- Submissions

Full Text
Examines in Marine Biology & Oceanography
Beaks And Gladii of Oegopsid Squids to Understand Their Trophic Ecology Around American Waters: A Review of The Stable Isotope Analysis
Rosas-Luis Rigoberto*
CONACYT, Tecnológico Nacional de México, I. T. de Chetumal, México
*Corresponding author: Rosas-Luis Rigoberto, CONACYT, Tecnológico Nacional de México, I. T. de Chetumal, Chetumal, Quintana Roo, México
Submission: June 09, 2022;Published: August 16, 2022

ISSN 2578-031X Volume4 Issue5
Abstract
The trophic ecology of squids has been studied using traditional methods of stomach content identification, there are reports of the diet of species of the order Oegopsida that described the prey and consumption of these predators in oceanic waters. Recently, the trophic ecology studies have included the analysis of hard tissues and the δ13C and δ15N to identify changes in the contribution of prey to the diet of squids, and to describe the trophic niche and overlap probability between species. This review analyzed the bibliographic references that used beaks and gladii to understand the trophic ecology of squids that inhabit in the marine waters of America. Results demonstrate that the beak of 14 oegopsid squid families have been included in this kind of analysis. The most studied squid was the Ommastrephid Dosidicus gigas in the Pacific Ocean, but many squid families (13) were analyzed in the Atlantic Ocean. The gladius was included in the hard tissue analysis, and it was reported that in addition to beaks, they are useful to describe the habitat, use of prey for squids, and feeding changes through ontogeny. As squids are fast moving predators, the use of these tissues in the isotope analysis help researchers to identify hot spots in the ocean, because these areas are the main refuges for feeding and for the development of these populations. Sympatric species can be identified by using hard tissue and isotopic analysis, as these tissues recorded the use of habitat and feeding behavior.
Keywords: Isotopes; Oegopsid squids; Trophic niche; Trophic ecology; American oceans
Introduction
Squids are widely distributed in the oceans and are important sources for fisheries [1,2] they are abundant species from the south to the north hemisphere [1]. The relative increase of the squid abundance has been explained by the fishing down of the food web that causes the depletion of stocks of groundfish and other predatory fishes [3], by the expansion of fisheries into new areas such as South America countries [1,4] and by the cephalopod`s adaptability to climate change [5,6]. Thus, it is necessary to find relations or differences in habitat use of these species that favor the creation of management strategies of their fisheries.
The most important squid species caught in the Pacific and Atlantic Oceans correspond to the order Oegopsida: the family Ommastrephidae with Dosidicus gigas (d’Orbigny 1835), Todarodes pacificus (Steenstrup 1880), Illex argentinus (Castellanos, 1960), and the order Myopsida: the family Loliginidae with the genus Doryteuthis and Loligunculla reported in fisheries [1]. These pelagic species are prey of sharks, fish, seabirds, marine mammals, and other squids [7-11] and they are active predators of fish, mollusks, and crustaceans [12-18]. These species are important transfers of energy and mass from basic to high levels of the food chain [19].
The study of distribution, migration, and trophic ecology of squids is difficult because they are fast-moving species, the large area they inhabit, and their horizontal and vertical movements [20]. A proxy to understand movements, use of habitat, and trophic interactions is the stable isotope analysis of carbon and nitrogen [21]. Stable isotopes of nitrogen (δ15N)and carbon (δ13C) have been reported to understand the trophic ecology of squids [17,22-24]. According to McCutchan et al. [25] and Vanderklift & Ponsard [26], the δ15N values show the trophic position of consumers, the δ15N values are higher in consumers relative to consumed prey. In addition, the latitudinal variation of δ15N is relatively low in oceanic waters of the Southern Ocean, and seasonal variation is integrated and buffered throughout the food web, from short-lived phytoplankton to long-lived predators [27]. The (δ13C) values indicate the inshore/pelagic versus offshore/ benthic contribution to food in the diet of consumers [22,23,28,]. In the giant squid Architeuthis dux (Steenstrup 1857), the more positive (δ13C) values indicate the use of waters to the north of the subtropical front to the Kerguelen waters in Southern Oceans [22], and for Doryteuthis gahi (d’Orbigny 1835) the more positive (δ13C) values indicate the use of shallow waters [17].
The isotope analysis in squids has been performed using mantle muscle tissue [17,29-31] beaks [32-36], gladii [37-39], statoliths [40], and eye lenses [41-43]. Each tissue allows the isotope analysis in a determinate time; two months approximately for muscle tissue, and life history for beaks, gladii, and eye lenses [29,41,44]. In general, the use of hard tissue in the isotope analysis resulted in the description of the trophic preferences and ontogenetic changes [40], the habitat characterization [34], spatial variation [45], and the life history [37,38,46]. Beaks and gladii are hard structures composed of chitin, which grow adding chemical components during ontogeny, these components could be related to the food and areas where squids inhabits and growth [22].
Since beaks and gladii are good indicators and recorders of the life history, the use in the isotope analysis resulted adequate to understand the behavior and trophic ecology of squids. Thus, this review revised the published information for oegopsid squids and δ13C and δ15N values in the American Pacific and Atlantic Oceans to resume and compare the data published and evidence research necessities and future challenges in the isotope studies with hard structure of squids.
Materials And Methods
Data
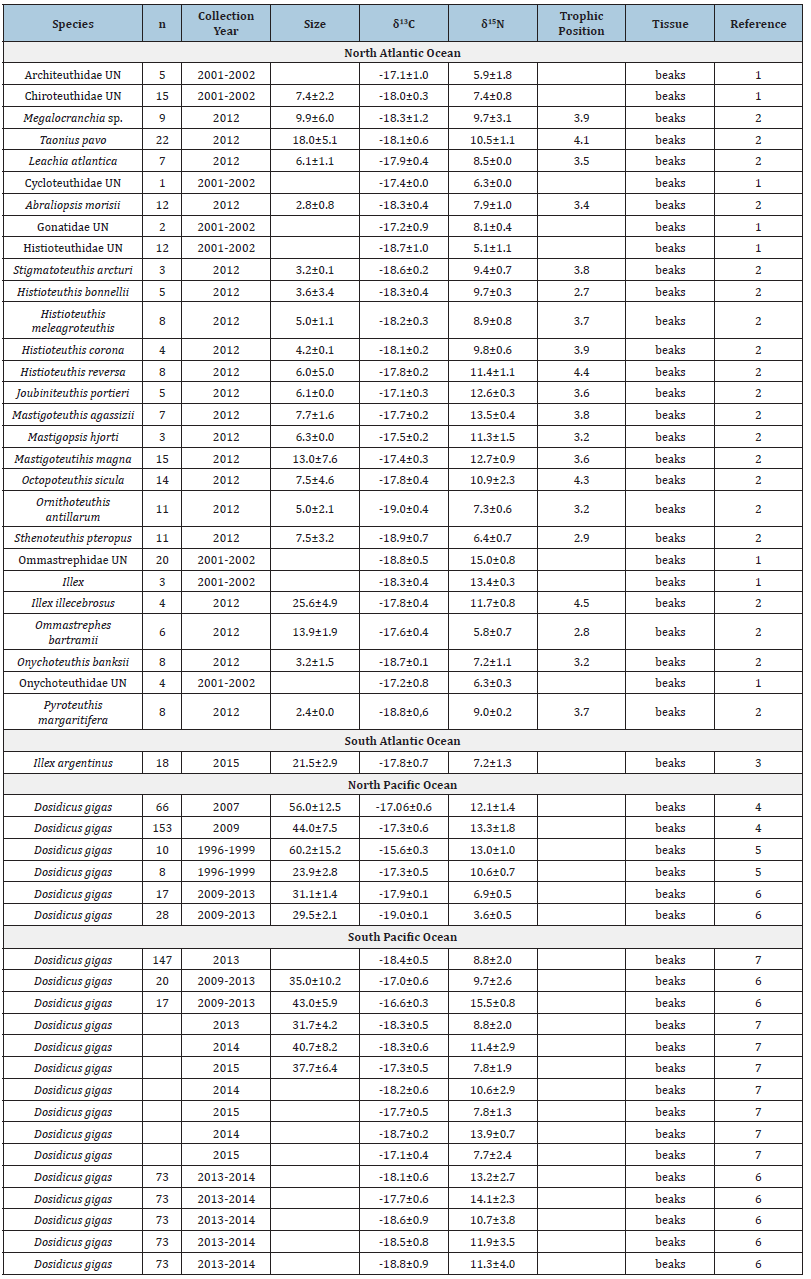
Data related to δ13C and δ15N values of beaks and gladii were compiled from published sources (Table S1 & S2). Data were organized by Atlantic and Pacific oceans. The mean and standard deviation (SD) were calculated for δ13C and δ15N values for each species. For D. gigas δ13C and δ15N values allowed the analysis by size group and by country. For gladii, the myopsid squid D. gahi was included in the analysis to compare with other species.
Supplementary material Table 1:Mean±standard deviation SD of δ13C and δ15N values of squid species. UN means unidentified. Logan and Lutcavage, 2013. 2 Staudinger et al. [32], Queirós et al. [36], Trasviña-Carrillo et al. [40], Ruiz- Cooley et al. [47], Liu et al. [34], Hu [33].
Prey groups
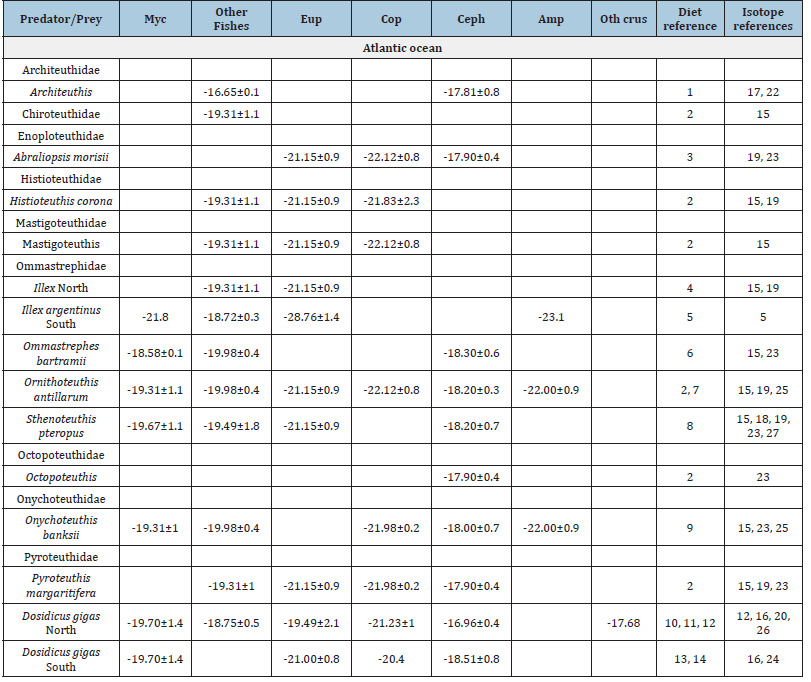
Reports of the feeding habits of squids were used to identify prey species and groups (Table S2 & S3). Prey species were organized in Myctophid fishes, other fishes, Euphausiids, Copepods, Amphipods, and other crustaceans. After the revision, the mean and SD of δ13C and δ15N values of prey were compilated (Table S2 & S3) [9,10, 12,14,15,16,18, 30,33,34,36, 35,40,47-60].
Supplementary material Table 2:Mean±standard deviation SD of C values of prey groups reported for squids, and bibliographic references. Myc is Myctohpid fish, Eup is euphausiid, Cop is copepods, Ceph is cephalopod, Amp is amphipods, and Oth crus is other crustacean. Regueira et al. 2014, Passarella & Hopkins 1991, Guerra-Merrero et al. [51], Squires [59], Rosas-Luis et al. [16], Parry 2006, Arkhipkin et al. [49], Merten et al. [18], Arkhipkin & Nigmatullin [12], Markaida et al. [14], Trasviña-Carrillo et al. [40], Ruiz-Cooley et al. [47], Argüelles et al. [30], Rosas-Luis et al. [48], Olivar et al. [56], Miller TW et al. [55], Loch and Hily [53], Bode et al. [50], Silva et al. [58], Olson et al. [15], López-Ibarra et al. [54], Logan & Lutcavage [32], Staudinger et al. [35], Liu et al. [34], Kaufman et al. [52], Tripp-Valdez et al. [60], Paiva et al. [57].
Supplementary material Table 3:Mean±standard deviation SD of N values of prey groups reported for squids. Myc is Myctohpid fish, Eup is euphausiid, Cop is copepods, Ceph is cephalopod, Amp is amphipods, and Oth crus is other crustaceans.

Isotopic mixing model
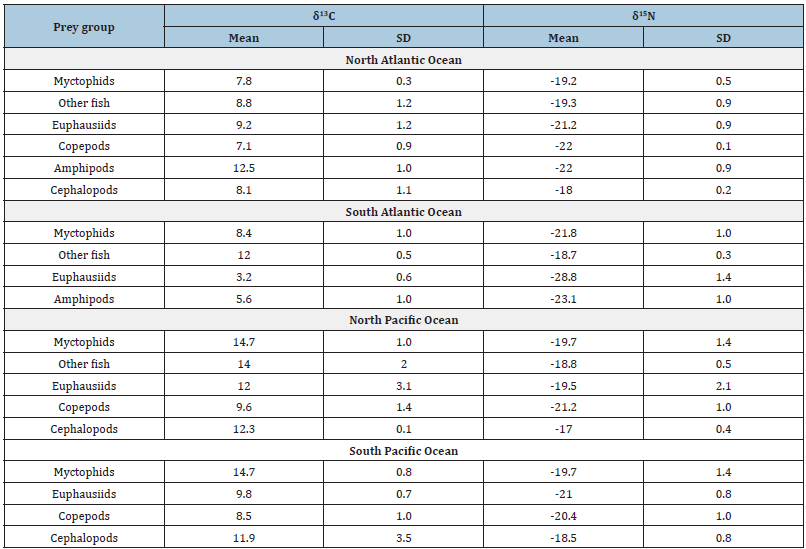
A stable isotope analysis in R (SIAR) was applied [61] to describe the potential contributions of different prey groups to the beak of each squid species. The SIAR model uses δ13C and δ15N values of the consumer and its potential prey group to calculate the assimilation in proportion of each prey in the tissue analyzed. This model runs in the free software R [62] and allows the inclusion of variability in the stable isotope ratios of the predator and the potential prey [61]. To run the SIAR model, values of six prey groups (myctophid fish, other fish, euphausiids, copepods, cephalopods, and amphipods; Table 1) reported as prey in the diet of each species were used (bibliographic references are included in the Table S2). Trophic enrichment factors (TEF) of 3.4±1 for δ15N and 0.4±1.3 for δ13C were used due to the facility to compare these results with previous reports of cephalopod species [63,64]. Potential prey contribution was used to generate a graphical network using the Food Web Designer software [65].
Table 1:Mean±standard deviation SD of C and N values of prey groups reported as components in the diet of squids.
Results
Beaks reports
A total of eight reports of δ13C and δ15N values related to beaks of oegopsid squids in America were accounted, with three related to the Atlantic Ocean and five to the Pacific Ocean (Table S1).
Gladii reports
Four reports of δ13C and δ15N values in gladii, Lorrain et al. [37] and Li et al. [66], Rosas-Luis et al. [38], and Kato et al. [39] were included in the revision. For the Pacific Ocean the squid D. gigas and Ommastrephes bartramii (Lesueur, 1821), and for the Atlantic Ocean I. argentinus and D. gahi were used in isotope reports.
General description of δ13C and δ15N values
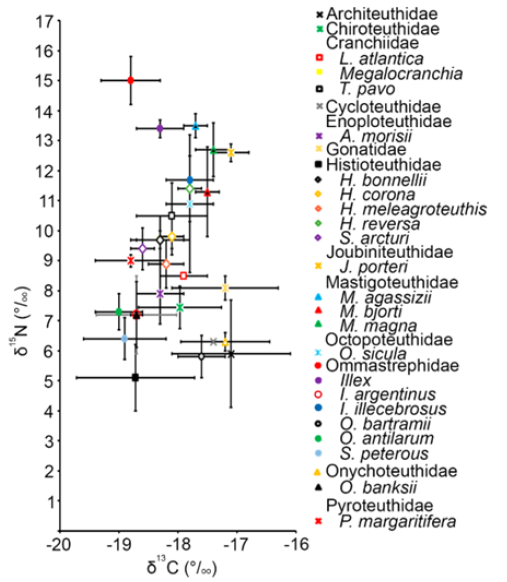
The δ13C and δ15N values of 14 oegopsid squid families were described in the Pacific and Atlantic Oceans around America. The Atlantic Ocean has been better studied with the families, Architeuthidae, Chiroteuthidae, Cranchiidae, Cycloteuthidae, Enoploteuthidae, Gonatidae, Histioteuthidae, Joubiniteuthidae, Mastigoteuthidae, Octopoteuthidae, Ommastrephidae, Onychoteuthidae, and Pyroteuthidae, included in the isotope analysis. For the Pacific Ocean only two families, Ommastrephidae and Ancistrocheiridae, had reports of δ13C and δ15N values. Mean values of δ13C ranged from -19.0 and -17.0% (Figure 1 & 2). Ancistrocheirus lesueurii (d’Orbigny, 1842) and Joubiniteuthis portieri (Joubin, 1916) showed the highest δ13C, D. gigas, Octopoteuthis sicula (Rüppell, 1844), Illex illecebrosus (Lesueur, 1821), Histioteuthis reversa (Verrill, 1880), Leachia atlantica (Degner, 1925), Taonius pavo (Lesueur, 1821), Histioteuthis corona (N. A. Voss & G. L. Voss, 1962), Histioteuthis meleagroteuthis (Chun, 1910), and Megalocranchia sp. (Pfeffer, 1884) showed medium values around -18.0%, and Ornithoteuthis antillarum (Adam, 1957), Sthenoteuthis pteropus (Steenstrup, 1855), Pyroteuthis margaritifera (Rüppell, 1844), and Onychoteuthis banksi (Leach, 1817) showed the lowest values around -19.0% (Figure 1).
Figure 1: Mean and standard deviation of Carbone and Nitrogen isotope values for the lower beak of Oegopsid squids analyzed around America.
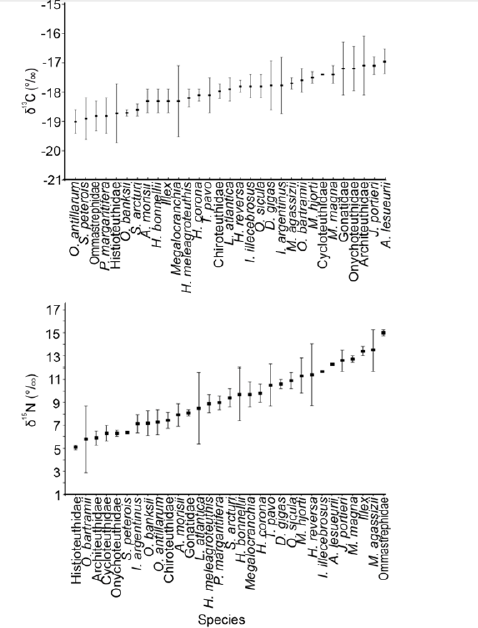
The mean δ15N values ranged from 5 to 15% (Figure 1). Mastigoteuthis agassizii (Verrill, 1881), Illex sp. (Steenstrup, 1880), Mastigoteuthis magna (Joubin, 1913), J. portier, and A. lesueurii showed the highest vales (between 12 and 15%), H. meleagroteuthis, P. margaritifera, Stigmatoteuthis arcturi (Robson, 1948), Histioteuthis bonnellii (Férussac, 1834), Megalocranchia sp. (Pfeffer, 1884), and H. corona showed medium values between 9 and 10%, and O. bartramii and S. pteropus with the lowest values between 5 and 7% (Figure 1).
δ13C and δ15N values of Atlantic Ocean squids
Squids, S. peterous, I. argentinus, Architeuthidae UN, Histioteuthidae UN, Onychoteuthidae UN, Gonatidae UN, showed the highest standard deviation for δ13C and δ15N values (Figure 2), the lowest values were reported for M. agassizii, J. portieri, and S. arcturi (Figure 2). The most positive δ13C and δ15N values were reported for J. portieri, M. magna, M. agassizii; the highest δ13C and the lowest δ15N values were reported for Architeuthidae UN, Onychoteuthidae UN, and Cycloteuthidae UN; the lowest δ13C and the highest δ15N values were reported for Ommastrephidae UN and Illex; the lowest δ13C and the lowest δ15N values were reported for Histioteuthidae UN, S. pterous, O. antillarum, O. banksi, and I. argentinus; the other squid species had medium values (Figure 2). The Ommastrephidae UN segregated from the other species, with the highest δ15N values, and O. bartramii had low δ15N values close to 5% (Figure 2).
Figure 2: Mean and standard deviation of C and N values of cephalopod´s lower beaks of the America´s Atlantic Ocean.
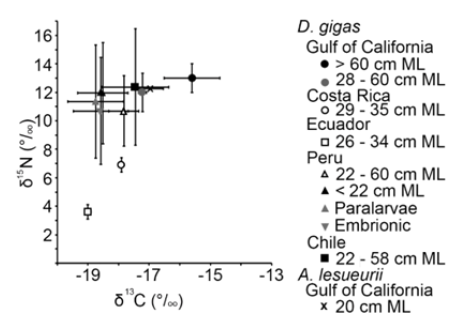
δ13C and δ15N values of Pacific Ocean squids
In the Pacific Ocean, two species were reported for isotope analysis, D. gigas and A. lesueurii. The Gulf of California showed the highest δ13C and δ15N values for D. gigas and A. lesueurii (Figure 3). For D. gigas, Costa Rica and Ecuador showed the lowest values; Chile and Peru showed medium values (Figure 3). The graphical analysis by size allowed the presentation of δ13C and δ15N values for D. gigas, the δ13C values increases according to the size of squids, from embryonic to adult stage (Figure 3). Medium sized squids from Costa Rica and Ecuador showed the lowest δ15N values (Figure 3).
Figure 3: Mean and standard deviation of C and N values of Dosidicus gigas and Ancistrocheirus lesueurii lower beaks of the America´s Pacific Ocean.
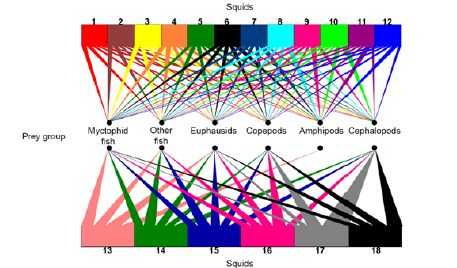
Potential prey contribution to the diet of squids
The amphipods were found as the most important contributor prey group for Architeuthidae UN, Chiroteuthidae UN, A. morisii, H. corona, Octopoteuthis sp., O. antillarum, S. pteropus, O. bartramii, O. banksii, and P. margaritifera (Figure 4). Cephalopods were the most important contributor prey for Mastigoteuthis spp. (Verrill, 1881), Illex spp., and D. gigas (in the Gulf of California, Costa Rica, Peru and Chile) (Figure 4). Euphausiids were the most important contributor prey for I. argentinus (Figure 4). Copepods were the most important contributor prey for D. gigas in Ecuador (Figure 4).
The second most important contributor prey group for A. morisii, H. corona, Octopoteuthis sp., O. antillarum, S. pteropus, O. banksii, P. margaritifera, D. gigas in Ecuador, and Architeuthidae UN, Chiroteuthidae UN, was the myctophid fishes (Figure 4). Amphipods were the second contributor group in importance for Mastigoteuthis spp., Illex spp., and I. argentinus (Figure 4). Other fishes were the second most important contributor prey group for O. bartramii and for D. gigas in the Gulf of California and Costa Rica, and copepods for D. gigas in Peru and Chile (Figure 4).
The third contributor group in importance for A. morisii, H. corona, Mastigoteuthis spp. Octopoteuthis sp., O. antillarum, S. pteropus, Illex spp., O. banksii, P. margaritifera, D. gigas in Ecuador, Architeuthidae UN, and Chiroteuthidae UN, were other fishes (Figure 4). Myctophids were the third contributor group in importance for O. bartramii, I. argentinus, and D. gigas in Costa Rica, Peru and Chile (Figure 4). Euphausiid were the third contributor group in importance for D. gigas in the Gulf of California (Figure 4).
Figure 4: Potential prey contribution to the beak tissue of squid. 1 Architeuthidae UN, 2 Chiroteuthidae UN, 3 Abraliopsis morisii, 4 Histioteuthis corona, 5 Mastigoteuthis spp., 6 Octopoteuthis sp., 7 Ornithoteuthis antillarum, 8 Sthenoteuthis pteropus, 9 Ommastrephes bartramii, 10 Illex spp., 11 Onychoteuthis banksii, 12 Pyroteuthis margaritifera. 13 Illex argentinus, 14 Dosidicus gigas (Gulf of California), 15 Dosidicus gigas (Costa Rica), 16 Dosidicus gigas (Ecuador), 17 Dosidicus gigas (Peru), 18 Dosidicus gigas (Chile).
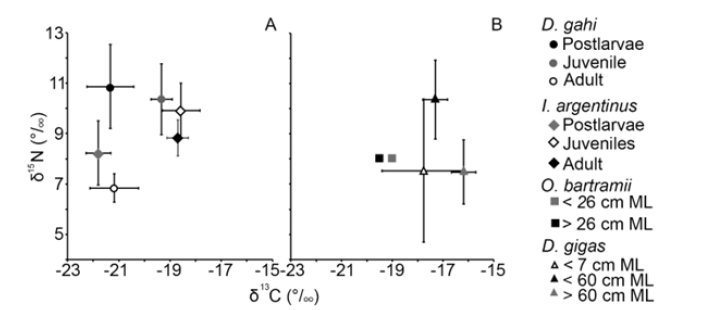
The δ13C and δ15N values in gladii
The δ13C and δ15N values of gladii of three oegopsid squids have been reported. In the south Atlantic Ocean, the isotope values of I. argentinus were reported (Figure 5). A segregation between postlarvae and juveniles and adult stages was reported; the δ13C values were lowest for postlarvae stage (Figure 5). In the same ecosystem, the juvenile stage of D. gahi was found with higher δ13C values than the juvenile and adult stage (Figure 5). The δ15N values of the adult stage for both species was lower than the other stages (Figure 5).
In the Pacific Ocean, the δ13C and δ15N values of O. bartramii and D. gigas were reported. For O. bartramii, the δ13C and δ15N values allowed the analysis between two groups, squids larger than 26cm ML and shorter than 26cm ML (Figure 5), values were similar for both size groups. For D. gigas, the δ13C and δ15N values showed an overlap with large SDs (Figure 5). The δ15N values of the adult stage of D. gigas was lower than the medium size group (Figure 5).
Figure 5: Mean and standard deviation of C and N values of oegopsid squid´s gladius (Illex argentinus, Ommastrephes bartramii, and Dosidicus gigas) and the myopsid quid Doryteuthis gahi. A represents values for the South Atlantic Ocean, and B represents values for the North Pacific - Ommastrephes bartramii and South Pacific - Dosidicus gigas. ML means mantle length. The values of O. bartramii were reported by Kato et al. [39], for D. gigas were reported by Lorrain et al. [37] and Li et al. [66], and for I. argentinus and D. gahi were reported by Rosas-Luis et al. [38].
Discussion
The revision of reports of δ13C and δ15N values, in hard tissues (beaks) of oegopsid squids related to America, highlights the need to increase research in the Pacific Ocean and to include more species in this kind of analysis. Since the first report in 2006 in the North Pacific Ocean for D. gigas [47], only three squids have been used to describe the isotope concentrations for the Pacific Ocean, they include D. gigas, O. bartramii and A. lesueurii, while in the Atlantic Ocean 27 squids have been reported. The highest number of species for the Atlantic were reported by Staudinger et al. [35] and corresponded to the North Atlantic Ocean. In the Pacific Ocean, all the reports used D. gigas as main squid in the analysis. This difference in the number of species is a result of the importance of squid species in fisheries. In the Pacific Ocean, Dosidicus gigas is the main fishery source in the Humboldt Current, in the Gulf of California and California Current, and a well-developed fishery industry is reported for Chile, Peru, Ecuador and Mexico [1,67]. In the Atlantic Ocean, a formal fishery industry is reported for I. argentinus, but no for other species [68]. Despite the need of more research, this revision organized the δ13C and δ15N values of squids and the discussion is based in the differences of values between species reported in the Atlantic Ocean and the values of different size groups and countries where D. gigas and A. lesueurii were captured in the Pacific Ocean.
General description of δ13C and δ15N
Values of δ13C in beaks showed a wide range indicating that squid species may use different δ13C baseline areas as previously reported by Navarro et al. [23]. In this review the results showed that the highest values were reported for A. lesueurii and J. portieri, and the lowest values were for O. antillarum, S. peterois and P. margaritifera. Squid species are voracious organisms looking for food at any time, this behavior could be evidenced with the δ13C values reported for A. lesueurii and J. portieri, which are nerito-oceanic species that can occur in association with seamounts and submarine ridges where upwelling events promote concentration of primary producers [69,70] and coinciding with the assumption that the highest values of δ13C are related to high productive areas in the ocean [63,71]. The lowest δ13C values reported for O. antillarum, S. peterois and P. margaritifera could be related to the oceanic areas where these species dwell, including the epipelagic to mesopelagic zones, and related to general patters for these species that are related to the use of the surface layer between 0 to 100m depths during early and juvenile stages [72]. Medium δ13C values were reported for D. gigas. I. argentinus, T. pavo, L. atlantica, among others, these values could be related to the habitat where these species are caught. For D. gigas, the distribution area included the east Pacific Ocean from the southern Chile to the North in Canada [73], this species is characterized to inhabit areas with high primary production as the Gulf of California, the Humboldt Current and the California current, and probably medium δ13C values vary in function of the vertical and horizontal migrations that these squid perform during day and night and between different areas for feeding as it was reported for D. gigas that moves from the coast to oceanic waters in the east Pacific Ocean [74].
Regarding the δ15N values, the highest values were reported for M. agassizii, M. magna, Illex sp., and the lowest values were reported for O. bartramii and Architeuthidae, indicating that the δ15N source is not related to the size of predator. Large species didn’t show the highest values. These results indicate that the general rule that the size of prey increases with the size of predators does not apply for squids. Reports on the feeding habits of Ommastrephid’s squids documented the variation in prey consumption between small to large squids, highlighting the consumption of small crustaceans in adult stages [74]. As the δ15N is accumulated through the food chain and depends in the source consumed by predators, the use of different feeding sources during squid ontogeny modified the mean δ15N values.
The description of δ13C and δ15N in squids of the American waters confirmed their plasticity, natural behavior and predatory strategies which are variate between species and promoting their interaction in the ecosystem through different trophic levels [23], minimizing competition and allowing their coexistence.
δ13C and δ15N values of Atlantic Ocean squids
An overlap in the δ13C for squids in the Atlantic Ocean is evidenced and related to the area where they were caught (Northwest Atlantic Ocean) for almost all species, the difference was found in the δ15N values. The highest values were showed for the squids of the family Ommastrephidae in the north Atlantic and the lowest values for the families Histioteuthidae and Architeuthidae. It is important to mention that the standard deviation reported for squids allowed the segregation between species, and that the accumulation in δ15N isotopes resulted in the increase of the trophic level of species, but for squids it cannot be related to the size of predators. The trophic levels assumed with the δ15N allowed the categorization of these species as medium trophic level predators, which corresponds with similar trophic levels calculated by Cherel & Hobson [22]. These squids showed a large range of δ15N with small variances that encompasses at least three trophic levels suggesting a greater diversity in their feeding habits. The exploratory analysis using potential prey indicated that segregation in prey consumption can be identified, squids feeding mainly on myctophids, squids feeding mainly on amphipods and euphausiids, and squids feeding mainly on copepods. The feeding partitioning resulted as the main factor that allows the coexistence of squid species. Feeding segregation was previously reported by Cherel & Hobson [22] for squid species in the subantarctic Kerguelen Islands, and results for the American waters coincide with their conclusion asseverating that the main feeding strategy of squids are related to transitions between crustaceans and fish-eaters, with omnivore the dominant strategy. In contrast, the findings in this review indicates that the contribution of Cephalopods in the diet of the Ommastrephids Illex sp. in the Northwest Atlantic Ocean is important. This finding must be analyzed in future research and include a better description of the feeding habits of this and other species, a lack in the trophic ecology studies was identified for the Northwest Atlantic Ocean resulting in a bad description of their feeding sources and predatory strategies.
δ13C and δ15N values of Pacific Ocean squids
Dosidicus gigas is the main species used in the isotope analysis for the Pacific Ocean, this species has been described as a squid with high abundances in areas where the upwelling events favored the enrichment of primary production in the Humboldt Current and in the Gulf of California [75]. Thus, their highest δ13C values in the Gulf of California are good indicators of the feeding activity that this squid maintains with coastal areas in their distribution area [76]. D. gigas is a squid species that consumes prey at deep waters during daytime [48] and during night this species moves to surface water where their diet could include other prey related to coastal and surface waters [48,74], that was evidenced with a wide range of isotope values compared with other species in this review. As A. lesueurii was collected at the same time that the samples of D. gigas [47] it can be assumed that they are inhabiting the same area as isotopes coincided for both species.
Isotope values of D. gigas of Ecuador and Costa Rica segregated from the other samples in Chile, Peru and Mexico, the difference was found in the δ15N values. These samples showed the lowest δ15N values, probably related to the area where these samples were taken (open oceanic waters) [45]. Unfortunately, there are no feeding reports of this species in open waters in these two countries, thus the δ13C and δ15N contributions found in this review must be confirmed with feeding description of the diet, and the confirmation of these prey as important for D. gigas. The findings in this review indicates that the contribution of cephalopods in the diet of D. gigas for the eastern Pacific Ocean is relevant for Peruvian and Chilean waters reinforcing the assumption made by Bruno et al. [77] where crustaceans and cephalopods are the main prey consumed by this species in Chile. This finding must be analyzed in future research because it is coincident with reports of the importance of cannibalism in D. gigas [77,78], and generally it is discarded in trophic ecology studies, enforcing the idea that this species prey on fishes.
The δ13C and δ15N values in gladii
Reports of the δ13C and δ15N descriptions in gladii are scarce in American waters, four reports including I. argentinus [38], O. bartramii [39] and D. gigas [37,52] were found. These reports highlighted the importance to analyze segments of the hard tissue to compare the values through ontogeny. In general, the analysis of gladii allowed the identification of habitat use, in the three oegopsid species analyzed and in the myopsid squid Doriteuthis gahi [44], related to different mature stages. Segments related to the postlarvae stage of I. argentinus had the lowest δ13C values suggesting an habitat with low primary production, and a migratory movement to highly productive areas for juveniles and adults [63,71]. For D. gigas a segregation in the habitat use could not be evidenced, but it is important to note that segments related to the largest size (˃60cm ML) had lower δ15N values that medium size squids, confirming the finding reported in stomach contents [74,76].
Final Considerations
As the isotope analysis in beaks has been applied using a single sample (a mixed sampled) it is not possible to identify the size or the stage that is represented in the isotope values, thus an error can be assumed quantifying the isotopes and relating them to a specific size or stage. The analysis of isotope contribution using prey reported in the diet and generating potential groups of prey allowed the identification of the crustaceans and fishes as main feeding sources for many squid species, but cephalopods resulted as important contributors for the diet of the Ommastrephids Illex sp. in the Northwest Atlantic Ocean and D. gigas for the eastern Pacific Ocean. That highlights the necessity to describe the feeding habits and isotope contribution through ontogeny using a wide range of sizes and increase the isotope analysis along the beaks, gladii, or eye lenses. As the author mentioned before, this finding must be analyzed in future research because it is coincident with reports of the importance of cannibalism in D. gigas [77,78]. Another topic that must be included in future research is the analysis of segments along the beak, this will allow the comparison between stages and the inferences will result in the characterization of movements, feeding habits, and areas where squids inhabit, as it was performed in eye lenses [46] and gladii [38,79].
Acknowledgement
Author thanks the support of the CONACYT Program “Investigadoras e Investigadores por México”.
References
- Markaida U, Gilly WF (2016) Cephalopods of Pacific Latin America. Fisheries Research 173(Part 2): 113-121.
- González AF, Pierce GJ (2021) Advances in the study of cephalopod fisheries and ecosystems. Fisheries Research 242: 105975.
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres-Jr F (1998) Fishing down marine food webs. Science 279(5352): 860-863.
- Boyle P, Rodhouse P (2005) Fishery resources. In: P Boyle, P Roshouse (Edt.), Cephalopods: ecology and fisheries. Blackwekk Science LTD, Oxford, Pp. 309-365.
- Gilly WF, Beman JM, Litvin SY, Robison BH (2013) Oceanographic and biological effects of shoaling of the oxygen minimum zone. Annual Review of Marine Science 5: 393-420.
- Stewart JS, Hazen EL, Bograd SJ, Byrnes JE, Foley DG, et al. (2014) Combined climate- and prey-mediated range expansion of Humboldt squid (Dosidicus gigas), a large marine predator in the California Current System. Glob Chang Biol 20(6): 1832-1843.
- Arizmendi-Rodríguez DI, Abitia-Cárdenas L, Galván-Magaña F, Trejo-Escamilla I (2006) Food habits of sailfish Istiophorus platypterus off Mazatlán, Sinaloa, Mexico. Bulleting of Marine Science 79(3) 777-791.
- Galván-Magaña F, Polo-Silva C, Hernández-Aguilar SB, Sandoval-Londoño A, Ochoa-Díaz MR, et al. (2013) Shark predation on cephalopods in the Mexican and Ecuadorian Pacific Ocean. Deep-Sea Research II 95: 52-62.
- Logan JM, Toppin R, Smith S, Galuardi B, Porter J, et al. (2013) Contribution of cephalopod prey to the diet of large pelagic fish predators in the central North Atlantic Ocean. Deep-Sea Research Part II: Topical Studies in Oceanography 95: 74-82.
- Staudinger MD, Juanes F, Salmon B, Teffer AK (2013) The distribution, diversity, and importance of cephalopods in top predator diets from offshore habitats of the North west Atlantic Ocean. Deep-Sea Research Part II: Topical Studies in Oceanography 95: 182-192.
- Rosas-Luis R, Navarro J, Loor-Andrade P, Forero MG (2017b) Feeding ecology and trophic relationships of pelagic sharks and billfishes coexisting in the central eastern Pacific Ocean. Marine Ecology Progress Series 573: 191-201.
- Arkhipkin AI, Nigmatullin N (1997) Ecology of the oceanic squid Onychoteuthis banksii and the relationship between the genera Onichoteuthis and Chaunoteuthis (Cephalopoda: Onichoteuthidae). Journal of the Marine Biological Association of the United Kingdom 77: 839-869.
- Arkhipkin AI, Laptikhovsky VV, Nigmatullin CH, Bestyatykh AV, Murzov SA (1998) Growth, reproduction and feeding of the tropical squid Ornithoteuthis antillarum (Cephalopoda: Ommastrephidae) from the central-east Atlantic. Scientia Marina 62(3): 273 -288.
- Markaida U, Gilly WF, Salinas-Zavala CA, Rosas-Luis R, Booth JAT (2008) Food and feeding of jumbo squid Dosidicus gigas in the central Gulf of California During 2005-2007. CalCOFI Reports 49: 90-104.
- Olson RJ, Popp BN, Graham BS, López-Ibarra GA, Galván-Magaña F, et al. (2010) Food-web inferences of stable isotope spatial patterns in copepods and yellowfin tuna in the pelagic eastern Pacific Ocean. Progress in Oceanography 86(1-2): 124-138.
- Rosas-Luis R, Chompoy-Salazar L (2016) Description of food sources used by jumbo squid Dosidicus gigas (D’Orbigny, 1835) in Ecuadorian waters during 2014. Fisheries Research 173(Part 2): 139-144.
- Rosas-Luis R, Navarro J, Sánchez P, Del Río JL (2016) Assessing the trophic ecology of three sympatric squid in the marine ecosystem off the Patagonian Shelf by combining stomach content and stable isotopic analyses. Marine Biology Research 12(4): 402-411.
- Merten V, Christiansen B, Javidpour J, Piatkowski U, Puebla O, et al. (2017) Diet and stable isotope analyses reveal the feeding ecology of the orangeback squid Sthenoteuthis pteropus (Steenstrup 1855) (Mollusca, Ommastrephidae) in the eastern tropical Atlantic. PLoSOne 12(12): e0189691.
- Rosas-Luis R, Salinas-Zavala C, Koch V, Del Monte-Luna P, Morales-Zárate V (2008) Importance of jumbo squid Dosidicus gigas (Orbigny, 1835) in the pelagicecosystem of the central Gulf of California. Ecological Modelling 218(1-2): 149-161.
- Semmens JM, Pecl GT, Gillanders BM, Waluda CM, Shea EK, et al. (2007) Approaches to resolving cephalopod movement and migration patterns. Reviews in Fish Biology and Fisheries 17: 401-423.
- Navarro J, Sáez-Liante R, Albo-Puigserver M, Coll M, Palomera I (2017) Feeding strategies and ecological roles of three predatory pelagic fish in the western Mediterranean Sea. Deep-Sea Research Part II: Topical Studies in Oceanography 140: 9-17.
- Cherel Y, Hobson KA (2005) Stable isotopes, beaks and predators: a new tool to study the trophic ecology of cephalopods, including giant and colossal squids. Proc Biol Sci 272(1572): 1601-1607.
- Navarro J, Coll M, Somes CJ, Olson RJ (2013) Trophic niche of squids: Insights from isotopic data in marine systems world wide. Deep-Sea Research Part II: Topical Studies in Oceanography 95: 93-102.
- Martínez-Baena F, Navarro J, Albo-Puigserver M, Palomera I, Rosas-Luis R (2016) Feeding habits of the short-finned squid Illex coindetii in the western Mediterranean Sea using combined stomach content and isotopic analysis. Journal of the Marine Biological Association of the United Kingdom 96(6): 1235-1242.
- Mccutchan JH, Lewis WM, Kendall C, Mcgrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulphur. Oikos 102(2): 378-390.
- Vanderklift A, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichments: a meta-analysis. Oecologia 136(2): 169-182.
- Cherel Y, Ducatez S, Fontaine C, Richard P, Guinet C (2008) Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands. Marine Ecology Progress Series 370: 239-247.
- Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. Journal of Animal Ecology 63: 786-798.
- Ruiz-Cooley RI, Gendron D, Aguíñiga S, Mesnick S, Carriquiry JD (2004) Trophic relationships between sperm whales and jumbo squid using stable isotopes of C and N. Marine Ecology Progress Series 277: 275-283.
- Argüelles J, Lorrain A, Cherel Y, Graco M, Tafur R, et al. (2012) Tracking habitat and resource use for the jumbo squid Dosidicus gigas: a stable isotope analysis in the Northern Humboldt Current System. Marine Biology 159: 2105-2116.
- Gong Y, Li Y, Chen X, Yu W (2020) Trophic Niche and Diversity of a Pelagic Squid (Dosidicus gigas): A Comparative Study Using Stable Isotope, Fatty Acid, and Feeding Apparatuses Morphology. Frontiers in Marine Science 7: 642.
- Logan JM, Lutcavage ME (2013) Assessment of trophic dynamics of cephalopods and large pelagic fishes in the central North Atlantic Ocean using stable isotope analysis. Deep-Sea Research Part II: Topical Studies in Oceanography 95: 63-73.
- Hu G, Boenish R, Gao C, Li B, Chen X, et al. (2019) Spatio-temporal variability in trophic ecology of jumbo squid (Dosidicus gigas) in the southeastern Pacific: Insights from isotopic signatures in beaks. Fisheries Research 212: 56-62.
- Liu B, Chen X, Qian W, Jin Y, Li J (2019) δ13C and δ15N in Humboldt squid beaks: understanding potential geographic population connectivity and movement. Acta Oceanologica Sinica 38: 53-59.
- Staudinger MD, Dimkovikj VH, France CAM, Jorgensen E, Judkins H, et al. (2019) Trophic ecology of the deep-sea cephalopod assemblage near Bear Seamount in the Northwest Atlantic Ocean. Marine Ecology Progress Series 629: 67-86.
- Queirós JP, Phillips RA, Baeta A, Abreu J, Xavier JC (2019) Habitat, trophic levels and migration patterns of the short-finned squid Illex argentinus from stable isotope analysis of beak regions. Polar Biology 42: 2299-2304.
- Lorrain A, Arguelles J, Alegre A, Bertrand A, Munaron J, et al. (2011) Sequential isotopic signature along gladius highlights contrasted individual foraging strategies of Jumbo Squid (Dosidicus gigas). PlosOne 6(7): e22194.
- Rosas-Luis R, Navarro J, Martínez-Baena F, Sánchez P (2017a) Differences in the trophic niche along the gladius of the squids Illex argentinus and Doryteuthis gahi based on their isotopic values. Regional Studies in Marine Science 11: 17-22.
- Kato Y, Sakai M, Nishikawa H, Igarashi H, Ishikawa Y, et al. (2016) Stable isotope analysis of the gladius to investigate migration andtrophic patterns of the neon flying squid (Ommastrephes bartramii). Fisheries Research 173(2): 169-174.
- Trasviña-Carrillo LD, Hernández-Herrera A, Torres-Rojas YE, Galván-Magaña F, Sánchez-González A, et al. (2018) Spatial and trophic preferences of jumbo squid Dosidicus gigas (D´Orbigny, 1835) in the central Gulf of California: ecological inferences using stable isotopes. Rapid Communications in Mass Spectrometry 32(15): 1225-1236.
- Onthank KL (2013) Exploring the life histories of cephalopods using stable isotope analysis of an archival tissue [dissertation]. Pullman, Witman County, Washington State University, USA.
- Meatha B, Peeblesa EB, Seibela BA, Judkins H (2019) Stable isotopes in the eye lenses of Doryteuthis plei (Blainville 1823): Exploring natal origins and migratory patterns in the eastern Gulf of Mexico. Continental Shelf Research 174: 76-84.
- Xu W, Chen X, Li B, Chen Y, Huan M, et al. (2019) Inter-individual variation in trophic history of Dosidicus gigas, as indicated by stable isotopes in eye lenses. Aquaculture and Fisheries 4(6): 261-267.
- Rosas-Luis R, Navarro J, Loor-Andrade P, Forero MG (2017b) Feeding ecology and trophic relationships of pelagic sharks and billfishes coexisting in the central eastern Pacific Ocean. Marine Ecology Progress Series 573: 191-201.
- Liu B, Fanga Z, Chen X, Chen Y (2015) Spatial variations in beak structure to identify potentially geographic populations of Dosidicus gigas in the Eastern Pacific Ocean. Fisheries Research 164: 185-192.
- Liu B, Xua W, Chena X, Huana M, Liu N (2020) Ontogenetic shifts in trophic geography of jumbo squid, Dosidicus gigas, inferred from stable isotopes in eye lens. Fisheries Research 226: 105507.
- Ruiz-Cooley RI, Markaida U, Gendron D, Aguíñiga S (2006) Stable isotopes in jumbo squid (Dosidicus gigas) beaks to estimate its trophic position: comparison between stomach contents and stable isotopes. Journal of the Marine Biological Association of the United Kingdom 86(2): 437-445.
- Rosas-Luis R, Tafur-Jiménez R, Alegre-Norza AR, Castillo-Valderrama PR, Cornejo-Urbina RM, et al. (2011) Trophic relationships between the jumbo squid (Dosidicus gigas) and the lightfish (Vinciguerria lucetia) in the Humboldt Current System off Peru. Scientia Marina 75(3): 549-557.
- Arkhipkin AI, Laptikhovsky VV, Nigmatullin CH, Bestyatykh AV, Murzov SA (1998) Growth, reproduction and feeding of the tropical squid Ornithoteuthis antillarum (Cephalopoda: Ommastrephidae) from the central-east Atlantic. Scientia Marina 62(3): 273 -288.
- Bode A, Olivar MP, Hernández-León S (2021) Natural abundance of stable isotopes in micronekton fish species from the BATHYPELAGIC cruise (North Atlantic, June 2018): carbon and nitrogen values in bulk tissues and nitrogen values in amino acids. PANGAEA.
- Guerra-Merrero A, Hernández-García V, Sarmiento-Lezcano A, Jiménez-Alvarardo D, Santana-Del Pino A et al. (2020) Migratory patterns, vertical distributions and diets of Abralia veranyi and Abraliopsis morisii (Cephalopoda: Enoploteuthidae) in the eastern North Atlantic. Journal of Molluscan Studies 86(1): 27-34.
- Kaufman MR, Gradinger RR, Bluhm BA, O Brien DM (2008) Using stable isotopes to assess carbon and nitrogen turnover in the Arctic sympagic amphipod Onisimus litoralis. Oecologia 158(1): 11-22.
- Loch FL, Hily C (2005) Stable carbon and nitrogen isotope analysis of Nephrops norvegicus / Merluccius merluccius fishing grounds in the Bay of Biscay (Northeast Atlantic). Canadian Journal of Fisheries and Aquatic Sciences 62(1): 123-132.
- López-Ibarra GA, Bode A, Hernández-Trujillo S, Zetina-Rejón MJ, Arreguín-Sánchez F (2018) Trophic position of twelve dominant pelagic copepods in the eastern tropical Pacific Ocean. Journal of Marine Systems 187: 13-22.
- Miller TW, Bosley KL, Shibata J, Brodeur RD, Omori K, et al. (2013) Contribution of prey to Humboldt Current, revealed by stable isotope analyses. Marine Ecology Progress Series 477: 123-134.
- Olivar MP, Bode A, López-Pérez C, Hulley PA, Hernández-Leon S (2019) Trophic position of lanternfishes (Pisces: Myctophidae) of the tropical and equatorial Atlantic estimated using stable isotopes. ICES Journal of Marine Science 76(3): 649-661.
- Paiva V, Xavier J, Geraldes P, Ramirez I, Garthe S, et al. (2010) Foraging ecology of Cory’s shearwaters in different oceanic environments of the North Atlantic. Marine Ecology Progress Series 410: 257-268.
- Silva MA, Borrell A, Prieto R, Gauffier P, Bérubé M, et al. (2019) Stable isotopes reveal winter feeding in different habitats in blue, fin and sei whales migrating through the Azores. Royal Society Open Science 6: 181800.
- Squires HJ (1966) Feeding habits of the squid, Illex illecebrosus. Nature 211: 1321.
- Tripp-Valdez A, Galván-Magaña F, Ortega-García S (2014) Food sources of common dolphinfish (Coryphaena hippurus) based on stomach content and stable isotopes analyses. Journal of the Marine Biological Association of the United Kingdom 95(3): 579-591.
- Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLOS ONE 5: e9672.
- R Development Core Team (2021) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna.
- Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83(3): 703-718.
- Golikov AV, Ceia FR, Sabirov RM, Ablett JD, Gleadall IG, et al. (2019) The first global deep-sea stable isotope assessment reveals the unique trophic ecology of Vampire Squid Vampyroteuthis infernalis (Cephalopoda). Scientific Reports 9: 19099.
- Sint D, Traugott M (2016) Food web designer: a flexible tool to visualize interaction networks. J Pest Sci 89: 1-5.
- Li Y, Gonga Y, Zhang Y, Chen X (2017) Inter-annual variability in trophic patterns of jumbo squid (Dosidicus gigas) off the exclusive economic zone of Peru, implications from stable isotope values in gladius. Fisheries Research 187: 22-30.
- Morales-Bojórquez E, Pacheco-Bedoya JL (2016) Jumbo squid Dosidicus gigas: a new fishery in Ecuador. Reviews in Fisheries Science & Aquaculture 24(1): 98-110.
- Arkhipkin AI, Rodhouse PGK, Pierce GJ, Sauer W, Sakai M, et al. (2015) World Squid Fisheries. Reviews in Fisheries Science & Aquaculture 23(2): 92-252.
- Furuya K, Odate T, Taguchi K (1995) Effects of a seamount on phytoplankton production in the western Pacific Ocean. In: Sakai H, Nozaki Y (Edt.), Biogeochemical Processes and Ocean Flux in the Western Pacific. Terra Scientific Publishing Company, Tokyo, Japan, Pp. 255-273.
- Fernandez-Arcaya U, Ramirez-Llodra E, Aguzzi J, Allcock L, Davies JS, et al. (2017) Ecological Role of Submarine Canyons and Need for Canyon Conservation: A Review. Frontiers in Marine Science 4(5): 1-26.
- France RL, Peters RH (1997) Ecosystem differences in the trophic enrichment of 13C in aquatic food webs. Canadian Journal of Fisheries and Aquatic Sciences 54(6): 1255-1258.
- Roper CFE, Nigmatullin C, Jereb P (2010) Family Ommastrephidae. In: Jereb P, Roper CFE (Eds.), Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Myopsid and Oegopsid Squids, 2: 269-347. FAO Species Catalogue for Fishery Purposes, Rome, Italy.
- Zeidberg LD, Robison BH (2007) Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proceedings of the National Academy of Sciences 104(31): 12948-12950.
- Bazzino G, Gilly WF, Markaida U, Salinas-Zavala CA, Ramos-Castillejos J (2010) Horizontal movements, vertical-habitat utilization and diet of the jumbo squid (Dosidicus gigas) in the Pacific Ocean off Baja California Sur, Mexico. Progress in Oceanography 86(1-2): 59-17.
- Nigmatullin CM, Nesis KN, Arkhipkin AI (2001) A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fisheries Research 54(1): 9-19.
- Markaida U, Gilly WF, Salinas-Zavala CA, Rosas-Luis R, Booth JAT (2008) Food and feeding of jumbo squid Dosidicus gigas in the central Gulf of California During 2005-2007. CalCOFI Reports 49: 90-104.
- Bruno C, Cornejo CF, Riera R, Ibáñez CM (2021) What is on the menu? Feeding, consumption and cannibalism in exploited stocks of the jumbo squid Dosidicus gigas in south-central Chile. Fisheries Research 233: 105722.
- Ibarra-García LI, Camarillo-Coop S, Salinas-Zavala CA (2014) Cannibalism assessment of jumbo squid Dosidicus gigas from the Gulf of California. Hidrobiológica 24(1): 51-56.
- Hu GY, Li JH, Liu BL, Liu N, Chen XJ (2021) Trophic ecology of Humboldt squid (Dosidicus gigas) in the oceanic waters off Ecuador: insight from isotopic signature analysis on beaks. Marine and Freshwater Research 73(4): 469-477.
© 2022 Rosas-Luis Rigoberto. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






