- Submissions

Full Text
Examines in Marine Biology & Oceanography
An Environmentally Friendly Solution Related to the Use of Fish Production Waste to Manufacture New Materials for Biomedicine
Semenycheva LL1, Egorikhina MN1*, Chasova VO1, Valetova NB2, Fukina DG2, Sukhareva AA2, Ostrosablin AN2, Kobyakova II1, Farafontova EA1 and Yu P Rubtsova1
1Federal State Budgetary Educational Institution of Higher Education, The Ministry of Health of the Russian Federation (FSBEI HE PRMU MOH), Russia
2Faculty of Chemistry, Lobachevsky State University of Nizhny Novgorod, Russia
*Corresponding author: Egorikhina Marfa N, Candidate of Biology Science, Federal State Budgetary Educational Institution of Higher Education, Minin and Pozharsky Square 10/1, 603950 Nizhny Novgorod, Russia
Submission: October 28, 2022;Published: November 16, 2022

ISSN 2578-031X Volume5 Issue2
Abstract
New graft copolymers of methyl methacrylate with cod collagen were derived with Azobisisobutyronitrile (AIBN) enhancement. The author studied the copolymer’s composition, molecular weight characteristics, morphological properties, and cytotoxicity, these indicating the successful grafting of synthetic fragments onto the collagen. It was demonstrated that the curves of the copolymer’s Molecular Weight Distribution (MWD) were shifted towards the area of high Molecular Weights (MW), whereas the values of its molecular weight increased relative to the original collagen. At that, the nitrogen content-nitrogen was originally present only in the collagen-was noticeably lower in the copolymer compared to that in pure collagen. A new, structurally-textured organization of the copolymer was demonstrated by comparison of sample sponges of the copolymer’s with the original collagen, using the Scanning Electron Microscopy (SEM). Water-diluted copolymer solutions (~1-2%) showed no cytotoxicity. The results indicate the promising character of this approach for the development of new materials based on graft copolymers of Methyl Methacrylate (MMA) with Cod Collagen (CC) for use in coatings for medical wound healing and in scaffolds.
Keywords:Cod collagen; Methyl methacrylate; AIBN; Graft copolymer; Cytotoxicity; Regenerative medicine
Abbreviations:MWD: Molecular Weight Distribution; MW: Molecular Weights; SEM: Scanning Electron Microscopy; CC: Cod Collagen; GPC: Gel Permeation Chromatography; MM: Molecular Mass; HDFs: Human Dermal Fibroblasts; DMSO: Dimethyl Sulfoxide
Introduction
Studies of environmental problems related to the processing of captured aquatic wildlife are relevant and attract the attention of biologists, chemists and other scientists [1-5]. Wastes from fish product processing, such as skin, bones, air bladders, etc. contain the natural protein, collagen. Isolation of collagen from commercial fish processing solves an important environmental problem of production waste. Furthermore, such waste is a technical renewable raw material. Collagen from fish skin is an especially promising resource for use in food, medical, and cosmetic products [6-11]. For medical purposes, the use of fish collagen is becoming more popular than collagen derived from other animal tissues. Unlike animal skins, there are no known fish skin viruses that can be passed from fish to humans, thus excluding the possibility of corresponding infections. Moreover, fish collagen is 96% similar to human protein, and characterized by hypoallergenic and transdermal properties, and, furthermore, its use does not contradict specific religious considerations [6,12,13]. Due to its biocompatibility, biodegradability and low antigenicity, it is already widely recognized and used in dressings and coatings [12-19]. The development of new technologies requires new materials with certain performance characteristics. In this regard, research is being conducted to create hybrid materials based on collagen and synthetic polymers for regenerative medicine [20-30]. The fundamental distinctive feature of such studies is their development at the intersection of medicine, biology, physics, chemistry, and other sciences.
The purpose of this study is to derive data on the chemical properties and cytotoxicity of graft copolymers using scaffold precursors based on MMA and CC with enhancement by the frequently-used radical initiator AIBN. In previous studies, the author obtained graft copolymers of acrylic monomers based on cod collagen using photocatalysis, either involving rather complex RbTe1.5W0.5O6 oxide enhancement and irradiation with visible light λ=400-700nm at a temperature of 20-25 °C [31-33], or with catalysts based on trialkylboranes [34,35]. The visualized morphology of the lyophilized samples of such graft copolymers allowed their morphological peculiarities to be established and compared to the original collagen, indicating the inclusion of synthetic polymer fragments in the fibrillar collagen structure. This was confirmed by the results of testing the elemental composition and molecular weight characteristics. The low level of cytotoxicity of such samples allows them to be considered as promising precursors for the manufacture of new biomedical products.
Materials and Methods
The following commercially available reagents were used in the study: MMA was preliminarily purified by washing with a 10% sodium hydroxide solution to remove the polymerization inhibitor, then it was purged with water to reach neutrality, dried over calcium chloride, and distilled under vacuum. AIBN preliminarily purified by recrystallization in alcohol and then dried to constant weight was used as the polymerization initiator. Marine collagen was isolated from cod cover tissues by extraction with acetic acid over the period of one day at room temperature, in line with the method used in [36]. Synthesis of the PMMA-CC graft copolymer was performed in an argon flow by mixing MMA and CC in a mass ratio of 1:1 (mass fraction of collagen 2.5%). The resulting emulsion was bubbled with argon for 15 minutes at room temperature in a magnetic stirrer (stirring at 750rpm). Then, the AIBN initiator (1% of the collagen mass) was added into the argon flow, and the emulsion heated to 50 °C while maintaining constant stirring to allow synthesis over a period of 5 hours. Upon completion of the reaction, the mixture was separated into aqueous and organic phases. The resulting aqueous phase, the graft copolymer, was analyzed by Gel Permeation Chromatography (GPC), SEM and elemental (CHNS) analysis, while the cytotoxicity was assessed using an MTT assay.
The molecular weight characteristics of the aqueous solutions were determined by GPC using a Shimadzu CTO20A/20AC highperformance liquid chromatograph (Japan) with an LC-Solutions- GPC software module; the isolation was conducted with a Tosoh Bioscience TSK gel G3000SWxl device with a pore diameter of 5μm; a ELSD-LT II low-temperature light-scattering detector was used, with 0.5M acetic acid solution used as the eluent at a flow rate of 0.8ml/min, while narrowly dispersed dextran samples with a Molecular Mass (MM) range of 1-410kDa (Fluca) were used for calibration. Examination of the surface of the lyophilized PMMACC graft copolymer on AAD was performed using a JSM-IT300 scanning electron microscope (JEOL Ltd., Japan) with an electron probe diameter of up to 5nm (operating voltage of 20 kV) and detectors for low-energy secondary electrons and reverse scattered electrons, in a low vacuum mode used to discharge the samples. Elemental analysis of the samples was conducted after evaporation of the liquid phase to achieve constant weight. CHNS analysis of the samples on a vario EL cube elemental analyzer provided for simultaneous determination of the CHNS(O).
To assess the effects of the graft copolymers on both metabolic activity and cell viability an MTT-assay was performed on Human Dermal Fibroblasts (HDFs) in passages 5-6. An active, morphologically homogeneous culture was used with the cells adhering well to the plastic. The immunophenotype of the cell culture corresponded to the immunophenotype of mesenchymal cells, and the viability of the culture was 95-98 %. The HDF culture used in this study was tested for sterility and infection. The MTT-assay is a colorimetric quantitative test based on the reaction of the watersoluble dye 3-(4,5-dimethylthiazol-2-yl)-2,5-tetrazolium bromide (MTT) being converted by living cells to insoluble formazan, which is purple. This conversion of MTT depends on the metabolic activity of NADP-H-dependent oxidoreductase enzymes, so, live and actively dividing HDFs demonstrate a high degree of MTT conversion, whereas toxically damaged, low metabolic-activity, and dead HDFs show a low degree of MTT conversion. For the test, Dimethyl Sulfoxide (DMSO) is added as a solvent and becomes colored by the intracellular formazan crystals. Quantitative measurement of the color intensity of the studied samples with added DMSO is performed on a tablet reader at a wavelength of 540nm. During the MTT assay the samples were examined in the following dilutions: Control 0:1: Extract 1:0; extract dilutions 1:1; 1:2; 1:4 and 1:8, being the ratio of extract to growth medium, respectively. The level of cytotoxicity was determined by the relative growth intensity (calculated by the following formula: Average optical density in the test culture/average optical density in the controlx100%). Here, 0-1 indicates a lack of cytotoxicity, 2-3 shows moderate cytotoxicity, and 4-5, high cytotoxicity. Characterized Human Dermal Fibroblasts (HDF) in passages 5-6 with a viability of 98-99% were used as the test cultures to study the samples with the MTT assay [16,37].
Result and Discussion
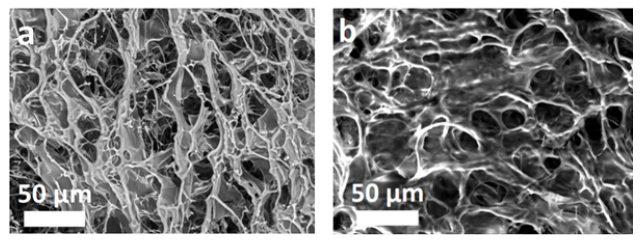
In accordance with the aims of the study, the synthesis of the graft copolymers was conducted with AIBN at a temperature of 50 °C. This temperature allows partial denaturation of the collagen into gelatin [38-41]. However, according to the author’s data [40] and the results of other scientists [12,41-46], this should not significantly impact the biomimetic properties of the final product. CC dissolved in water is a nonionic emulsifier and a macromolecular component onto which the synthetic fragments become grafted. The protein macromolecules (collagen) form radical cores due to interaction of the initiating radical with the amino acid residuesthe structural elements of collagen, with hydrocarbon fragments, as shown in diagrams (1) and (2) - as well as with elements containing hydroxyl groups: (hydroxyproline (~15 %), serine (~4 %), hydroxylysine (~1 %)) [46]. The radical interaction with the monomer results in PMMA becoming grafted onto the collagen (Figure 1). The results of physicochemical analysis of the polymers formed in the aqueous phase confirm the graft formation. A shift of the MWD curves of the reaction product towards a higher molecular weight region in comparison with the initial sample was established by GPC (Figure 2). Overall, there is an increase in the reaction product MW compared with the initial sample, and elemental analysis evidences a decrease in the nitrogen content in the reaction product compared with the native collagen (Figure 3). It is obvious that such changes in the molecular weight parameters and nitrogen content in the copolymer samples are related to the grafting of synthetic fragments (containing no nitrogen) onto the collagen. SEM microphotographs of the reaction products differ significantly from the collagen photographs (Figure 4). Collagen has clear lines of collagen fibers and well-formed pores (Figure 4a), whereas the grafted copolymer has denser contours of the collagen matrix due to the grafted synthetic fragments (Figure 4b).
Figure 1:Scheme of hydroxyl radical’s interaction with a collagen macromolecule on the example of hydroxyproline (1) and alanine (2).
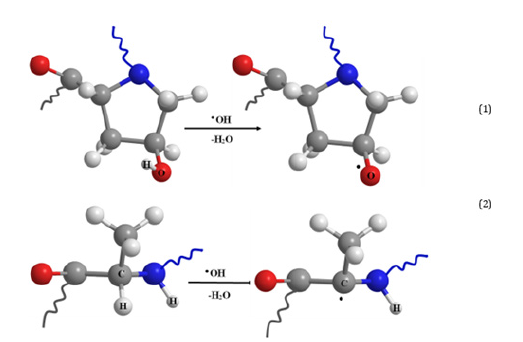
Figure 2:Molecular weight distribution curves of the original collagen and of its AIBN-enhanced PMMA-CC graft copolymer.
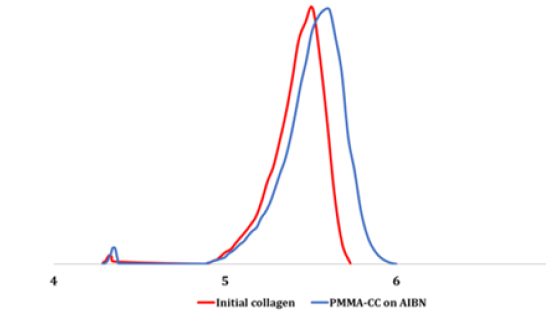
Figure 3:Changes in the nitrogen and molecular weight content in the PMMA-CC graft copolymer compared to the initial collagen.
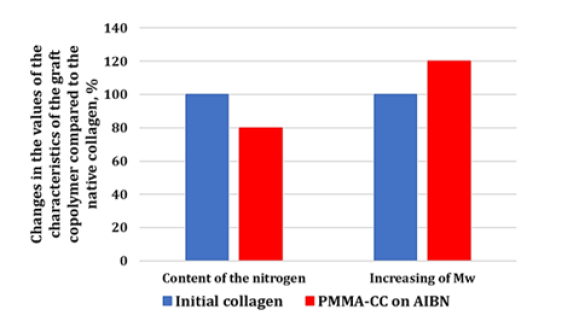
Figure 4:SEM image of lyophilized sponges of initial collagen (a) [31] and PMMA-CC graft copolymer (b).
Medical use of the resulting copolymers as scaffold precursors requires that there is no cytotoxicity. The study of the cytotoxicity in the MTT-assay of the PMMA-CC copolymer samples obtained directly from the aqueous phase of the synthesis (the sample taken with a collagen concentration of 10 % and a ratio of collagen: MMA=1:1) demonstrated the expressed cytotoxicity: here, the relative growth rate of the cell culture was 20%, which corresponded to a cytotoxicity of level 4. When the samples were diluted with growth medium (1:1 and 1:2), the cytotoxicity decreased to level 2. At higher dilutions (1:4,1:8) it was completely eliminated-the cytotoxicity was 0. A similar change in cytotoxicity was seen for the reaction product, after being lyophilized and then washed with chloroform over a period of 10 hours in a Soxhlet extractor (The result, now termed: PMMA-CC-S). Here, it should be emphasized that the PMMA-CC-S solution (a lyophilized sample of PMMA-CC-S dissolved in growth medium) had moderate, level 3, cytotoxicity. With dilution (1:1 and 1:2), the cytotoxicity decreased to level 2. With further dilution (1:4,1:8), the cytotoxicity reached level 1, which indicated an effective lack of cytotoxicity. Based on these findings, one can conclude that diluted solutions of the graft copolymer are appropriate for use as precursors for developing target materials for regenerative medicine.
Conclusion
Thus, the results of the study of the chemical properties and cytotoxicity of graft copolymers formed from methyl methacrylate with cod collagen using radical initiator azobisisobutyronitrile, demonstrate the promising character of the new material for the production of medical wound healing coatings and scaffolds. Previously, graft copolymers of acrylic monomers on cod collagen have been formed using photocatalysis with rather complex enhancement using RbTe1.5W0.5O6 oxide and irradiation with visible light λ=400-700nm [31-33] or with catalysts based on trialkylboranes [34,35]. The advantages of these initiators include their ability to run the processes at room temperature. In this study, PMMA-CC graft copolymers were obtained with an initiator (AIBN) that is widely used in the production of polymeric materials and represents a much more economic approach to the practical implementation of producing safe, new medical materials as demonstrated in this study.
Acknowledgement
The work was carried out within the framework of the program “Priority-2030”, by Minister of Science and Higher Education of the Russian Federation on the equipment of the Collective Usage Center “New Materials and Resource-saving Technologies” (Lobachevsky State University of Nizhny Novgorod).
References
- Saguin KK (2022) Making manila's resource frontier. Urban Ecologies on the Edge, University of California Press, USA, pp. 124-139.
- Hou EJ, Huang CS, Lee YC, Chu HT (2022) Upcycled aquaculture waste as textile ingredient for promoting circular economy. Sustainable Materials and Technologies 31.
- Ghamkhar R, Hartleb C, Rabas Z, Hicks A (2022) Evaluation of environmental and economic implications of a cold-weather aquaponic food production system using life cycle assessment and economic analysis. Journal of Industrial Ecology 26(3): 862-874.
- D’Orbcastel ER, Lutier M, Floc’h E, Ruelle F, Triplet S, et al. (2022) Marine ecological aquaculture: A successful Mediterranean integrated multi‑trophic aquaculture case study of a fish, oyster and algae assemblage. Aquaculture International.
- Chouhan H, Parthasarathy D, Pattanaik S (2017) Urban development, environmental vulnerability and CRZ violations in India: impacts on fishing communities and sustainability implications in Mumbai coast. Environment Development and Sustainability 19(3): 971-985.
- Felician FF, Xia C, Qi W, Xu H (2018) Collagen from marine biological sources and medical applications. Chemistry and Biodiversity 15(5):
- Song E, Kim SY, Chun T, Byun H-J, Lee YM (2006) Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 27(15): 2951-2961.
- Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML (2018) Collagen: A review on its sources and potential cosmetic applications. Journal of cosmetic dermatology 17(1): 20-26.
- Silvipriya KS, Kumar K, Bhat A, Kumar B, John A, et al. (2015) Collagen: Animal sources and biomedical application. Journal of applied pharmaceutical science 5(3): 123-127.
- Peng YY, Stoichevska V, Vashi A, Howell L, Fehr F, et al. (2015) Non-animal collagens as new options for cosmetic formulation. International journal of cosmetic science 37(6): 636-641.
- Bernhardt A, Paul B, Gelinsky M (2018) Biphasic scaffolds from marine collagens for regeneration of osteochondral defects. Marine drugs 16(3): 91-107.
- Oliveira V, Assis CR, Costa BAM, Neri RCA, Monte F, et al. (2021) Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. Journal of Molecular Structure 1224: 129023.
- Toledano M, Toledano-Osorio M, Carrasco-Carmona A, Vallecillo C, Lynch CD, et al. (2020) State of the art on biomaterials for soft tissue augmentation in the oral cavity. Part I: Natural polymers-based biomaterials. Polymers 12(8): 1850.
- Egorikhina MN, Aleynik DY, Rubtsova YP, LevinGY, Charykova IN, et al. (2019) Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioactive Materials 4: 334-345.
- Egorikhina MN, Aleynik DY, Rubtsova YP, Charykova IN (2020) Quantitative analysis of cells encapsulated in a scaffold. MethodsX 7:
- Shanmugam S, Gopal B (2014) Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Applied Surface Science 303: 277-281.
- Fan C, Wang D-A (2017) Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering. Tissue Engineering Part B: Reviews 23(5): 451-461.
- Caliari SR, Burdick JA (2016) A practical guide to hydrogels for cell culture. Nature Methods 13(5): 405-414.
- Egorikhina MN, Rubtsova YP, Charykova IN, Bugrova ML, Bronnikova II, et al. (2017) Biopolymer hydrogel scaffold as an artificial cell niche for mesenchymal stem cells. Polymers 12(11): 2550.
- Shpichka AI, Koroleva AV, Deiwick A, Timashev PS, Semenova EF, et al. (2017) Evaluation of the vasculogenic potential of hydrogels based on modified fibrin. Cell and Tissue Biology 11(1): 81-87.
- Galler KM, Cavender AC, Koeklue U, Suggs LJ, Schmalz G, et al. (2011) Bioengineering of dental stem cells in a PEGylated fibrin gel. Regenerative Medicine 6(2): 191-200.
- Zhang G, Wang X, Wang Z, Zhang J, Suggs L (2006) A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Engineering 12(1): 9-19.
- Ivanov AA, Popova OP, Danilova TI, Kuznetsova AV (2019) Strategies for selecting and use of scaffolds in bioengineering. Biology Bulletin Reviews 139(2): 196-205.
- Zhang D, Wu X, Chen J, Lin K (2018) The development of collagen based composite scaffolds for bone regeneration. Bioactive Materials 3(1): 129-138.
- Kayal TA, Losi P, Pierozzi S, Soldani G (2020) A new method for fibrin-based electrospun/sprayed scaffold fabrication. Scientific Reports 10(1): 5111.
- Sousa RO, Martins E, Carvalho DN, Alves AL, Oliveira C, et al. (2020) Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. Journal of Polymer Research 27: 73.
- Castilho M, Hochleitner G, Wilson W, Rietbergen B, Dalton PD, et al. (2018) Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Scientific Reports 8: 1245.
- Jiang HJ, Xu J, Qiu Z, Ma XL, Zhang ZQ, et al. (2015) Mechanical Properties and cytocompatibility improvement of vertebroplasty PMMA bone cements by incorporating mineralized collagen. Materials 8(5): 2616-2634.
- Vedhanayagam M, Ananda S, Nair BU, Sreeram KJ (2020) Polymethyl Methacrylate (PMMA) grafted collagen scaffold reinforced by PdO-TiO2 Materials Science and Engineering: C 108: 110378.
- Carrion B, Souzanchi MF, Wang VT, Tiruchinapally G, Shikanov A, et al. (2016) The synergistic effects of matrix stiffness and composition on the response of chondroprogenitor cells in a 3D precondensation microenvironment. Advanced Healthcare Materials 5(10): 1192-1202.
- Semenycheva LL, Chasova VO, Fukina DG, Koryagin AV, Valetova NB, et al. (2022) Synthesis of polymethyl-methacrylate–collagen-graft copolymer using a complex oxide RbTe5W0.5O6 photocatalyst. Polymer Science, Series D 15: 110-117.
- Chasova V, Matkivskaya J, Fukina D, Koryagin A, Belaya T, et al. (2021) Features of polymerization of methyl methacrylate using a photocatalyst-the complex oxide RbTe5W0.5O6. Journal of Inorganic and Organometallic Polymers and Materials 31(8): 3572-3583.
- Semenycheva LL, Uromicheva MA, Chasova VO, Fukina D, Koryagin AV, et al. (2022) Synthesis of a graft copolymer of polybutyl acrylate on fish collagen substratum using the RbTe5W0.5O6 complex oxide photocatalyst. Proceedings of Universities. Applied Chemistry and Biotechnology 12(1): 97-108.
- Kuznetsova Y, Gushchina K, Sustaeva K, Mitin A, Egorikhina M, et al. (2022) Grafting of methyl methacrylate onto gelatin initiated by tri-butylborane-2,5-di-tert-butyl-p-benzoquinone system. Polymers 14(16): 3290.
- Uromicheva MA, Kuznetsova YL, Valetova NB, Mitin AV, Semenycheva LL, et al. (2021) Synthesis of grafted polybutyl acrylate copolymer on fish collagen. Proceedings of Universities. Applied Chemistry and Biotechnology 11(1): 16-25.
- Semenycheva LL, Egorikhina MN, Chasova VO, Valetova NB, Kuznetsova YL, et al. (2015) Enzymatic hydrolysis of marine collagen and fibrinogen proteins in the presence of thrombin. Mar Drugs 18(4): 208.
- Yudin VV, Kovylin RS, Baten'kin MA, Kulikova TI, Chesnokov SA, et al. (2020) Visible-light induced synthesis of biocompatible porous polymers from Oligocar-Bonatedimethacrylate (OСM-2) in the presence of dialkyl phthalates. Polymer 192: 122302.
- Schweizer TA, Shambat SM, Haunreiter VD, Mestres CA, Weber A, et al. (2020) Polyester vascular graft material and risk for intracavitary thoracic vascular graft infection. Emerging Infectious Diseases 26(10): 2448-2452.
- Gulevsky AK, Shcheniavsky II (2020) Collagen: Structure, metabolism, production and industrial application. Biotechnologia Acta 13(5): 42-61.
- Chasova V, Semenycheva L, Egorikhina M, Charykova I, Linkova D, et al. (2022) Cod gelatin as an alternative to cod collagen in hybrid materials for regenerative medicine. Macromolecular Research 30(3): 212-221.
- Finer EG, Franks F, Phillips MC, Sugget A (1975) Gel formation from solutions of single chain gelatin. Biopolymers 14(10): 1995-2005.
- Olsen D, Yang C, Bodo M, Chang R, Leigh S, et al. (2003) Recombinant collagen and gelatin for drug delivery. Advanced Drug Delivery Reviews 55(12): 1547-1567.
- Shah BM, Palakurthi SS, Khare T, Khare S, Palakurthi S (2020) Natural proteins and polysaccharides in the development of micro/nano delivery systems for the treatment of inflammatory bowel disease. International Journal of Biological Macromolecules 165: 722-737.
- Cao J, Wang P, Liu Y, Zhu C, Fan D (2020) Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. International Journal of Biological Macromolecules 155: 625-635.
- Cruz-López H, Rodríguez-Morales S, Enríquez-Paredes LM, Villarreal-Gómez LJ, Olivera-Castillo L, et al. (2021) Comparison of collagen characteristic from the skin and swim bladder of Gulf corvina (Cynoscion othonopterus). Tissue and Cell 72: 101593.
- Sun B, Li C, Mao Y, Qiao Z, Jia R, et al. (2021) Distinctive characteristics of collagen and gelatin extracted from dosidicus gigas skin. International Journal of Food Science and Technology 56(7): 3443-3454.
© 2022 Egorikhina MN. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






