- Submissions

Full Text
Examines in Marine Biology & Oceanography
Reproductive, Spawning, and Sexual Strategies in Fish
Paschalis Gavriilidis*
Head of the Department of General Surgery, Saint Helena General Hospital, UK
*Corresponding author: Paschalis Gavriilidis, Head of the Department of General Surgery, Saint Helena General Hospital, UK
Submission: December 5, 2022;Published: December 14, 2022

ISSN 2578-031X Volume5 Issue2
Abstract
The reproductive strategies in fish varies widely. However, the majority of them release a large amount of unfertilized eggs into the water. Simultaneously, the male fish releases a large amount of sperm which fertilizes some of the released eggs. Mainly, the reproduction methods of the fish can be divided into two methods. In the first method the female fish releases eggs either on the leaves of the sea plants or on the seabed. In the second method, the male by using the anal fin as a copulative organ introduces sperm into the female; therefore, the eggs are fertilized internally. Consequently, the female delivers live fry. The aim of the present study is to present a review of the reproductive, spawning and sexual strategies in fish.
Keywords: Reproductive; Spawning; Strategies; Fish
Reproductive Strategies
Testes and ovaries are the reproductive organs of fish. In most species these are paired organs. The testes are smooth and white and account for up to 12% of the mass of the fish. On the other hand, the ovaries are granular and yellow or orange, accounting for up to 70% of the fish’s mass. The produced eggs or the sperm are released through the genital papilla a small fleshy tube behind the anus; the shape of this papilla can be used to determine the sex of the fish (Figure 1-3) [1]. Fish have two testes of similar size. Many of the features found in the ovaries of fish are usually common to all vertebrates, including the presence of the tunica albuginea and the follicular cells. However, the corpus luteum is found only in the mammals. The eggs of the fish are jelly like and do not have shell: if exposed to the air they will dry out (Figure 1 & 2). The eggs of cartilaginous fish (elasmobranchs) such as skates, sharks, rays and chimaeras are fertilized internally and undergo a wide variety of both internal and external embryonic development [2]. Some cartilaginous fish (rays and sharks) have fins that have been modified to function as a movable sexual intromittent organs which facilitate internal fertilization. These organs are called claspers; they are the posterior part of the pelvic fins and are used to channel the sperm into the female’s cloaca. In the ray finned fish, they are called gonopodiums or andropodiums (Figure 3 & 4) [3]. According to Thierry Lode reproductive strategies, based on the development of the zygote and its interrelationship with the parents, can be classified into five categories: 1. Ovuliparity, 2. Oviparity, 3. Ovo-viviparity, 4. Histotrophic viviparity, and 5. Hemotrophic viviparity [4].
Figure 1:Diagram of a fish egg: A. Vitelline membrane B. Chorion C. Yolk D. Oil globule E. Perivitelline space F. Embryo.
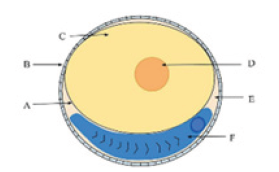
Figure 2:Organs of a female Atlantic cod: 1. Liver 2. Swim bladder 3. Roe 4. Duodenum 5. Stomach 6. Intestine.
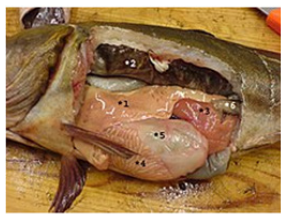
Figure 3:The male mosquito fish has a characteristic anal fin which is called gonopodium and function as an intromittent sexual organ.

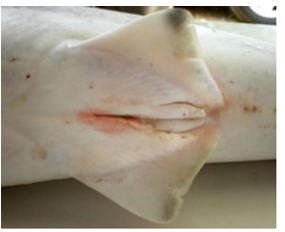
Figure 4:A young male spinner shark with characteristic claspers which function as intromittent organs.
Ovuliparity
Typical examples of ovuliparous fish are goldfish, salmon, cichlids, tuna and eels. Female and male fish shed their eggs and sperm into the surrounding water, and therefore, fertilization takes place outside the mother’s body [4].
Oviparity
In this type of reproduction, the male fish uses his intromittent organ (clasper) to deliver the sperm into the female. Therefore, fertilization occurs internally but the female delivers her embryos into the water. These embryos are called larvae are very different in appearance from juvenile and adult specimens and carry a large yolk sac for their nourishment [4].
Ovo-viviparity
Typical examples of ovo-viviparous fish are angel sharks, guppies and coelacanths. In this type of fertilisation each embryo develops in its own egg and receives little or no nourishment from the mother and relies on the yolk of the egg for its nourishment [4].
Viviparity
Based on the way the offspring receives its nutrients viviparity
is classified into histotrophic and haemotrophic.
a) Histotrophic type (tissue eating). This type is observed
mainly among sharks but is reported also in a few bony fish [4,5].
In particular, grey nurse shark embryos develop by consuming
their smaller and weaker siblings; this phenomenon is called
adelphophagy (adelphos=brother, phagy=eating in Greek).
This has also been reported in Nomorhaphus abrardtii [5]. In
general, embryos of this type develop by obtaining nutrients by
either consuming eggs (oophagy) (oon=egg, in Greek) or their
siblings (zygotes) [4,5].
b) Haemotrophic (blood eating), (haema=blood, in Greek).
Characteristic representatives include the surfperches,
seahorses, lemon shark, split fins and pipefish. Their embryos
develop by obtaining nutrients directly from the parent through
a placenta analogous to mammals [4]. Ichyologists commonly
define ovo-viviparous and viviparous fish as live bearers.
Hermaphrotidism
Fish can be divided to gonochorists and hermaphrodites. The majority are gonochorists. Hermaphroditism has been reported in 14 families of teleost fish [6]. Gonochoristic fish are either male or female and remains that way throughout their lives. Hermaphrodite fish can switch sex, usually from female to male (protogynous hermaphrodite) and more rarely from male to female (protandrous hermaphrodite), [7-9] these fish are called sequential hermaphrodites. Typical examples of protogynous hermaphrodite are groupers and wrasses (Figure 5). Another characteristic of hermaphroditism is that it allows for complex mating systems. In particular, wrasses exhibit three different mating systems: polygynous, lek-like, and promiscuous mating systems; group spawning and pair spawning occur within mating systems [9]. Anemone fish typical of protandrous hermaphroditism live monogamously in an anemone, protected by the anemone stings. The female is typically larger and the male does not need to compete with other males. If the female dies a young male anemone fish moves in and the resident male then turns into a female (Figure 6) [10]. Less commonly fish can be synchronous hermaphrodites, meaning they possess both ovaries and testicles and can function as either sex at one time. The black hamlet is typical of this type [11]. The Kryptolebias marmoratus (mangrove rivulus) is a typical example of self-fertilization; it produces both eggs and sperm simultaneously by meiosis and fertilizes itself when an egg and sperm that it has produced by an internal organ unite inside the fish’s body [12]. The individuals produced by this mode of reproduction can yield highly homozygous lines and are genetically uniform [13,14]. The capacity for selfing in these fish provides the benefit of fertilization assurance at each generation. Further studies are needed to compare the effects of the resulting inbreeding that leads to expression of deleterious recessive alleles with the self-fertilization.
Figure 5:Groupers are characteristic representatives of protogynous hermaphroditism. Grouper changes its sex to male if no male is available.

Figure 6:Anemone fish is protandrous hermaphrodite and live in pairs; if the female dies then a young male moves into anemone and the resident male changes sex from male to female.

Sexual parasitism
The most acceptable explanation for sexual parasitism is that the relative low density of females in deep-sea waters leaves little opportunity for mate choice. So far, the almost unique representative of this mode of sexual production is the anglerfish (Figure 7). These fish are very thinly distributed in deep-sea environments. Therefore, finding a mate is problematic. Variable methods have been described by which the male anglerfish locate female mate. Species with well-developed eyes so by vision and those with developed nostrils by olfaction [15]. When a male encounters a female, he bites into her skin, and releases an enzyme that digests the skin of his mouth and her body, fusing the pair down to blood-vessel level [16]. Thus, the male becomes dependent on the female host for survival by receiving nutrients through now-shared circulatory system, and in return provide sperm to the female continuously. They live and remain reproductively functional as long as the female stays alive and can take part in multiple spawning’s [15].
Figure 7:Haplophryne mollis, female anglerfish is a typical representative of sexual parasitism, the picture demonstrates a female with atrophied males attached.
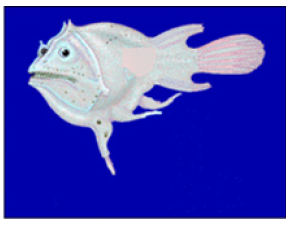
When scientists first started capturing ceratioid anger fish they noticed that many “parasites” attached to female; these were reduced male ceratioid angler fish. This phenomenon indicates that the angler fish use a polyandrous mating system.
Parthenogenesis
Figure 8:Amazon molly. In this fish, first described parthenogenesis.
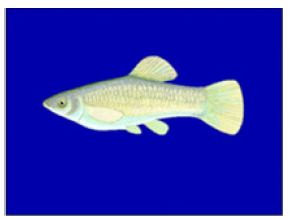
It is defined as a form of sexual reproduction in which growth and development of embryos occur without fertilization. Limitations of this mode of reproduction are low genetic diversity and therefore, increased rate of adverse mutations. A benefit is the possibility of reproduction without the need for a male [17,18]. The first case of this type of reproduction was described in the Amazon molly in 1932 (Figure 8) [17]. It reproduces by gynogenesis. In this type of parthenogenesis, the eggs are stimulated to develop simply by the presence of sperm. The sperms do not contribute any genetic material to offspring [17-21]. It has been confirmed that bonnethead, hammerhead, zebra and blacktip sharks can be reproduced parthenogenetically [19-21].
Internal Gametic association
So far, this unique reproductive mode has only been described in Alcichthys elongates (the Elkhorn fish). The sperm is introduced into the ovary. However, actual sperm-egg fusion does not occur until the eggs have been released into sea [22].
Spawning Strategies
The noun “spawn” means the release or depositing of eggs and sperm into water by aquatic animals. The verb “to spawn” refers to the process of releasing eggs and sperm, and the act of both sexes is called spawning. Most aquatic animals, except aquatic mammals and reptiles, reproduce through the process of spawning. Balon EK in 1975 proposed a classification of the spawning behaviour of fish. This classification is based on how the eggs are fertilized (internal and external spawners), where the eggs are deposited (pelagic or benthic spawners), and whether and how the parents look after the eggs (bearers, guarders and non-guarders) [23].
Non-guarders
In this category are fish that do not protect their eggs and offspring after spawning.
Open substrate spawners:These fish usually spawn in shoals without complex rituals, males outnumber the females and the eggs are scattered into the sea.
Broadcast spawners:This category releases their eggs
and sperm into water for external fertilization and there is no
subsequent parental care [24].
A. Pelagic spawners: This is a type of broadcast spawner.
They spawn mostly near the surface of the open sea. Typical
examples are pelagic fish (pelagos=open sea in Greek) such as
sardines and tuna. Some demersal fish such parrotfish, coral
reef fish and wrasses leave the bottom to spawn pelagically.
The main characteristic of the eggs of the pelagic spawners is
that they contain oil globules or have high water content as a
result of which they are buoyant and thus, are widely dispersed
by the currents. This means that they can drift into unsuitable
areas or can be eaten by pelagic predators. To compensate for
this the females, spawn large numbers of eggs and extend their
spawning periods [24].
B. Benthic spawners: (benthos, bathos=depth of the sea in
Greek) these fish spawn their eggs on the bottom of the sea.
Typical representatives of benthic (demersal) fish are the cod
and flatfish. Typically, these fish spawn without sexual rituals.
Usually, each female is followed by several males who fertilize
the spawn as they released. The eggs can adhere to rocks or
plants. Some eggs by absorbing water can be deposited into the
cracks where they swell and wedge themselves in place [23,24].
Benthic spawners can be subdivided into the following three
categories:
a. Egg scatterers: These are schooling fish which spawn in
groups or pairs. Usually scattering non-adhesive or adhesive
eggs to float to the surface or to fall to the substrate, into plants.
b. Egg depositors: They deposit eggs on a substrate such as
rocks, plants, wood, and tank glass. Usually, they deposit fewer
eggs compared to egg-scatters. This category can be further
divided into those that care for their eggs, and those that do not.
Representatives of this category are cichlids and some catfish.
c. Cavity spawners: deposit their eggs in a cavity or cave.
These fish form pairs and demonstrate advanced brood care
by cleaning and defending their eggs. Consequently, when the
eggs hatch, the fry are often guarded by the parents. Cyprinidae,
catfish and killifish belong to this category [23,24].
C. Brood hiders
Typical representatives of this group are the salmon and trout.
The female digs a nest with her tale into the gravel where she lays
her eggs while the male fertilizes them. This nest is called Redd.
The pair defend the Redd if necessary. Subsequently, the female
buries the nest, and the nest site is abandoned. The annual killifish
deposits its eggs in mud. The eggs remain in a dormant stage until
rain stimulates hatching. A remarkable reproductive strategy is
demonstrated by bitterling. The female deposits her eggs between
the gill filaments of mussels. The male then spawns his sperm into
the mussel’s inhalant water current and fertilization takes place
within the gills of the host (Figure 9).
Figure 9:Bitterlings deposit their eggs in live mussels.

Guarders
Parental care, also called brood care characterizes guarder-fish that care for their eggs and offspring after spawning. This parental care can be expressed in a variety of ways including, brood-pouch egg carrying, nest building, fanning, internal gestation, oral brooding, ectodermal feeding, removal of dead eggs, retrieval of straying fry, external egg carrying, etc. [23-25]. The offspring are usually defended by the males. Territorial behaviour is demonstrated by competition for the best egg-laying sites and consequently, by defending the site where offspring are being looked after [23-25].
Bearers
These fish carry their offspring either internally or externally around with them.
External bearers:Mouth brooders carry their eggs or larvae in their mouth and can be defined as ovophiles or lavrophiles. Ovophile fish lay their eggs in a pit, which is sucked up into the mouth of the female. The eggs then hatch in the mother’s mouth, and the fry remain there for a period of time. Lavrophiles deposit their eggs on substrate and guard them until the eggs hatch. After hatching, the female picks up the fry and keeps them in her mouth until they are able to care for themselves (Figures 10 & 11) [23,24].
Figure 10:A female cichlid mouth-brooding fry which can be seen looking out her mouth.

Figure 11:Male seahorse is a pouch brooder.

Internal bearers:Internal bearers are divided further to facultative (optional) and obligate (by necessity).
Spawning, feeding, and nursery grounds:The readership will further understand the reproductive and spawning strategies of fish if they become familiar with the terms spawning, feeding and nursery grounds. Spawning grounds are defined as the areas of water where fish spawn their eggs. After spawning the fish may or may not migrate to new grounds which become its nursery grounds. Fish which migrate from the sea to a river to spawn their eggs are called anadromous; a typical representative of this category is the salmon. The eel migrates from the river to sea to spawn and is called catadromous. Of note, some fish migrate in a triangle between spawning, feeding and nursery grounds. These triangular migrations might be protective for the offspring because when feeding parents cannot distinguish their own offspring. Typical example is a stock of herrings that have their spawning ground in southern Norway, their nursery ground in northern Norway and their feeding ground in Iceland [26]. Unfortunately, nowadays the construction of weirs and hydroelectric dams obstruct the migration of the fish to spawning grounds.
Sexual Strategies
Sexual strategies in fish can be defined into following four basic mating systems: monogamy, polygyny, polyandry, and polygynandry.
Monogamy or pair spawning
Monogamy or pair spawning, occurs when one female mates with one male exclusively [27]. Usually, conditions such as, low likelihood to find another potential male, small feeding and breeding grounds promote monogamy [27,28]; fishes that both sexes care for the offspring are monogamous and fiercely defend their young against predators, typical representatives are the tropical cichlids [27,28]. In the pipefishes and seahorses, the close timing of hatching the eggs in the brood pouch of a male and the maturation of a new batch of eggs by the female promotes monogamy [27,28].
Polygyny
Polygyny occurs when one male is mating with multiple females and usually defends them from other males [27]. In polygyny, females choose large male capable to defend the breeding site. Another interesting method that used by females to choose the male that they want to be their mate is the method of leks. Leks are called the places where many fish come together where the males display to each other. Based on these displays each female has the possibility to choose mate partner [27,28]. During the mating period, in Lake Malawi, the cichlid Cyrtocara eucinostomous males display together on a lek four kilometres long; then the females, which are mouth brooders, can select which male they want to fertilize their eggs [29].
Polyandry
Polyandry occurs when one female is mating with multiple males. Usually, it occurs in pipefishes when males do the brooding but are not able to manage all the eggs; then the female chooses another male to deposit the eggs [7]. Another characteristic representative is the deep-sea anglerfish. When the male finds a female it bites into her skin, then by releasing an enzyme that digests the skin fusing down to the blood-vessel level. Consequently, his digestive system, heart, brain, and eyes atrophy completely and at the end remains of him nothing more than a pair of gonads, which release sperm in response to hormones in the female’s blood indicating egg release [30].
Polygynandry
Polygynandry occurs when multiple females mate indiscriminately with multiple males. This approach most commonly used by spawning fishes and most probably is the “original fish mating system”. Common representatives are forage fish such as herrings [7].
Discussion
Although, the reproductive methods of fish are divided into two principal methods. In particular, the external more common and characteristic for most of fish reproduction and the less common the internal. On the other hand, the reproductive strategies as proposed by Thierry Lode can be classified into five categories. Namely, 1. Ovuliparity, 2. Oviparity, 3. Ovo-viviparity, 4. Histotrophic viviparity, and 5. Hemotrophic viviparity [4]. Ovoviviparous and viviparous are commonly defined by Ichthyologists as live bearers. The majority of fish are gonochorists. So far, only 14 families of teleost fish have been reported as hermaphrodite [6]. Hermaphroditism allows complex mating systems such as lek-like, polygynous and promiscuous. Physical and social changes usually, trigger the sex change in hermaphrodites [7-9]. Usually, spawning strategies are categorised according to classification proposed by Baron EK [23].
In particular, firstly, they classified as internal and external spawners based on how the eggs are fertilised, secondly, as pelagic and benthic spawners depending where the eggs are deposited and thirdly, as bearers, guarders and non-guarders based on how the parents cared for the fertilised eggs [23,31]. Sexual strategies are divided into four mating systems: monogamy, polygyny, polyandry and polygynandry. Usually, when breeding and feeding grounds are small and when is difficult to find partners monogamy occurs [32]. On the other hand. In polygyny the large male that successfully defend prime breeding sites and females from other males became attractive to females and consequently, mates with many of them [32]. Interestingly, when a single fish cannot brood all the eggs that the female produces then the eggs are deposit in more than one male this phenomenon is called polyandry and is common in pipe fish [7].
Conclusion
Interesting questions that should be addressed by future studies will be the following what is the success rate of the external fertilization in different fishes in a variable environment and how climate change may impact on it. Taking into account that internal fertilization relays on hormones, mating rituals and behavioural factors it will be interesting to study whether pollution, climate change and overfishing impact on fish production. Moreover, further investigation needed about ecological advantages of hermaphroditism. In addition, special attention should be given on the impact of fishing pressure on protogynous hermaphroditism. Further understanding of reproductive strategies may help in more effective fish farming. Usually, monosex farming helps to achieve uniformity of fish size during harvesting and better market price.
Glossary
a) Adelphophagy:Adelphos: brother, -phagy: denoting the practice of eating a specified food.
b) Andropodium or Gonopodium:Anir, andras: male in Greek, -podium: modified extremity as an anal fin used as copulative organ.
c) Benthos:The flora and fauna found on the bottom, or in the bottom sediments of a sea or lake, from Greek benthos (the depths of the sea).
d) Elasmobranch:Any of a subclass of cartilaginous fishes that have five to seven lateral to ventral gill openings on each side and that comprise the sharks, rays, and skates
e) Gonochorist:Separate sex. In biology, gonochorism is a sexual system where there are only two sexes and each individual organism is either male or female.
f) Gynogenesis:Gynogenesis is a process in which the embryo genome originates exclusively from female origin, following embryogenesis stimulation by a male gamete. In contrast, androgenesis is the development of embryos that contain only the male nuclear genetic background.
g) Haemotrophic:(Blood eating) haema: blood, trophy: food in Greek.
h) Hermaphrodite:The term “hermaphrodite” is derived from the Greek mythological God “Hermaphroditos” son of Hermes and Aphrodite, whose body after being merged with nymph Salmakis assumed a form with both male and female attributes.
i) Histotrophic:(Tissue eating) Histos: tissue
j) Monogamous:Monogamy derives from the Greek monos (alone) and gamos (marriage).
k) Parthenogenesis:Reproduction from an ovum without fertilization.
l) Pelagic:Relating to open sea, pelagos: open sea in Greek.
m) Protandrous:First male.
n) Protogynous:First female.
o) Vitellus:Yolk.
p) Zygote:From Greek zygotos “yoked”- joining two things together. The diploid cell resulting from the union of a haploid spermatozoon and ovum.
Acknowledgement
In particular, I would like to express many thanks to my professional diving instructors and buddies Lourens Malan, E Seretakis, Anthony Thomas, and Craig Yon because thanks to their professional lessons and advices, have the possibility to be in contact with the beauty and indefinite wisdom of the kingdom of Poseidon.
References
- Rodrigo JG, Jose EDS, Gilmar BS (2005) Gonadal structure and gametogenesis of Loricaria lentiginosa Isbrucker (pisces, teleostei, siluriformes). Brazilian Journal of Zoology 22(3): 556-564.
- Brito MFG, Bazzoli N (2003) Reproduction of the surubim catfish (pisces, pimelodidae) in the Sao Francisco river, Pirapora region, Minas Gerais, Brazil. Brazilian Archive of Veterinary Medicine and Animal Science 55(5): 624-633.
- Kapoor BG, Khanna B (2004) Ichthyology Handbook. 2004th (Edn.), Springer Publishers, USA, pp. 497-498.
- Lode T (2012) Oviparity or viviparity? That is the question…. Reproductive Biology 12(3): 259-264.
- Meisner A, Burns J (1997) Viviparity in the halfbeak genera dermogenys and nomorhamphus (Teleostei: Hemiraphidae). Journal of Morphology 234(3): 295-317.
- Shapiro DY (1984) Sex reversal and sociodemographics processes in coral reef fish. Fish Reproduction: Strategies and Tactics 1984: 103-106.
- Moyle PB, Cech JJ (2004) Fishes: An introduction to ichthyology. 5th (Edn.), Pearson Publishers, UK, pp. 1-752.
- Robertson DR, Warner RR (1978) Sexual patterns in the labroid fish of the Western Caribbean II, the parrotfish (Scaridae). Simthsonian Contributions to Zoology 255: 1-26.
- Colin PL, Bell LJ (1992) Aspects of the spawning of labrid and scarid fish (Pisces, Labroidei) at Enewetak atoll, Marshall Islands) with notes on other families. Enviromental Biology of Fish 31: 229-260.
- Fricke H, Fricke S (1977) Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266(5605): 830-832.
- Fischer EA, Peterson CW (1987) The evolution of sexuality in the seabasses. Bioscience 37(7): 482-489.
- Sakakura Y, Soyano K, Noakes DLG, Hagiwara A (2006) Gonadal morphology in the self-fertilizing mangrove killifish, Kryptolebias marmoratus. Ichthyological Research 53: 427-430.
- Avise JC, Tatarenkov A (2012) Allard’s argument versus Baker’s contention for the adaptive significance of selfing in hermaphroditic fish. Proc Natl Acad Sci U.S.A. 109(46): 18862-18867.
- Earley RL, Hanninen AF, Fuller A, Garcia MJ, Lee EA (2012) Phenotypic plasticity and integration in the mangrove rivulus (Kryptolebias marmoratus): A prospectus. Integr Comp Biol 52(6): 814-827.
- Pietsch TW (2005) Dimorphism, parasitism, and sex revisited: Modes of reproduction among deep-sea ceratioid anglerfish (Teleostei: Lophiiformes). Ichthyological Research 52: 207-236.
- Gould SJay. Hen’s Teeth and Horse’s toes : Further reflections in natural history. W.W. Norton & Publishers, USA, pp. 1-416.
- Hubbs CL, Hubbs LC (1932) Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science 76(1983): 628-630.
- Vrijenhoek RC, Dawley RM, Cole CJ, Bogart JP (1989) A list of the known unisexual vertebrates. In: Dawley RM, Bogart JP (Eds.), Evolution and ecology of unisexual vertebrates. USA, pp. 19-23.
- Chapman DD, Shivji MS, Louis Ed, Sommer J, Fletcher H, et al. (2007) Virgin birth in hammerhead shark. Biology Letters 3(4): 425-427.
- Robinson DP, Baverstock W, Al-Jaru A, Hyland K, Khazanedhari KA (2011) Annually recurring parthenogenesis in a zebra shark Stegostoma fasciatum. Journal of Fish Biology 79(5): 1376-1382.
- Chapman DD, Firchau B, Shivji MS (2008) Parthenogenesis in large body requiem shark, the blacktip Carcharhinus limbatus. Journal of Fish Biology 73(6): 1473-1477.
- Koya Y, Munehara H, Takano K (2002) Sperm storage and mobility in the ovary of the marine sculpin Alichthys alcicornis (Teleostei: Scorpaeniformes), with internal gametic association. Journal of Experimental Zoology 292(2): 145-155.
- Balon EK (1975) Reproductive guilds of fish: A proposal and definition. Journal of Fish Biology 32(6): 821-864.
- Moyle PB, Cech JJ (2003) Fishes: An Introduction to Ichthyology. 5th (), Benjamin Cummings Publishers, USA.
- (2022) Parental care archived 2016-01-25 at the Wayback machine fish base glossary.
- Dragesund O, Johannessen A, Ultang O (1997) Variation in migration and abundance of Norwegian spring spawning herring (Clupea herengus L). Sarsia 82(2): 97-105.
- Berglund A (1997) Mating systems and sex allocation. In Gordon JJ, (Ed.), Behavioural ecology of teleost fishes. Oxford University Press, UK, pp. 237-265.
- Barlow G (2002) The cichlid fishes: Nature’s grand experiment in evolution. Perseus Publishing, USA, pp. 1-352.
- McKaye (1983) Ecology and breeding behavior of a cichlid fish, Cyrtocara eucinostomous, on a large lek in Lake Malawi, Africa. Environ Biol Fish 8(2): 81-96.
- Pietsch TW (1975) Precocious sexual parasitism in the deep sea ceratoid anglerfish, Cryptopsaras couesi Nature 256(5512): 38-40.
- Balon EK (1984) Patterns in the evolution of reproductive styles in fishes. In GW Potts, Wootton RJ, (Eds.), Fish reproduction: Strategies and tactics. Academic Press, UK, pp. 35-53.
- Berglund A (1997) Mating systems and sex allocation. In: Gordon JJ (Ed.), Behavioural ecology and teleost fishes. Oxford University Press, UK, pp. 237-265.
© 2022 Paschalis Gavriilidis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






