carbohydrate [9]. In India, states like Tamil Nadu, Gujarat, Kerala and Maharashtra involves in the production of agar on an industrial scale basis.
Method of extraction:The industrial process of agar production follows the steps like washing and soaking of raw material, heat extraction in dilute acid (CH3COOH) / alkali solution (NaOH), filtration, freezing and thawing, bleaching and then drying. During washing, the dried raw materials are cleaned from debris, sand and shells and other extraneous material using running water. Then they are soaked in fresh water and dried under the sun. The soaking and drying are repeated till the seaweeds are bleached. The dried seaweed is boiled with dilute acid/alkali with continuous stirring. The boiled slurry is subjected to filtration using filter paper or nylon mesh. The filtrate is allowed to form a gel at room temperature. Repeated freezing-thawing of the agar gel is practiced to remove the impurities and reduce the moisture content. Further, the jelly material is bleached in the bleaching agent for improving the colour and further dried to get the agar strips. Before marketing, the agar strip is pulverized into powder and packed in a suitable container [10,11]. Different authors described different temperatures and times for extraction of agar (Table 1).
Table 1: Process parameter available in literature for agar extraction.

Chemistry of agar and its uses: Agar is composed of two polysaccharides i.e., agarose which is a linear polymer of anaerobioses and present at the level of 70% in agar preparation (Figure 3). Another polysaccharide is agaropectin composed of D-glucuronic acid, ester of sulphate and pyruvic acid and contributes around 30% on the wet composition of agar. Agarose built up, on the basis of an alternative unit like 1, 3 linked ᵦ-D-glucopyranose units and 1, 4 linked 3, 6 anhydro- ᾳ-L-glucopyranose (Figure 4) [12]. Agar is used predominantly for its stabilizing and gelling characteristics. It has the unique ability to hold a massive amount of moisture. It is mainly employed as a stabilizer in, piping gels, pie fillings, cookies, icings, cream shells etc. Agar is also used in forensic medicine, prosthetic dentistry pharmaceutical, photographic stripping films, electrophoresis, cosmetics, papers, and lotions as well as biodegradable thin films for various application [13].
Figure 3:Classification of agar.
Figure 4:Chemical structure of agar.

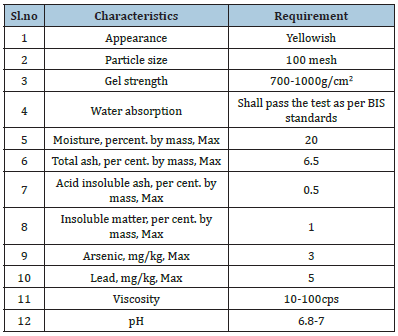
Specification of agar: The common name of agar is Agar agar or Gelées. Specification of food grade agar has been prescribed under IS 5707: 1996. The International Numbering System for food additives (INS) for agar is 406 [14]. The specification of agar is presented in Table 2.
Table 2:Specification of agar [14].
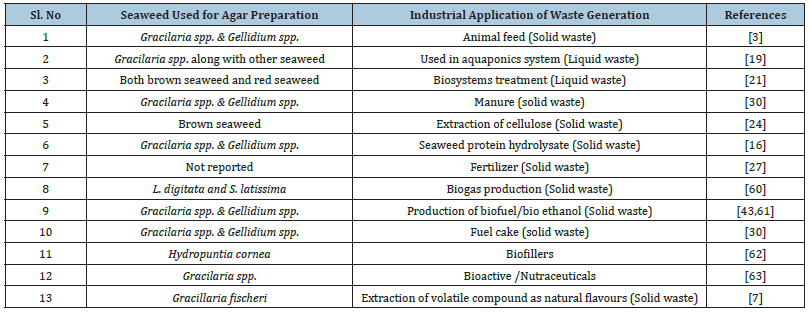
Waste generation during agar processing: Seaweed processing industries discharge huge amounts of wastewater during washing that contains residual chemicals like NaOH, H2O2, KOH, and KCl which pollute the environment and natural water bodies upon discharging untreated [15]. Two types of seaweed wastage are found in the agar industry i.e., solid waste (Figure 5) and liquid waste. Most of the time, the liquid waste is used for irrigation purpose whereas the solid waste is directly incorporated in feed formulation and agricultural fertilizer. The highest application in the food and medical industry involves the extraction of a specified bioactive compounds, some purification and characterization as shown from solid waste which can be incorporated into food products, medicinal usages for health benefits, feed material for animals etc., as shown in Table 3.
Figure 5:Waste generated from agar processing industry, Madurai, Tamil Nadu, India. Original photo of agar processing waste captured at agar processing industry, Tamil Nadu, India.
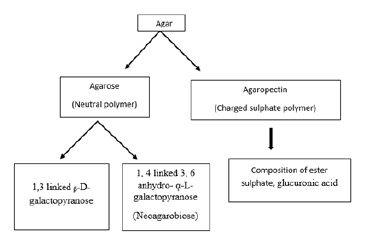
Table 3:Industrial application of waste generates from agar processing waste.
Utilization of waste: Valorization of agar processing industrial
waste used for food and non-food uses has been discussed as
follows.
A. Non-food uses
a) Uses in animal feed
b) Uses in the aquaponics system
c) Uses Biosystems
d) Uses in the extraction of cellulose assisted by cellulase
e) Uses in Fertilizer/Manure
f) Uses in Biogas/Bio-methane production
g) Uses in bio fillers as bio composites
B. Food uses
a) Protein from agar processing waste and its hydrolysate
b) Bioactive /Nutraceuticals
c) Extraction of the volatile compound as a flavor enhancer
Non-food uses
Uses in animal feed: Seaweed waste can be used to produce animal feed for livestock. The dried seaweed waste generated from the agar processing industry contains 40-50% of organic compounds, mainly cellulose (raw fibers), 4-5% proteins, 50-60% of inorganic compounds, including about 55% of inorganic insoluble salt and many micro and macro elements [3]. It can be used directly as a feed supplement otherwise can be fermented using microorganisms. Liu et al. [16] have reported the fermentative conversion of seaweed processing waste from algin, mannitol, iodine, fucoid and other products within 15 days to use as animal feed upon adding corn powder (0.5%) and microbial agent (1%). Hence it is proposed that agar processing waste can be converted into animal feed through fermentation. This area requires extensive research to optimize fermentation parameters and suitable microbial agents in the near future to realize the full utilization of the resources of Gellidium Spp. and Gracilaria Spp. The better shelf life of the feed obtained in fermentative conversion would be achieved by drying at the ambient temperature (37 °C) or the temperature of 65 °C in the blast drier. So far, the available reports indicate whole seaweed as feed ingredients. On the same line by-products from seaweed processing industries are also a promising source of feed stuff.
Uses in the aquaponics system: The aquaponics system is a form of bio integration which correlates with aquaculture patterns using the principle of recirculation with hydroponic plants and vegetable production [17]. It is a time and space-saving cultivation where plants serve as a filter of vegetation that decomposes the toxins into harmless substances released by fish. On the other hand, it increases the supply of oxygen in the water which is indirectly used to conserve the fish [18]. Alamsjah [19] has used the crude wastage of Gracilaria sp. along with Kappaphycus sp. and Sargassum sp. in an aquaponics system. The wastage is fermented using probiotic bacteria, Lactobacillus sp. where the microbes perform the fermentation process by converting complex organic matter into a simpler molecule in the aquaponics system. Liquid waste of Gracilaria sp. was found to increase the growth of plants and vegetables in the aquaponics system. This system is having advantages of improving the host immune response to disease as well as improving the environmental quality of the aquatic system [20].
Uses in biosystems: A biosystem is a set of processes which treat the wastewater (domestic, industrial, agricultural activities, surface runoff water) using biological life like micro-organisms and plants. Micro-organism (cellulolytic bacteria and Bacillus spp.) helps /involved in the degradation of organic matter whereas the root system of plants (Ipomea classicals) functions as a biofilter [21]. After treatment, the treated water obtained from the biosystem can be reused in plantation and other industrial sectors. There is no record available for the utilization of seaweed processing wastewater after treatment. To the best of the author’s knowledge, the literature available on the utilization of liquid waste from the agar processing industry is scared.
Figure 6:Flow chart of extraction of cellulolytic enzyme (cellulase) from agar processing industry.
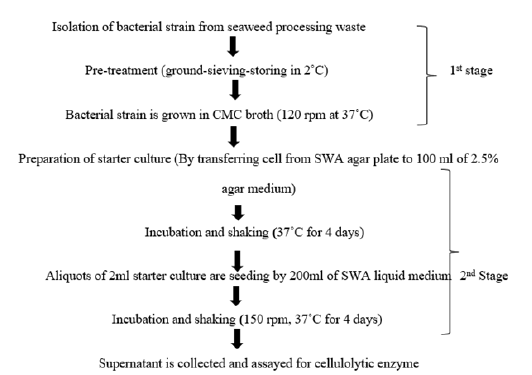
Uses in the extraction of cellulose assisted by cellulase: Cellulose (C6H10O5) n is an organic compound, a polysaccharide, consisting of a linear chain of several hundred to many thousands of β (1→4) linked D-glucose units. The raw materials such as cotton, recycled paper, wood cellulose, and sugarcane are used in making cellulose. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and oomycetes. Cellulose is mainly used in the paper industry, textile industry, pharmaceutical sector, building material, and laboratory and also used as an emulsifier and thickener in the food processing industry. The worldwide demand for cellulose each year is estimated at around 1.6 million tonnes [22]. Increasing demand for derivatives of plant cellulose causes several detrimental effects like deforestation and global environmental issues [23]. So seaweed is an alternative source for the production of cellulose and can be useful raw material. Cellulose was first extracted from seaweed of the class valonia, helicities, cladophora and also from some brown seaweed. After the agar preparation, the leftover pulp (refuse) is found to contain 62-68% holocellulose, which is enzymatically extracted using commercial cellulase. The Solid Waste of the Agar processing industry (SWA) can be used as an alternative source for cellulose production. Bacterial strain, Bacillus poilus LA4P, isolated from decaying industrial solid seaweed processing wastage of G. Verrucosa (agar) has been used to degrade the solid waste and produce a valuable product in the processing industry (Figure 6) [24]. The solid wastage of the agar seaweed processing industry can be utilized as a source of indigenous cellulolytic enzymes and as their substrate for the production of cellulose enzymes in turn, the cellulase enzyme can be used to extract the cellulose in oligosaccharide form.
Uses in fertilizer/manure: The present utilization practice of seaweed waste from the agar industry is mainly converted into fertilizer through decomposition at an atmospheric condition which tends to obtain fertilizer over the land. Fertilizer made from seaweed waste has macro-elements such as Nitrogen (N), Phosphorus (P), and Potassium (K) and microelements such as B, Cu, Ca, Mg and Fe which are expected to meet the needs in most of the agricultural crops. The drift-seaweed washed up on beaches has been used for centuries as a natural fertilizer in many coastal regions throughout the world [25]. Seaweed improves soil structure and provides it with trace elements and growth activators particularly potassium, nitrogen, calcium and magnesium [26]. Mosquera L et al. [27] studied that the co-composting of fish offal with drift-seaweed and pine bark allows to reduce greatly the volume of fisheries byproducts. This product might be used as a fertilizer in agriculture systems, as it is of natural origin and has the potential to be widely adopted. Seaweed waste from Cystoseira spp. and Laminaria spp. fish processing waste and pine bark were used as a fertilizer for the horticultural crop without any addition of chemical fertilizer ingredients [28]. The seaweed fertilizer has also been used as the nutrient uptake and growth promoter for soybean (Glycine max) under rainfed conditions [29]. Fresh Agar factory discharge (AFD) has been used as a manure for the seedling of cowpea. AFD can be processed and marketed to use as the manure for field crops [30].
The composting of seaweed fertilizer is an easy process. A compost pile is created using fish waste, seaweed waste and pine bark to increase the C/N ratio with different proportions. To avoid nutrient leaching, the pile is set on an impermeable base and sheltered above. The total duration of composting process lasts four months. The compost pile is turned weekly during the first two months and every 15 days during the last two months. The temperature and O2 levels are tested weekly to monitor the progressive development of the process. Water should not be added throughout the procedure. Once the compost is considered mature, it is sieved using a 20mm mesh screen before storage [31]. In the Indian market, few companies are manufacturing seaweed fertilizer in both solid and liquid forms. The cost of powder form of fertilizer varies from 200-500 rupees/kg whereas the cost of liquid fertilizer is about 50-200 rupees/ liter. Seaweed sap is a liquid fertilizer which is organic and biodegradable and applied to crops as root dips, soil drenches or foliar sprays [32]. Seaweed sap acts as an effective bio-stimulant in many crops including vegetables, trees, flowering plants, and grain crops. Sap contained plant growth regulators such as auxins and Cytokinins in varying amounts [33]. Seaweed sap from Gracilaria spp., popularly known as G-sap has been effectively used in the crop field of maize, wheat and rice etc. and has proven to be positively influenced in yield [34]..
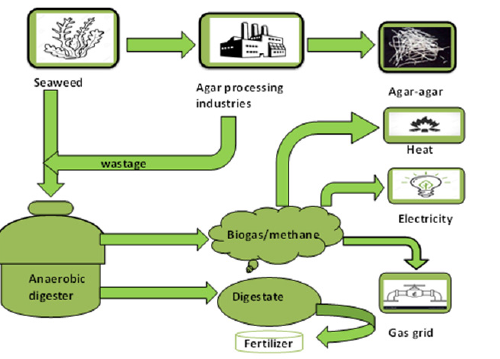
Uses in biogas/biomethane production: Recently different energy types have been appraised and are already in use to replace fossil fuels-e.g., solar, wind, tidal, nuclear, geothermal, biofuel, hydroelectric, etc. However, these alternative energy resources often possess specific disadvantages, like high cost, dependency on environmental conditions, damage to ecosystems, need for a lot of space and viability considerations. Biogas is a renewable energy source produced by the breakdown of organic matter such as agricultural waste, plant waste, municipal waste and manure and plant material in the absence of oxygen. The biogas digester system used to produce bio-energy can greatly contribute to reducing not only greenhouse gases but also the usage of fossil fuels [35].
Figure 7:A schematic diagram of biogas/bio methane production from agar processing industry.

Marine macro-algae (seaweed) conventionally have been used for environmental and commercial purposes. Recently a growing interest has been focused on seaweed detritus as a sustainable and cost-efficient feedstock for the anaerobic production of biogas (bio-ethane). Seaweed contributed a large proportion of carbohydrates, mainly polysaccharides, such as alginate, agar and cellulose. The monomeric sugars of these components, released through saccharification, are glucose, galactose (agar), glucuronic and mannuronic acid (alginate) etc. which are all suitable for the microbial metabolic pathway. The high proportion of carbohydrates and a low fraction of lignocellulosic compounds in macro-algae bodies (1:8) favors the microbial digestion or decomposition and conversion of algal biomass to methane-rich biogas (50-85% CH; 20-35% CO2; H2, N2 and H2S) [36]. In the process of converting seaweed biomass into biogas, the feedstock undergoes anaerobic digestion in biogas plants (Figure 7). During anaerobic digestion, four successive biochemical reactions namely hydrolysis (breaking saccharide bonds and addition of water molecule), acidogenesis (simple monomers are converted into volatile fatty acid), acetogenesis (acetate is produced by the reduction of CO2S)> or organic acid) and methanogenesis (formation of methane by microbes) transform the organic matter into methane (CH4) and carbon dioxide (CO2). The efficiency of conversion is largely dependent on hydrolysis. Easily degradable organic matter will undergo almost complete degradation, unlike complex and crystalline polymers, which are known to resist the breakdown in the presence of anaerobic bacteria and CO2 and accelerate the production of methane gas.
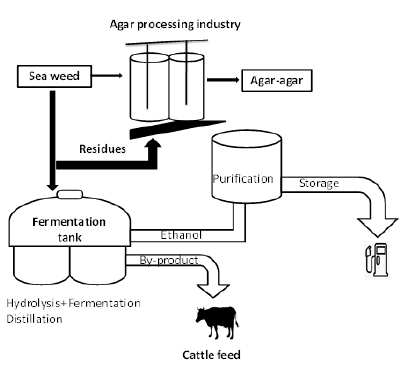
Uses in biofuel/bioethanol production: Energy production from biomass has emerged as one of the more attractive and promising alternatives to fossil fuels. In this connection, solid waste from the agar processing industry shows a potential feedstock for the production of bioethanol due to the presence of cellulose and hemicellulose in considerable quantity. The use of enzymatic hydrolysis to convert cellulose and hemicellulose into fermentable sugars has been studied extensively. The enzymatic process improves the economic feasibility of bioethanol production. Thus, much importance has been given to studying various enzymes and process parameters employed in the conversion of cellulose and hemicellulose to the form of fermentable sugars [37]. A feasible way of bioethanol production from solid seaweed waste is to use the method of dilute sulphury acid pre-treatment to find out the total sugar and total reducing sugar content followed by semi-continuous saccharification using cellulolytic enzyme and fermentation (Figure 8) [38]. It is an innovative step towards minimizing seaweed waste disposal and such process adds value to the industry.
Figure 8:A schematic diagram of bio-ethanol production from seaweed waste.
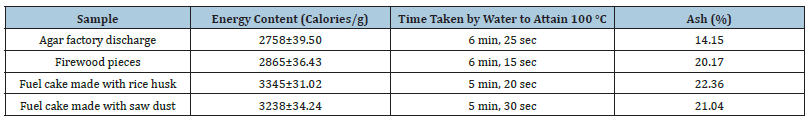
Fuel cake: A part of Agar Factory Discharge (AFD) is used as fuel in the form of fuel cake with an equal proportion of rice husk or sawdust which is a cost-effective source of fuel for cooking or boiling seaweed in the agar factory (Table 4) [30]. AFD can be incorporated into fuel cake and such a unit should be established in nearby agar processing factories for maximum utilization of wastage.
Table 4:Energy and ash content and combustibility of AFD fuel cakes and their ingredients [30].
Uses in bio-fillers as bio-composites: Bio-composite is a material formed by a matrix (resin) and a reinforcement of natural fibers. It can act as an artificial structure of living materials having and strengthening properties providing better biocompatibility [39]. The matrix is formed by polymers derived from renewable and non-renewable resources. The matrix is important to protect the fibers from environmental degradation and mechanical damage. Bio-fillers are the principal components of bio-composites that are derived from biological origins such as fibers from crops (cotton, flax or hemp), recycled wood, waste paper, crop processing by-products etc . Though it is renewable, cheap, biodegradable and recyclable, it has several applications like automobiles, railway coaches, aerospace, military applications, construction, and packaging [40]. In the agar processing industry, large quantities of solid seaweed waste are generated during the filtration step which is usually discarded. The seaweed waste is mainly enriched with cellulose and hemicellulose and contains small amounts of Floridian starch, diatomite earth [41]. Therefore, the solid seaweed waste is a material enriched with different polysaccharides that can be used as an inexpensive hybrid fillers in bio-composites. The seaweed waste reinforcement with Poly-Lactic Acid (PLA) as a bio-composite can be used as a bio-fillers in a packaging industry (Figure 9) [42]. The use of seaweed waste as a filler in PLA provides an interesting alternative for the production of low-cost and eco-friendly bio-composites with good thermal and mechanical properties [43].
Figure 9:Extraction of bio-fillers as a bio-composites from agar processing industry.
Food uses
Figure 10:Structure of tetrapyrrole groups.
Proteins from agar processing waste and its hydrolysate: There are two groups of functionally active proteins found in seaweed, such as lectin and phycobiliproteins [44]. Lectins are glycoproteins, which bind with carbohydrates and participate in biological processes like intercellular communication. Lectins are generally divided into four main categories, namely, legume lectins, chitin-binding lectins, monocot mannose-binding lectins, and type-2 ribosome inactivating proteins. Lectins are used for blood grouping as an anti-viral and antibacterial agent, cancer biomarkers etc. [45]. Phycobiliproteins are highly soluble fluorescent proteins found in red seaweed. These proteins contain covalently linked tetrapyrrole groups (Figure 10) that play a biological role in collecting light through fluorescence resonance energy transfer and help in photosynthetic reaction. There are three major categories of phycobiliproteins such as phycocyanin’s, allophycocyanin’s and phycoerythrin’s where phycoerythrin’s work as a major lightharvesting pigment in red seaweeds and allophycocyanin’s and phycoerythrin’s absorb more light where chlorophyll absorbs poorly. Phycocyanin is used as a blue pigment manufacturing in some products such as ice-cream, chewing gum, confectionery, dairy products, soft drinks, and other cosmetic products [46].
Protein hydrolysates are derived from proteins through chemical hydrolysis (acid/alkali), enzymatic hydrolysis and fermentation (Figure 11). It consists of peptides with different molecular weights and free amino acids. Smaller peptides (less than 6kDa) present in hydrolysis have gained great interest due to their bioactive potential. In this context, the enzymatic hydrolysis is preferred industrially due to the advantages it offers over the control of the process. Some commercially important enzymes prepared from the bacterial origin, including alcalase [47,48], neutrals [49] and flavoenzyme [16,50] as well as from animals and plants, including trypsin [51,52] pepsin are used in hydrolysis of protein at a large scale. After the hydrolysis, bioactive peptides are extracted for pharmaceutical and nutraceutical uses. The hydrolyzed protein products from agar waste will have better utilities as functional ingredients, especially in bakery products and beverages. A general list of by-products obtained from seaweed protein hydrolysate is shown in Table 5.
Figure 11:Flow chart for isolation and purification of seaweed protein hydrolysates.
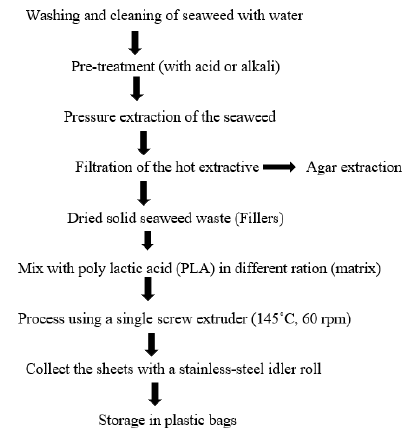
Table 5:A general list out of by-products obtaining from seaweed protein hydrolysate.
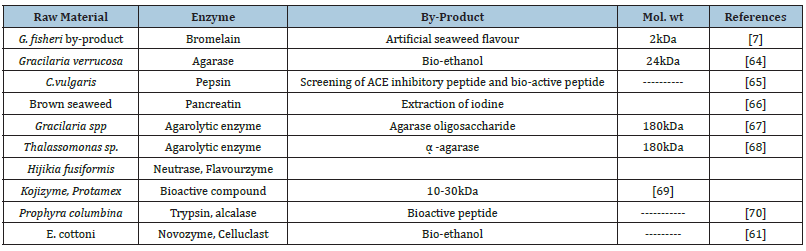
Bioactive/nutraceuticals: Food protein hydrolysates and bioactive peptides from macro-algae are given priority because of their organic nature [53,54]. It has antioxidant, antimicrobial, antitumor and antidiabetic activities which have been well established and already tested [54]. It can be used in the pharmaceutical and food processing industry. In the global market, no commercial products of nutraceutical interest from seaweed processing waste (by-product) are available. So more attention should be given towards the extraction of nutraceuticals and bioactive compounds from agar processing industrial waste. Such ingredients having health beneficial value can be introduced in the formulation of novel food products with specified health beneficial effects and pharmaceutical value [55-60].
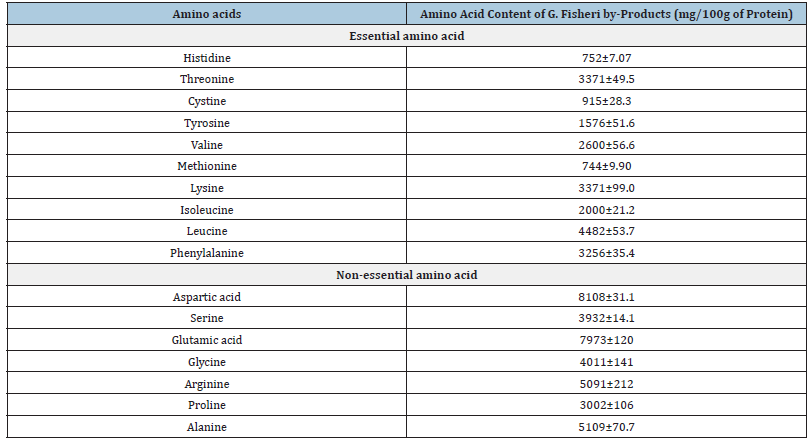
Extraction of volatile compound as a flavor enhancer: For centuries, seaweed has been used in the preparation of soup and foods in East Asia, South East Asia and the South African regions due to its unique flavor, especially in countries like Japan, Korea, Malaysia, Thailand and Ireland. Seaweed by-product after agar extraction is a good source of plant protein and it contains taste active amino acids like glutamic acid, aspartic acid, and arginine etc. (Table 6) [7]. The researcher found umami components like glutamic acid and aspartic acid in agar by-product which is responsible for savory with a meaty or broth-like taste [7].
Table 6:Amino acid composition of Gracillaria fisheri by- products [7].
Conclusion
Large quantities of liquid and solid residuals are produced every year by the seaweed processing industry. Seaweed wastage contains principally biodegradable organic matter that can be harmful to the environment if untreated or recovered [61-65]. The utilization of agar processing by-products for recovery of various food and non-food uses can be a management strategy for yielding the greatest benefit to the industry, society and environment. The pollution loads at the processing plant can be reduced significantly by switching towards valorization to the development of by- products or food product. Many opportunities exist for the recovery of agar wastage for human and animal uses. A variety of processes have been developed for converting waste into fuels, food ingredients, nutraceuticals and pharmaceutical based products [66-70]. Progress in research and development is expected in the near future to improve the method of utilization, commercial manufacture and public awareness with immediate effect in this sector.
Acknowledgement
References
- Food and Agriculture Organisation (2018) The State of World Fisheries and Aquaculture‐Meeting the Sustainable Development Goals.
- Food and Agriculture Organisation (2020) The state of World Fisheries and Aquaculture: Contributing to Food Security and Nutrition for all.
- Thuy HL (2017) Study on hydrolysis of seaweed waste (Gracilaria verrucosa) by cellulase enzymes from bacteria to apply in feed production for unisexual tilapia. PhD thesis. NHA Trang University, Vietnam, pp. 1-3.
- TIFAC Technical report (2018) Seaweeds cultivation and utilisation: Prospects in India. India.
- Gunasekera C (1963) Small scale manufacture of crude agar from Gracilaria seaweeds. Bull Fisk Res Stn Ceylon 16(1): 49-52.
- Kaliaperumal N, Kalimuthu S, Ramalingam JR (2004) Present scenario of seaweed exploitation and industry in India. Seaweed Research and Utilisation 26(1&2): 47-53.
- Laohakunjit N, Selamassakul O, Kerdchoechuen O (2014) Seafood-like flavour obtained from the enzymatic hydrolysis of the protein by-products of seaweed (Gracilaria sp.). Food Chemistry 158:162-170.
- Rasyid A, Ardiansyah A, Pangestuti R (2019) Nutrient composition of dried seaweed Gracilaria gracilis. Indonesian Journal of Marine Sciences 24(1): 1-6.
- Ashoush YA, Sayed SME, Farid HE, Elwahab MAA (2017) Biochemical studies on red algae Gelidium sp. grown in Egypt. Chemistry Research Journal 2(5): 334-341.
- Mathew PT, Prabhu PV, Gopakumar K (1993) Agar from gelidiella acerosa by alkali treatment. Seaweed Resource and Utilization 16(1&2): 177-182.
- Nahdi ZA, Alawi AA, Marhobi IA, Zefiti AA (2015) Optimization of yield and chemical properties of agar extracted from Melanothamnus somalensis from Oman Sea. Journal of Environmental Science and Engineering 4: 302-14.
- Stewart WDP (1974) Algal physiology and biochemistry. University of California Press, USA, 10: 10-11.
- Abraham A, Afewerki B, Tsegay B, Ghebremedhin H, Teklehaimanot B, et al. (2018) Extraction of Agar and Alginate from Marine Seaweeds in Red Sea Region. Int J Marine Biol Res 3(2): 1-8.
- Food safety and standards Authority of India (2011) Food products standards and food additives regulations, pp. 1-437.
- Sedayu BB, Basmal J, Fithriani D (2007) Reuse of waste water on alkali treated cottoni; Coagulation and filtration. Journal of Fishery of biotechnology 2(2): 107-115.
- Liu X, Ping ZS, Han L, Li Y (2012) Influence of several fermentation on seaweed waste of feed. Journal of Sustainable Bioenergy Systems 2(4): 108-111.
- Foh MBK, Kamara MT, Amadou I, Foh BM, Wenshui X (2011) Chemical and physicochemical properties of tilapia (Oreochromis niloticus) fish protein hydrolysate and concentrate. International Journal of Biological chemistry 5(1): 21-36.
- Ahmad T, Sofiarsih L, Rusmana (2007) The Growth of Pangasiodon hypopthalmus in a close system tank. Indonesian Aquaculture Journal 2(1): 67-73.
- Alamsjah MA (2013) Gracilaria sp. waste, Lactobacillus sp. and Chlorella sp. integration on intensive aquaculture with aquaponics system. Journal of Natural Sciences Research 3(11): 66-77.
- Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64(4): 655-671.
- Suyasa WB, Dwijani WS (2015) Biosystems treatment approach for seaweed processing wastewater. Journal of Environment and Waste Management 2(2): 59-62.
- Italia C (2015) The international cellulose market. IOP Publishing Paper industry world.
- Esa F, Tasirin SM, Rahman NA (2014) Overview of bacterial cellulose production and application. Agriculture and Agricultural Science Procedia 2: 113-119.
- Munifah I, Sunarti TC, Irianto HE, Meryandini A (2015) Biodegradation of solid wastes of agar seaweed processing industry by indigenous cellulolytic Bacillus Pumilus Biosciences Biotechnology Research Asia 12(3): 1957-1964.
- McHugh DJ (2003) A guide to the seaweed industry. FAO Fisheries Technical Paper 441. Food and Agriculture Organization of the United Nations, Italy.
- Blunden G, Guiry MD (Eds.), (1991) Seaweed resources in Europe: Uses and potential. J Wiley and Sons, USA.
- Mosquera MEL, Lema EF, Villares R, Corral R, Alonso B, et al. (2011) Composting fish waste and seaweed to produce a fertilizer for use in organic agriculture. Procedia Environmental Sciences 9: 113-117.
- Vives MI, Labandeira SS, Brito LM, Fabal AL, Mosquera MEL (2015) Evaluation of compost from seaweed and fish waste as a fertilizer for horticultural use. Scientia Horticulturae 186:101-107.
- Rathore SS, Chaudhary DR, Boricha GN, Ghosh A, Bhatt BP, et al. (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South African Journal of Botany 75(2): 351-355.
- Kunda SK, Kaladharan P (2003) Agar factory discharge as fuel and manure. Seaweed Res Utiln 25(1&2): 165-168.
- Heo SJ, Ko SC, Cha SH, Kang DH, Park HS, et al. (2009) Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol in Vitro 23(6): 1123-1130.
- Shah MT, Zodape ST, Chaudhary DR, Eswaran K, Chikara J (2013) Seaweed sap as an alternative liquid fertilizer for yield and quality improvement of wheat. Journal of Plant Nutrition 36(2): 192-200.
- Basavaraja PK, Yogendra ND, Zodape ST, Prakash R, Ghosh A (2018) Effect of seaweed sap as foliar spray on growth and yield of hybrid maize. Journal of Plant Nutrition 41(14): 1851-1861.
- Singh S, Singh MK, Pal SK, Trivedi K, Yesuraj D, et al. (2016) Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. Journal of Applied Phycology 28(3): 2099-2112.
- Vanegas C, Bartlett J (2013) Anaerobic digestion of Laminaria digitata: The effect of temperature on biogas production and composition. Waste and Biomass Valorisation 4(3): 509-515.
- Barbot YN, Al-Ghaili H, Benz R (2016) A review on the valorisation of macro algal wastes for bio-methane production. Marine Drugs 14(6): 120.
- Tan IS, Lee KT (2014) Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 78: 53-62.
- Kumar G, Sivagurunathan P, Sen B, Mudhoo A, Davila-Vazquez G, et al. (2017) Research and development perspectives of lignocellulose-based biohydrogen production. International Biodeterioration & Biodegradation 119: 225-238.
- Fazeli M, Florez J, Simao R (2018) Improvement in adhesion of cellulose fibres to the thermoplastic starch matrix by plasma treatment modification. Composites Part B: Engineering 163: 207-216.
- Michael Legault (2015) Bio-composites update: Beyond eco-branding. Composites World, Gardner Business Media, USA.
- Kraan S (2012) Algal polysaccharides, novel applications and outlook. Carbohydrates-Comprehensive studies on glycobiology and Glyco Technology.
- Marinho-Soriano E, Bourret E (2005) Polysaccharides from the red seaweed gracilaria dura (Gracilariales, Rhodophyta). Bioresource Technology 96(3): 379-382.
- Martosuyono P, Hakim A, Fawzya YN (2015) Chemical pre-treatment and enzymatic saccharification of seaweed solid wastes. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology 10(2): 61-71.
- Pangestuti R, Kim SK (2015) Seaweed proteins, peptides and amino acids. Seaweed Sustainability, Academic Press, USA, pp. 125-140.
- Bleakley S, Hayes M (2017) Algal proteins: Extraction, application and challenges concerning production. Foods 6(5): 33.
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. Journal of Bioscience and Bioenergy 101(2): 87-96.
- Sila A, Sayari N, Balti R, Martinez-Alvarez O, Nedjar-Arroume N, et al. (2014) Biochemical and antioxidant properties of peptidic fraction of carotene proteins generated from shrimp by-products by enzymatic hydrolysis. Food Chemistry 148: 445-452.
- Je JY, Qian ZJ, Byun HG, Kim SK (2007) Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochemistry 42(5): 840-846.
- Hammershoj M, Nebel C, Carstens JH (2008) Enzymatic hydrolysis of ovomucin and effect on foaming properties. Food Research International 41(5): 522-531.
- Foh MBK, Kamara MT, Amadou I, Foh BM, Wenshui X (2011) Chemical and physicochemical properties of tilapia (Oreochromis niloticus) fish protein hydrolysate and concentrate. International journal of Biological chemistry 5(1): 21-36.
- Aleman A, Gimenez B, Montero P, Gomez-Guillen MC (2011) Antioxidant activity of several marine skin gelatins. LWT-Food Science and Technology 44(2): 407-413.
- Praiboon J, Chirapart A, Akakabe Y, Bhumibhamon O, Kajiwara T (2006) Physical and chemical characterization of agar polysaccharides extracted from the Thai and Japanese species of Gracilaria. Science Asia 32(1): 11-17.
- Karavita R, Senevirathne M, Athukorala Y, Affan A, Leey J, et al. (2007) Protective effect of enzymatic extracts from microalgae against DNA damage induced by H2O2. Marine Biotechnology 9(4): 479-490.
- Balti R, Bougatef A, El Hadj Ali N, Ktari N, et al. (2011) Comparative study on biochemical properties and antioxidative activity of cuttlefish (Sepia officinalis) protein hydrolysates produced by alcalase and Bacillus licheniformis NH1 Journal of Amino Acids 2011: 107179.
- Kumar V, Fotedar R (2009) Agar extraction process for gracilaria cliftonii. Carbohydrate Polymers 78(4): 813-819.
- Roleda MY, Montano NE, Ganzon-Fortes ET, Villanueva RD (1997) Acetic acid pre-treatment in agar extraction of Philippine Gelidiella acerosa (Forsskaal) Feldmann et Hamel (Rhodophyta, Gelidiales). Botanica Marina 40(1-6): 63-69.
- Veeragurunathan V, Prasad K, Vizhi JM, Singh N, Meena R, et al. (2019) Gracilaria debilis cultivation, agar characterization and economics: bringing new species in the ambit of commercial farming in India. Journal of Applied Phycology 31: 2609-2621.
- Meena R, Siddhanta AK, Prasad K, Ramavat B, Eswaran K, et al. (2007) Preparation, characterization and benchmarking of agarose from gracilaria dura of Indian waters. Carbohydrate Polymers 69(1): 179-188.
- Wang L, Shen Z, Mu H, Lin Y, Zhang J, et al. (2017) Impact of alkali pre-treatment on yield, physico-chemical and gelling properties of high-quality agar from gracilaria tenuistipitata. Food Hydrocolloids 70: 356-362.
- Zaimis U, Jurmalietis R, Jansone A (2018) Processed seaweed and winemaking waste co-fermentation for biogas extraction: Pilot study. Latvia University of Life Sciences and Technologies 17: 1916-1919.
- Tan IS, Lee KT (2014) Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 78: 53-62.
- Madera‐Santana TJ, Freile‐Pelegrín Y, Encinas JC, Ríos‐Soberanis CR, Quintana‐Owen P (2015) Bio composites based on poly (lactic acid) and seaweed wastes from agar extraction: Evaluation of physicochemical properties. Journal of Applied Polymer Science 132(31).
- Francavilla M, Franchi M, Monteleone M, Caroppo C (2013) The red seaweed gracilaria gracilis as a multi products source. Marine Drugs 11(10): 3754-3776.
- Roseline TL, Sachindra NM (2018) Purification and characterization of agarose from marine bacteria Acinetobacter sp. PS12B and its use for preparing bioactive hydrolysate from agarophyte red seaweed gracilaria verrucosa. Applied Biochemistry and Biotechnology 186(1): 66-84.
- Sheih IC, Fang TJ, Wu TK (2009) Isolation and characterisation of a novel Angiotensin I-Converting Enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chemistry 115(1):279-284.
- Romaris-Hortas V, Bermejo-Barrera P, Moreda-Pineiro A (2013) Ultrasound-assisted enzymatic hydrolysis for iodinated amino acid extraction from edible seaweed before reversed-phase high performance liquid chromatography-inductively coupled plasma-mass spectrometry. Journal of Chromatography 1309: 33-40.
- Potin P, Richard C, Rochas C, Kloareg B (1993) Purification and characterization of the α‐agarose from alteromonas agarlyticus (Cataldi) comb nov strain GJ1 European Journal of Biochemistry 214(2): 599-607.
- Ohta Y, Hatada Y, Miyazaki M, Nogi Y, Ito S (2005) Purification and characterization of a novel α-agarase from a Thalasso monas sp. Current Microbiology 50(4): 212-216.
- Siriwardhana N, Jeon YJ, Kim SH, Ha JH, Heo SJ, et al. (2004) Enzymatic hydrolysis for effective extraction of anti-oxidative compounds from hizikia fusiformis. Algae 19(1): 59-68.
- Cian RE, Martinez-Augustin O, Drago SR (2012) Bioactive properties of peptides obtained by enzymatic hydrolysis from protein by-products of Porphyra columbina. Food Research International 49(1): 364-372.
© 2023 Elavarasan K,. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






