- Submissions

Full Text
Examines in Marine Biology & Oceanography
Deformities in Cirrhinus mrigala (Ham. -Buch.) Cultured in Fish Ponds of District Kathua, Jammu Region, of the Union Territory of Jammu & Kashmir
Nazir M1, Wangano AK1 and Dutta SPS2*
1Department of Limnology and Environmental Sciences, Barkatullah University, India
2Department of Environmental Sciences, University of Jammu, India
*Corresponding author: Dutta SPS, Professor Emeritus, Department of Environmental Sciences, University of Jammu, Jammu, 180006, Jammu & Kashmir, India
Submission: March 17, 2022;Published: August 05, 2022

ISSN 2578-031X Volume4 Issue5
Abstract
Seventeen adult anomalous specimens of Cirrhinus mrigala (Ham.-Buch.)were seen among fish collections from freshwater fish ponds of Kathua district and have been elaborated. Morphological deformities observed are truncated body with dorsal protuberance (twelve specimens), post dorsal truncated body with a depression on left side (one specimen), curved trunk (one specimen), injured truncated caudal peduncle (two specimens) and highly truncated deflexed caudal peduncle and aberrant anal and caudal fin (one specimen). Radiological study has revealed various vertebral anomalies viz. lordosis, kyphosis, scoliosis, ankylosis, trough, vertebral overlapping and duplication. Multiple factors known to induce anomalies in fishes have also been described.
Keywords: Cirrhinus mrigala; Morphological and skeletal deformities; Kathua; Fish ponds; Multiple factors
Introduction
Cirrhinus mirgala, a major Indian carp, is well distributed in rivers, streams and ponds of district Kathua of Jammu region [1-2]. In district Kathua, in order to increase fish production in inland waterbodies, various ponds have been renovated, under Central Govt. sponsored schemes, and stocked with Indian, including C. mrigala, and exotic carps. During the present survey seventeen deformed specimens of C. mrigala (Ham.-Buch.) were seen along with normal specimens and have been described. Teratology in natural and reared C. mrigala has earlier been reported [3-11] and their frequencies are rapidly increasing in cultured waters. These malformed fishes have unattractive shape, poor growth, low survival percentage and low price in fish market and are a big loss to the aquaculturists. There is no immediate record of human health problems by consumption of deformed fishes but due to rising water pollution may be a serious concern in future. The purpose of this study is to identify/diagnose the deformities and there causes among pond cultured fishes in Jammu region.
Material And Methods
Seventeen deformed specimens of C. mrigala were noticed among fish collections made by fishermen from fish ponds, Kathua district. These were studied for morphological characteristics, parasitic infestation and photographed. For radiological observations, deformed and normal specimens were X-rayed (AGFA). For water quality analysis samples from various fish ponds were collected in plastic containers and analyzed by standard methods [12].
Observations
Normal Cirrhinus mrigala (Ham. Buch.)
Body is streamlined. Dorsal fin installation is more towards snout tip than caudal fin base. There is a wide space between longest pectoral fin ray and pelvic fin origin, pelvic fin ray and anal fin origin and anal fin ray and caudal fin base (Figure 1a). X-ray analysis has revealed the presence of 35 amphicoleous vertebrae, after complex vertebrae, in streamlined vertebral column. Air bladder is bilobed, anterior lobe is globular and large and posterior lobe elongated (Figure 1b).
Deformed fishes
Deformities observed in seventeen adult specimens of Cirrhinus mrigala (Ham.-Buch.) reared in fish ponds of Kathua district, Jammu region, are described in Table 1 (Figure 2.1a - 2.17b).
Figure 1a: Normal Cirrhinus mrigala.

Figure 1b: Xray photograph of normal Cirrhinus mrigala.

Table 1:Morphological, vertebral and air bladder characteristics of deformed Cirrhinus mrigala collected from fish ponds in Kathua district.

Figure 2.1a: Cirrhinus mrigala showing minor dorsal protuberance.

Figure 2.1b: X ray photograph of Cirrhinus mrigala showing Vertebral kyphosis.
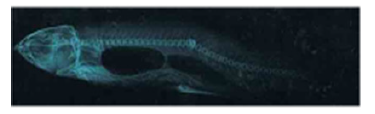
Figure 2.2a: Cirrhinus mrigala showing dorsal protuberance and displacement of pectoral and fins.

Figure 2.2b: X ray photograph of Cirrhinus mrigala showing vertebral Lordosis and kyphosis.
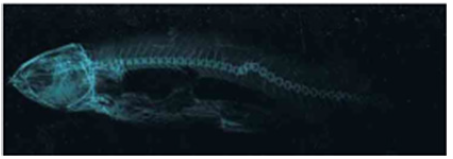
Figure 2.3a:Cirrhinus mrigala showing dorsal protuberance and displacement of pectoral, pelvic and anal fins./p>

Figure 2.3b: X ray photograph Cirrhinus mrigala showing aminor kyphosis.
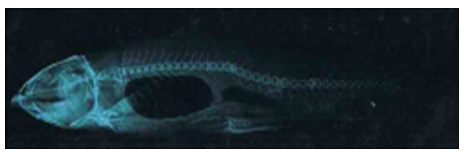
Figure 2.4a: Cirrhinus mrigala showing dorsal protubrance and displacement of pectoral, pelvic and anal fins.

Figure 2.4b: X ray photograph of Cirrhinus mrigala showing minor vertebral kyphosis.

Figure 2.5a: Cirrhinus mrigala showing dorsal protuberance and displacement of pectoral, pelvic and anal fins

Figure 2.5b: X ray photograph of Cirrhinus mrigala showing vertebral kypohosis.

Figure 2.6a: Cirrhinus mrigala showing a dorsal protuberance and displacement of pectoral, pelvic and anal

Figure 2.6b: X ray photograph of Cirrhinus mrigala showing a minor vertebral kyphosis.

Figure 2.7a: Cirrhinus mrigala showing dorsal protuberance and minor displacement of pectoral, pelvic and anal fins.

Figure 2.7b: X ray photograph of Cirrhinus mrigala showing 13th -16th overlapping vertebrae and vertebral kyphosis.

Figure 2.8a: Cirrhinus mrigala showing dorsal protuberance and minor displacement of pectoral fins.

Figure 2.8b:X ray photograph of Cirrhinus mrigala showing minor Vertebral kyphosis.
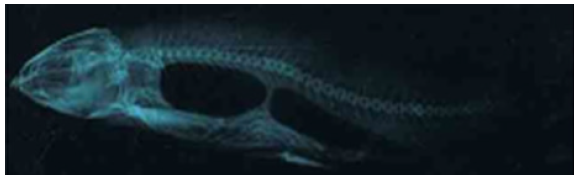
Figure 2.9a: Cirrhinus mrigala showing dorsal protuberance and displacement of pectoral and pelvic fins.

Figure 2.9b: X ray photograph of Cirrhinus mrigala showing vertebral kyphosis.
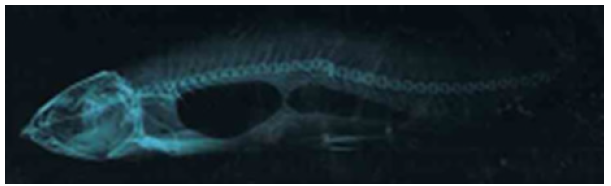
Figure 2.10a: Cirrhinus mrigala showing dorsal protuberance and displacement of pectoral, pelvic and anal fins.

Figure 2.10b: X ray photograph of Cirrhinus mrigala showing vertebral kyphosis.

Figure 2.11a: Cirrhinus mrigala showing dorsal protuberance and displacement of pectoral and pelvic fins.

Figure 2.11b: X ray photograph of Cirrhinus mrigala showing vertebral kyphosis.
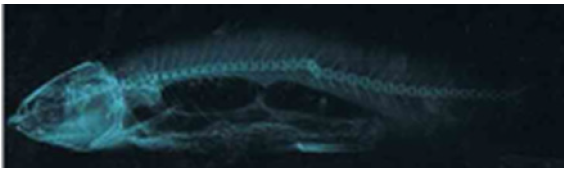
Figure 2.12a:Cirrhinus mrigala showing dorsal protuberance and left side bulge

Figure 2.12b: X ray photograph of Cirrhinus mrigala showing short displaced 17th – 19th vertebrae and vertebral kyphosis.
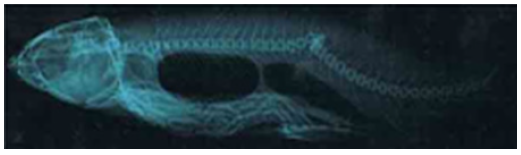
Figure 2.13a: Cirrhinus mrigala showing injured caudal peduncal, bulbous caudal fin base and displacement of anal fin.

Figure 2.13b: X ray photograph of Cirrhinus mrigala showing short and opaque 29th – 30th vertebrae.

Figure 2.14a: Cirrhinus mrigala showing injured truncated caudal Peduncal, compressed caudal fin base and displacement of anal fin.

Figure 2.14b: X ray photograph of Cirrhinus mrigala showing undifferentiated and fused 32nd – 35th vertebrae.

Figure 2.15a: Cirrhinus mrigala showing truncated caudal peduncal, a depression on left side and displacement of pectoral, pelvic and anal fins.

Figure 2.15b: X ray photograph of Cirrhinus mrigala showing irregular vertebral thickness and fusion.
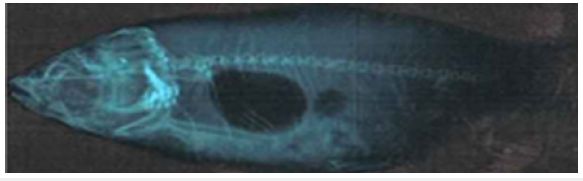
Figure 2.16a: Cirrhinus mrigala showing dorsally curved trunk and displacement of pectoral, pelvic and anal fins.

Figure 2.16b: X ray photograph of Cirrhinus mrigala showing dorsally curved vertebral column and minor kyphosis.
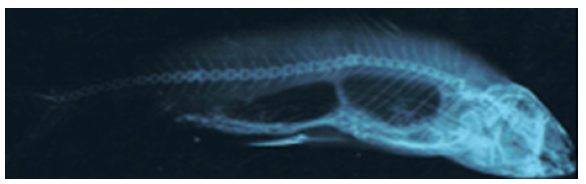
Figure 2.17a: Cirrhinus mrigala showing highly truncated, deflexed caudal peduncal, displaced dorsal and pelvic fins and aberrant anal and caudal fins.

Figure 2.17b: X ray photograph of Cirrhinus mrigala showing vertebral column deformities and abnormal air bladder lobes.
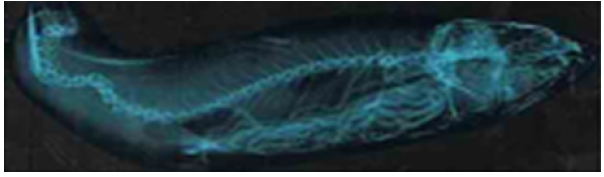
Discussion
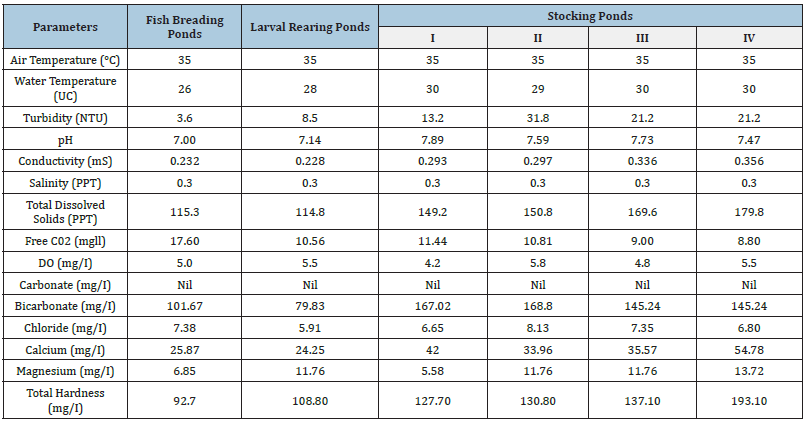
Record of only seventeen adult anomalous specimens of C. mrigala, among about 2000 fish specimens scanned, suggests a negligible percentage (>1%) of deformed fishes in Kathua ponds. This is either because they are less abundant or they have decreased survival as these easily fall prey to predators. Record of these adult deformed fishes suggests that these anomalies are not fatal, feeding is normal and are able to avoid predators. Several factors have been implicated for the appearance of fish deformities. Among abiotic factors alteration in one or more parameters like temperature, light, pH, salinity, low oxygen and inadequacy of key nutrients such as calcium and phosphorus. [12-20] have been attributed to cause fish deformities. Water quality characteristics of various fish ponds in Kathua district (Table 2) suggests that fish anomalies, under discussion, cannot be attributed to variation in abiotic characteristics of water or water pollution [21].
Table 2: Water quality of various fish ponds in Kathua district.
Fish deformities have also been attributed to currents [22,23], cultural techniques [24-26], faulty methods followed in induced breeding [27], intensive rearing [28], stress [29-31], effects of unfavorable environmental conditions [6, 32] and cannot be ruled out in the present case.
Fish anomalies due to inbreeding [33-35] and genetic defects [36-38] are on record. As these abnormal fish specimen were not analysed genetically, hence it could not be ascertained whether these abnormities were hereditary or non-hereditary. Parasitic infection, reported to be a possible factor in development of skeletal malformation by [39-43] is ruled out in the present case as there was no visible infection on any deformed specimen of Cirrhinus marigala.
Fish deformities ascribed to dietary factors [44-46] are unexplainable as there is no detailed analysis of supplementary diet given to these cultured fishes. Two specimens of Cirrhinus mrigala showed injured truncated caudal peduncle (Figure 2.13 - 2.16) and is caused by biological or mechanical injury and wound healing [9,10, 47-48]. Vertebral kyphosis in 12 fish specimens (Figure 2.12) and dorsally curved body in one fish specimen (Figure 2.15) is most probably caused by air bladder deformity [49-51].
Various types of anomalies in Cirrhinus mrigala, under discussion, can also be attributed to pesticides used in surrounding paddy fields and their ground water contamination and use of this contaminated water in fish ponds. Fish anomalies due to pesticides contaminated water/pesticides have also earlier been reported [52- 56]. From the foregoing discussion it is clear that fish deformities are induced by multiple factors. Therefore, more research is needed to exactly identify the factors causing such deformities among cultured fishes.
References
- Dutta SPS, Kour H (2005) Fish fauna of Kathua District, Jammu Region. In: Proc Nat Sem New Trends in Fishery Dev, Punjab University, Chandigarh, India. 16-18: 233-240.
- Dutta SPS (2021) Fish fauna of the river Ravi and its some tributaries with a new record of Ailia punctata and Clupisoma naziri for Punjab state and Union Territory of Jammu and Kashmir India of Kathua District, Jammu Region. Aquaculture and Fisheries Studies 3(1): 1-5.
- Sarkar HL, Kaushik NK (1958) Notes on two deformed specimens of the India carp Cirrhinus mrigala (Hamilton). Proc Zool Soc 11: 39-45.
- Banerji SR, Singh MN (1978) A truncated specimen of Cirrhinus mrigala (Ham.). Matsya 4: 80-82.
- Gupta SC, Dutta SPS, Sharma N, Balla N (2002) Morphological abnormalities in Cirrhinus migrala (Ham.) inhabiting lentic environments of Jammu. Aquaculture 3(2): 149-154.
- Narejo NT, Mastoi AM, Laghari MY, Lashari PK (2007) Incidence of crooked vertebral column in adult Cirhinus mrigala (Ham.) from Keenjhar lake (District: Thatta) Sindh, Pakistan. Pakistan J Zool 39(3): 199-201.
- Amitabh H, Firoz AM (2010) A wild specimen of Indian carp, Cirrhinus mrigala (Ham, 1822) with multiple vertebral deformities. World Jour Zoology 5(3): 167-171.
- Dutta SPS, Gupta N (2010) Morphological and skeletal deformities in Cirrhinus mrigala (Ham.-Buch.) from freshwater ponds of Gurdaspur District, Punjab. J Aquatic Biol 25(2): 165-172.
- Dutta SPS, Slathia D, Chander G, Kumar H (2011) Anomalies in Cirrhinus mrigala, a commercially important freshwater food fish from Gurdaspur district of Punjab. The Bioscan 6(3): 405-411.
- Dutta SPS (2018) Record of some deformed specimens of Cirrhinus mringala (Ham.-Buch) from river Tawi in Jammu City. The Bioscan 13(4): 831-834.
- APHA (1998) Standard methods for examination of water and waste water. (20thedn),. American Public Health Association New York, United States.
- Garside ET (1959) Some effects of oxygen in relation to temperature on development of lake trout embryos. Can J Zool 37(5): 689-698.
- Hubbs C (1959) High incidence of vertebral deformities in two natural populations of fishes inhabiting warm springs. Ecology 40: 154-155.
- Kwain WL (1975) Embryonic development, early growth and meristic variation in rainbow trout (Salmo gairdneri) exposed to combination of light intensity and temperature. J Fish Res Board Canada 32(3): 397-402
- Lee CS, Menu B (1981) Effects of salinity on egg development and hatching in grey mullet, Mugil cephalus, J Fish Biol 19(2): 179-188.
- Bolla S, HolmefJord I (1988) Effect of temperature and light on development of Atlantic halibut (Hippoglossus hippoglossus) larvae. Aquaculture 74(3-4): 355-358.
- Wiegand MD, Hataley JM, Kitchen C, Buchanan L (1989) Induction of developmental abnormalities in larval gold fish (Carassius auratus ) under cool incubation conditions. J Fish Biol 35(1): 85-95.
- Steingraeber MT, Gingerich WH (1991) Hatching, growth, ion accumulation and ossification of brook trout (Salvelinus alpinus) alevins in acidic soft waters. Can J Zool 69(8): 2266-2276.
- Al-Hassan LAJ (1982) Vertebral deformities in fishes from Iraq and the United Arab Emirates, Arabian Gulf. Iraqi Jour Mar Sci 1(1): 13-23.
- Lall SP, Lewis McCrea LM (2007) Role of nutrients in skeletal metabolism pathology in fish. An overview. Aquaculture 267(1-4): 3-19.
- Sun PL, Hawkins WE, Overstreet RM, Brown-Peterson NJ (2009) Morphological deformities as biomarkers in fish from contaminated rivers in Taiwan. Int J Envir Res Public Health 6(8): 2307-2331.
- Chatain B (1994) Abnormal swim bladder development and lordosis in sea bass (Dicentrachus labrax) and sea bream (Sparus auratus). Aquaculture 119(4): 371-379.
- Dutta SPS, Farooq S (2021) Some deformed specimens of Tor tor (Ham.-Buch.) and Tor putitora (Ham.-Buch.) from the torrential river Chenab, an important Himalayan River, draining Union Territory of Jammu and Kashmir, India. Aquaculture and Fisheries Studies 3(1): 6-10.
- Romanov NS (1984) Effect of cultural conditions on skull morphology in smolts of the masu salmon. Onchorhynchus masou (Brevort). Aquaculture 41(2): 147-153.
- Koumoundouros G, Divanach P, Kentori M (2001) The effect of rearing conditions on development of saddle back syndrome and caudal fin deformities in Dentex dentex (L) Aquaculture 200(3-4): 285-304.
- Leary RF, Allendorf FW, Kundsen KL (1991) Effect of rearing density on meristics and development stability of rainbow trout. Copeia 1991(1): 44-49.
- Bhattacharya S (1983) Abnormal silver carps Hypophthalmichthys molitrix in fish nurseries. Environment and Ecology 1: 15-16.
- Aydin I (2012) The external abnormalities of hatchery-reared black sea flounder (Platichthys flessus luscus Pallas, 1814) in Turkey. Turkish Journal of Fishries and Aquatic Sciences 12: 127-133.
- Raghunathan MB, Jayaram KC (1973) An abnormal specimen of Cirrhinus reba (Hamilton). The Indian Journal of Zootomy 14(2): 77-78.
- Pillai CT, Thampy DM (1990) Cases of deformities in some cultivable fishes. Indian J Fish 37(2): 171-173.
- Sharma KK (1996) Effects of mechanical stress on early embryonic stages of Tor tor. Advances in Fish and Wild life Ecology and Biology I: 151-154. In: Bansi Lal Koul, Daya Publishing House, Delhi, India.
- Yershov PN (2008) The vertebral abnormalities in eel pout Zoarces viviparous. Proceedings of the Zoological Institutes RAS 312(1/2): 74-82.
- Aulstad D, Kittelsen A (1971) Abnormal body curvature of rainbow trout (Salmo gairdneri) inbred fry. J Fish Res Bd Can 28(12): 1918-1920.
- Kinacid HL (1978) Inbreeding in rainbow trout (Salmo gairdneri). J Fish Res Board Can 33(11): 2420-2428.
- Piron RD (1978) Spontaneous skeletal deformities in the zebra danio (Brachidanio rerio) bred for fish toxicity test. J Fish Biol 13(1): 79-83.
- Isikawa Y (1990) Development of caudal structure of a morphogenetic mutant in the Telost fist medaka (Oryzias latipes). Journal Morphol 205(2): 219-232.
- Evans M, Neff BD (2009) Non-additive genetic effects contribute to larval spinal deformity in two populations of Chinook, salmon (Onchorhynchus tshawytscha) Aquaculture 216(1-2): 169-173.
- Fagbuaro O, Awopetu J, Oso JA (2006) Common deformities in hatchery bred Clarias gariepinus and its hybrids in Nigeria. Journal of Applied Environmental Science 2: 177-181.
- Hewitt GC, Little RW (1972) Whirling disease in New Zealand trout caused by Myxosoma cerebralis (Hoffer, 1903) (Protozoa : Myxosporida). NZ Journal of Marine and Freshwater Research 6(1-2): 1-10.
- Treasurer J (1992) Vertebral anomalies associated with Myxobolus sp. on perch, Perca flaviatilis L. in Scottish loch. Bull Eur Assoc Fish Pathol 12(2): 61-63.
- Kent ML, Watral VG, Whipps CM, Cunningham ME, Criscione CD, et al. (2004) A digenean metacercaria
(Apophallus ) and a myxozoan (Myxobolus sp.) associated with vertebral deformities in cyprinid fishes from the Williamette river, Oregon. Journal of Aquatic Animal Health 16(3): 116- 129. - Yokoyama H, Freeman MA, Bloch N, Fukuda Y (2005) Spinal curvature of cultured Japanese mackerel, Scomver japanicus associated with a brain myxosporean, Myxobolus acanthogobii. Dis Aquat Org 66(1): 1-7.
- Dutta SPS (2016) Some deformed specimens of Mystus bleekeri (Day) and Labeo bata (Ham.-Buch.) from the river Chenab in Pargwal wetland, Akhnoor, Jammu. Journal of Applied and Natural Science 8(1): 481-484.
- Kitamura S, Ohara S, Suwa T, Nakagawa K (1965) Studies on vitamin requirements of Rainbow trout, Salmo gairdenri. On the ascorbic acid. Bull Jap Soc Sci Fish 31(10): 818-826.
- Ashley LM (1972) Nutritional pathology. In: JE Helver (Edt.), Fish Nutrition. New York and London, Academic Press, p. 713.
- Newsome CS, Piron RD (1982) Aetiology of skeletal deformities in the zebra danio fish (Brachidanio rerio, Hamilton-Buchanan). J Fish Biol 21(2): 235-237.
- Jawad LA, Sadighzadeh Z, Valinasaab T (2010) Malformation of the caudal fin in the fresh water mullet, Liza abu (Actinopterygii: Mugilidae) collected from Karkhe river. Annales de Biologia 32: 11-14.
- Dutta SPS (2021) Rudimentary caudal peducal in Bagarius bagarius (Ham/-Buch) and blunt head, rudimentary snout and abnormal mouth in Labeo dero (Ham.-Buch) from river Tawi Jammu, J&K UT, India. Examines Mar Biol Oceanogr 4(2): 1-4.
- Kitajima C, Watambe T, Tsukashima Y, Fujita S (1994) Lordotic deformation and abnormal development of swimbladders in some hatchery bred marine physocolistous fish in Japan. J World Aquacult Soc 25(1): 64-77.
- Trotter AJ, Pankhurst PM, Buttagiene SC (2004) Morhological development of the swimbladder in hatchery reared striped trumpeter Latris lineata. Journal of Applied lcthyology 20(5): 395-401.
- Grotmol S, Kryvi H, Totland GK (2005) Deformities in the notochord by the pressure from the swimbladder may cause malformation of the vertebral column in cultured Atlantic cod Gadus morchua larvac: a case study. Dis Aquat Organ 65(2): 121-128.
- Couch JA, Winstead JT, Hansen DJ, Goodman LR (1979) Vertebral dysplasia in young fish exposed to the herbicide trifluralin. Journal of Fish Diseases 2(1): 35-42.
- Newsome CS (1980) A multigeneration fish toxicity test as an aid in the hazard evaluation of aquatic pollutants. Ecotoxicol Environ Saf 4(4): 362-369.
- Dulcic J (2004) Incidents of spinal deformities in natural populations of grass goby. Zosterisessor ophiocephalus from the Karin sea, Eastern middle Adriatic. Cybium 28(1): 7-11.
- Douglas PM, John WF, Michael JH (1990) Vertebral abnormalities in juvenile inland silversides, Menidia beryllina exposed to terbufos during embryogenesis. Diseases of Aquatic Organisms 9: 109-116.
- Celik ES, Kaya H, Yilmaz S (2012) Effects of phosalone on mineral contents and spinal deformities in common carp (Cyprinus carpio L 1758). Turkish Journal of Fisheries and Aquatic Sciences 12: 259-264.
© 2022 Dutta SPS. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






