- Submissions

Full Text
Examines in Marine Biology & Oceanography
Dichotomy of Thermal Desalination: “To Evaporate or to Freeze, that is the Question”
Woochul Lee1 and Albert S Kim2*
1Department of Mechanical Engineering, University of Hawaiʻi at Mānoa, Honolulu, HI 96822, USA
2Department of Civil and Environmental Engineering, University of Hawaiʻi at Mānoa, Honolulu, HI 96822, USA
*Corresponding author: Albert S Kim, Department of Civil and Environmental Engineering, University of Hawaiʻi at Mānoa, Honolulu, HI 96822, USA
Submission: December 15, 2022;Published: December 22, 2022

ISSN 2578-031X Volume5 Issue2
Opinion
In this article, we overview the present technologies for freshwater production, such as membrane separation, thermal desalination, and water reuse/recycling. Specifically, we discuss the thermodynamic aspects of the desalination technologies to suggest freezing desalination as an alternative or supplement to mature desalination technologies.
Overview
Energy resources consist of non-renewable and renewable, based on their replenishing periods. Non-renewable energy, such as coal, oil, and natural gas, have higher production power than renewable energy consisting of solar, wind, hydro, biomass, and geothermal resources. Renewable energy resources are unlimited over the long term but are less efficient than non-renewable resources. Desalination technologies, primarily through seawater desalination, are known as energy-intensive processes because of the requirement to overcome feed osmotic pressure. Due to the continuous demand for high (electrical) energy consumption, desalination research often aims at achieving high rejection and production at low energy consumption. Freshwater production and water supply technologies can be categorized into thermal desalination, membrane separation, and water reuse/recycling [1-3]. Commercially available Reverse Osmosis (RO) membranes were designed in the early 1960s by Loeb and Souriranjan and proactively applied to salt rejection processes, covering from freshwater production by seawater desalination to ultra-pure water production in the semiconductor industry [4-7]. Even though membrane technology became competitive with traditional thermal techniques, the membrane’s salt-rejection capability was often traded off with permeate flux, i.e., the production rate per unit membrane surface area [8,9].
Thermal energy utilization for freshwater production has a more than four-thousand-year history. Around 2000 B.C., Sanskrit writing recorded how to purify water through boiling, sunlight exposure, and charcoal filtering. In the 15th and 13th centuries B.C., Egyptian wall paintings depicted the earliest siphoning apparatus for clarifying liquids. In 98 A.D., the first engineering report on water supply and treatment was written by Roman Senator Sextus Julius Frontinus in Rome, Italy. In modern decades, thermal desalination primarily includes a series of efficient processes consisting of water evaporation followed by condensation.
Evaporative desalination
Distillation technologies have been developed to improve production efficiencies with smaller footprints. Vacuum distillation uses the basic thermodynamics principle of phase change [10,11], described by the Clausius-Clapeyron equation to link the evaporation enthalpy and vapor pressure [12]. Within a chamber, feed water evaporates at a pressure maintained lower than the vapor pressure at the chamber temperature. External condensers receive a vapor stream and produce distilled water with almost zero salinity, but thermal waste heat is unavoidable. Other processes are as follows. Vapor-compression distillation compresses the vapor present above the liquid, and the compressed vapor provides thermal energy higher than the evaporation enthalpy. Multi-Stage Flash Distillation (MSF) consists of a series of flash evaporators, fundamentally equivalent to vacuum distillation. Multiple-Effect Distillation (MED) is often used for seawater desalination, in which the incoming feed stream is sprayed and heated for efficient evaporation.
A hybrid technology of porous membrane and thermal desalination is Membrane Distillation (MD) of various operation types [13-16], depending on the condensation mechanisms, such as direct contact [12,17,18], vacuum [19,20], sweep gas [21-23], air gap [24-26], and liquid gap [27-29] MD processes. Porous membranes, used in microfiltration and ultrafiltration, play the role of the contacting barriers between the liquid and vapor phases of water, without any chemical reactions. After evaporation, vapor molecules migrate through membrane pores by competing Brownian [30-35] and Knudsen [20,36] diffusion and are either condensed or collected on the distillate side.
Water reuse/recycling has been less popular since it requires new development of or significant changes in social infrastructure while using conventional water treatment methods [37,38]. In reality, RO pretreatment is as essential as the RO process itself because imperfect pretreatment causes rapid fouling on membrane surfaces, potentially resulting in the process ceasing [39]. Thermal and membrane-based desalination produces warmer saline water than ambient water or highly concentrated brine stream, which may cause unexpected environmental impacts. Zero Discharge Desalination (ZDD) started as a critical research topic, which becomes the future universal requirement at the city or county level [40-42]. In conventional thermal or membrane-based desalination, the continuous salt rejection produces either dense liquid or dry salts. At the theoretical limit of perfect recovery, the dry salt production rate is calculated using the seawater salinity, i.e., 35kg per ton or 103,200lb per million gallons of product water. Steady disposal of this large salt amount can include direct ocean disposal, incineration, and reuse as a defrosting agent on traffic roads. In an efficient water reuse/recycling system, zero discharge can be more feasible if the sources and types of water utilization are better known.
Thermal desalination has focused on process optimization for effective water evaporation, followed by its condensation with minimum energy consumption. The ultimate obstacle to thermal desalination is the vapor pressure at operating temperature. For example, the gas pressure should be maintained below 0.03 atm in the chamber to evaporate water at 25 °C. Maintaining the low pressure state, closer to the vacuum phase, requires steady electrical consumption. Costs for the vacuum process are comparable to electricity consumption for high-pressure pumps for reverse osmosis processes. Nevertheless, the specific advantage of evaporation-based desalination is excellent salt rejection (even if the seawater is used as a feed stream) due to water’s intrinsic material properties.
In principle, thermal desalination is the traditional method that effectively excludes salt ions during the water evaporation process. Excessive thermal energy, higher than the latent heat demand for the phase change, breaks hydrogen bonds between two adjacent water molecules at the water-air interface. The latent heat of liquid water is represented as a function of absolute temperature T, such as H(T) = l0 − l1T , where l0 = 57.075kJ / mol and l1 4.3856 10-2 kJ / mol = × − K are estimated assuming the water is incompressible [12]. An accurate estimation of the evaporation enthalpy is 40.65kJ/mol or 2256.4kJ/kg at 100 °C. The vapor pressure is derived as
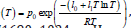
Freezing desalination
As briefly explained above, the freezing process removes salt ions in formed ice, consuming much less thermal energy. Freezing Desalination (FD) includes direct contact and indirect contact FD processes. In direct contact freeze desalination, the cold refrigerant directly contacts with the feed solution to vaporize the refrigerant at a lower pressure. The vaporization causes ice formation through heat removal from the feed solution. This method has a high ice production rate, but the created ice can be potentially contaminated due to volatile refrigerants. Refrigerants such as Butane have been studied due to its water-immiscible properties, but its flammability raises safety issues. Recently, Xie et al. enhanced heat transfer and phase separation in the freeze desalination process [43] by investigating the effects of initial refrigerant temperature, the ratio of produced ice to feed seawater mass, and ice production. In the spray freezing desalination studied by Liu et al. [44] warm seawater is sprayed at the top of a freezing tower and cold air (from the atmosphere or re-gasification of liquefied natural gas) is injected at the bottom of the tower. As the sprayed seawater droplets descend, heat transfer results in ice particle formation. A 200m high tower can yield 27.7kg/s fresh water (of 0.5% NaCl concentration) in an atmospheric temperature of -26 °C. An earlier study by Gao et al. [45] demonstrated that the possibility of ∼60% impurity removal using spray freezing [45]. Indirect contact FD consists of Suspension Freeze Crystallization (SFC) and Layer Freeze Crystallization (LFC) [46,47]. In contrast to direct contact FD, salt water and refrigerant/ coolant are separated by thermally conducting walls.
In SFC, seed ice crystals are produced in salt water and grow larger via the Ostwald ripening mechanism in suspension [48]. Shin et al. [49] introduced surface-scraped freeze-crystallizer process to remove the ice crystal seeds creating step using a U-shaped agitator. The two-staged freeze desalination process resulted in 40% water productivity with 0.18 wt% salinity (with feed salinity of 3.5 wt%) [49]. Erlbeck et al. [50-52] used various SFC crystallizers to treat salt water with a 3.75 wt% NaCl concentration to produce a potable drinking water level of 0.01wt% with 22% water productivity [50-52].They used ice pressing as a post-treatment with a force as large as 37.4kN (or 100 bar), which significantly decreased NaCl concentration to ∼0.01wt%. Sahu et al. [53] used a U-shaped crystallizer in the SFC process to enable continuous ice production. Their method integrated cooling, chilling, and freezing processes to result in water productivity of 15% and 0.8 wt% salinity of product water (with feed salinity ∼3.5 wt%) [53].
LFC employs a cold surface to form a large ice crystal and facilitates efficient salt removal due to smaller ice surface area. However, heat transfer between the plate and feed stream becomes less effective when ice layers are created, hindering further ice formation. Chen et al. [54] developed supercooled water dynamic ice making method to achieve 50% water productivity with 0.045wt% salinity using 3.5wt% feed stream with lower energy consumption due to smaller ice powder sizes [54,55]. Rich et al. [55] developed a dynamic layer crystallizer capable of obtaining water productivity of 19% with 0.03wt% of product water salinity with a sweating post-treatment. Vertical freezing apparatus were operated in a batch-wise mode, where ice was forcefully grown in one direction [56,57] to demonstrate water productivity of 51% and 0.91 wt% salinity of product water [57] and ∼25% water productivity and ∼0.1wt% salinity [56].
Future perspective: Enthalpy, blessing or obstacle?
As one of the freshwater production technologies, desalination aims to remove salt ions and impurities with lower energy consumption. During a desalination process, saline water enters a different phase from the liquid phase. The transition phases are membrane materials in the membrane separation and vapor phase in evaporative desalination, respectively. Evaporative desalination requires high energy consumption for the high rejection ratio; on the other hand, freezing desalination consumes a fractional thermal energy required for water evaporation but the salt rejection ratio is not high enough. The evaporation and melting enthalpies form energy barriers for water phase changes. The fixed enthalpy is a blessing under which materials and their phases in nature are balanced, but it is an obstacle to overcome for advanced thermal desalination. In freezing desalination, the temperature is adjusted for ice crystal formation, and pressure is maintained accordingly. Both the evaporative and freezing desalination are subject to the latent heat and vapor pressure, respectively, at the transition temperatures. Conventional thermal desalination aimed to develop more energy-efficient processes but is limited by enthalpies for melting and vaporization. Novel technologies that can reduce the latent heat and vapor pressure during a short period (i.e., of an order of seconds or shorter) can significantly reduce energy consumption and enhance phase change efficiencies.
References
- (2010) Sustainable water for the future: Water recycling versus desalination. In: Escobar IC, Schäfer A, (Eds.), 1st (Edn.), Sustainability Science and Engineering, Elsevier Science, Volume 2, USA.
- Quist-Jensen CA, Macedonio F, Drioli E (2015) Membrane technology for water production in agriculture: Desalination and wastewater reuse. Desalination 364: 17-32.
- Suwaileh W, Johnson D, Hilal N (2020) Membrane desalination and water re-use for agriculture: State of the art and future outlook. Desalination 491.
- Lonsdale HK, Merten U, Riley RL (1965) Transport properties of cellulose acetate osmotic membranes. Journal of Applied Polymer Science 9(4): 1341-1362.
- Loeb S (1981) The loeb-sourirajan membrane: How it came about. Synthetic Membranes, ACS Symposium Series, American Chemical Society, 153: 1-9.
- Glater J (1998) The early history of reverse osmosis membrane development. Desalination 117(1-3): 297-309.
- Rodríguez CA, Silva CGA, Osorio F, González López J, Calvo C (2015) Reverse Osmosis seawater desalination: Current status of membrane systems. Desalination and Water Treatment 56(4): 849-861.
- Murad S, Nitsche LC (2004) The effect of thickness, pore size and structure of a nanomembrane on the flux and selectivity in reverse osmosis separations: A molecular dynamics study. Chemical Physics Letters 397(1-3): 211-215.
- Kim AS (2017) Review of basics reverse osmosis process modeling: A new combined fouling index proposed. Membrane Journal 27(4): 291-312.
- Burch CR (1929) Some experiments on vacuum distillation. Proceedings of the Royal Society of London Series A, Containing Papers of a Mathematical and Physical Character 123(791): 271-284.
- Rashid A, Ayhan T, Abbas A (2016) Natural vacuum distillation for seawater desalination-A review. Desalination and Water Treatment 57(56): 26943-26953.
- Kim AS (2013) A two-interface transport model with pore-size distribution for predicting the performance of Direct Contact Membrane Distillation (DCMD). Journal of Membrane Science 428: 410-424.
- Curcio E, Drioli E (2005) Membrane distillation and related operations-A review. Separation & Purification Reviews 34(1): 35-86.
- Khayet M (2011) Membranes and theoretical modeling of membrane distillation: A review. Advances in Colloid and Interface Science 164(1-2): 56-88.
- Souhaimi MK, Matsuura T, Mohamed K (2011) Membrane distillation: Principles and applications. Elsevier Science, USA, pp. 1-477.
- Ki SJ, Kim HJ, Kim AS (2015) Big data analysis of Hollow Fiber Direct Contact Membrane Distillation (HFDCMD) for simulation-based empirical analysis. Desalination 355: 56-67.
- Lee JG, Kim YD, Kim WS, Francis L, Amy G, et al. (2015) Performance modeling of Direct Contact Membrane Distillation (DCMD) seawater desalination process using a commercial composite membrane. Journal of Membrane Science 478: 85-95.
- Kim AS (2014) Cylindrical cell model for Direct Contact Membrane Distillation (DCMD) of densely packed hollow fibers. Journal of Membrane Science 455: 168-186.
- Abu-Zeid MAER, Zhang Y, Dong H, Zhang L, Chen HL, et al. (2015) Comprehensive review of vacuum membrane distillation technique. Desalination 356: 1-14.
- Kim AS, Lee HS, Moon DS, Kim HJ (2017) Statistical theory of vapor transport through hollow fiber membranes in vacuum membrane distillation: Effusion analogy. Desalination 410: 77-90.
- Khayet M, Cojocaru C, Baroudi A (2012) Modeling and optimization of sweeping gas membrane distillation. Desalination 287: 159-166.
- Said IA, Chomiak T, Floyd J, Li Q (2020) Sweeping Gas Membrane Distillation (SGMD) for wastewater treatment, concentration, and desalination: A comprehensive review. Chemical Engineering and Processing- Process Intensification 153.
- Khayet M, Godino P, Mengual JI (2000) Theory and experiments on sweeping gas membrane distillation. Journal of Membrane Science 165(2): 261-272.
- Alklaibi AM, Lior N (2005) Transport analysis of air-gap membrane distillation. Journal of Membrane Science 255(1-2): 239-253.
- Meindersma GW, Guijt CM, Haan AB (2006) Desalination and water recycling by air gap membrane distillation. Desalination 187(1): 291-301.
- Im BG, Francis L, Santosh R, Kim WS, Ghaffour N, et al. (2022) Comprehensive insights into performance of water gap and air gap membrane distillation modules using hollow fiber membranes. Desalination 525.
- Ugrozov VV, Elkina IB, Nikulin VN, Kataeva LI (2003) Theoretical and experimental research of liquid-gap membrane distillation process in membrane module. Desalination 157(1): 325-331.
- Essalhi M, Khayet M (2014) Application of a porous composite hydrophobic/hydrophilic membrane in desalination by air gap and liquid gap membrane distillation: A comparative study. Separation and Purification Technology 133: 176-186.
- Khalifa AE (2015) Water and air gap membrane distillation for water desalination-An experimental comparative study. Separation and Purification Technology 141: 276-284.
- Brown R (1828) XXVII. A brief account of microscopical observations made in the months of June, July and August 1827, on the particles contained in the pollen of plants; and on the general existence of active molecules in organic and inorganic bodies. The Philosophical Magazine 4(21): 161-173.
- Langevin P (1908) On the theory of Brownian motion. Reports of the Academy of Sciences (Paris) 146: 530-533.
- Uhlenbeck GE, Ornstein LS (1930) On the theory of the Brownian motion. Physical Review 36(5): 823-41.
- Chandrasekhar S (1943) Stochastic problems in physics and astronomy. Reviews of Modern Physics 15(1): 1-89.
- Kubo R (1966) The fluctuation-dissipation theorem. Reports on Progress in Physics 29(1): 255-284.
- Batchelor GK (1976) Brownian diffusion of particles with hydrodynamic interaction. Journal of Fluid Mechanics 74(1): 1-29.
- Shi Y, Lee YT, Kim AS (2012) Knudsen diffusion through cylindrical tubes of varying radii: Theory and monte carlo simulations. Transport in Porous Media 93(3): 517-541.
- Gude VG (2017) Desalination and water reuse to address global water scarcity. Reviews in Environmental Science and Bio/Technology 16(4): 591-609.
- Salgot M, Folch M (2018) Wastewater treatment and water reuse. Current Opinion in Environmental Science & Health 2: 64-74.
- Henthorne L, Boysen B (2015) State-of-the-art of reverse osmosis desalination pretreatment. Desalination 356: 129-139.
- Tong T, Elimelech M (2016) The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environmental Science & Technology 50(13): 6846-6855.
- Chung HW, Nayar KG, Swaminathan J, Chehayeb KM, Lienhard V JH (2017) Thermodynamic analysis of brine management methods: Zero-discharge desalination and salinity-gradient power production. Desalination 404: 291-303.
- Tsai JH, Macedonio F, Drioli E, Giorno L, Chou CY, et al. (2017) Membrane-based zero liquid discharge: Myth or reality? Journal of the Taiwan Institute of Chemical Engineers 80: 192-202.
- Xie C, Zhang L, Liu Y, Lv Q, Ruan G, et al. (2018) A direct contact type ice generator for seawater freezing desalination using LNG cold energy. Desalination 435: 293-300.
- Liu Y, Ming T, Wu Y, Richter R, Fang Y, et al. (2020) Desalination of seawater by spray freezing in a natural draft tower. Desalination 496.
- Gao W, Smith DW, Sego DC (2004) Treatment of pulp mill and oil sands industrial wastewaters by the partial spray freezing process. Water Research 38(3): 579-584.
- Kalista B, Shin H, Cho J, Jang A (2018) Current development and future prospect review of freeze desalination. Desalination 447: 167-181.
- Kadi KE, Janajreh I (2019) Desalination by freeze crystallization: An overview. International Journal of Thermal and Environmental Engineering 15(2): 103-110.
- Najim A (2022) A review of advances in freeze desalination and future prospects. NPJ Clean Water 5(1):
- Shin H, Kalista B, Jeong S, Jang A (2019) Optimization of simplified freeze desalination with surface scraped freeze crystallizer for producing irrigation water without seeding. Desalination 452: 68-74.
- Erlbeck L, Rädle M, Nessel R, Illner F, Müller W, et al. (2017) Investigation of the depletion of ions through freeze desalination. Desalination 407: 93-102.
- Erlbeck L, Wössner D, Kunz T, Rädle M, Methner FJ (2018) Investigation of freeze crystallization and ice pressing in a semi-batch process for the development of a novel single-step desalination plant. Desalination 448: 76-86.
- Erlbeck L, Wössner D, Schlachter K, Kunz T, Methner FJ, et al. (2019) Investigation of a novel scraped surface crystallizer with included ice-pressing section as new purification technology. Separation and Purification Technology 228.
- Sahu P, Krishnaswamy S, Pande NK (2020) Process intensification using a novel continuous u-shaped crystallizer for freeze desalination. Chemical Engineering and Processing-Process Intensification 153.
- Chen D, Zhang C, Rong H, Wei C, Gou S (2020) Experimental study on seawater desalination through supercooled water dynamic ice making. Desalination 476.
- Rich A, Mandri Y, Mangin D, Rivoire A, Abderafi S, et al. (2012) Sea water desalination by dynamic layer melt crystallization: Parametric study of the freezing and sweating steps. Journal of Crystal Growth 342(1): 110-116.
- Fujioka R, Wang LP, Dodbiba G, Fujita T (2013) Application of progressive freeze-concentration for desalination. Desalination 319: 33-37.
- Moharramzadeh S, Ong SK, Alleman J, Cetin KS (2021) Parametric study of the progressive freeze concentration for desalination. Desalination 510.
© 2022 Albert S Kim. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






