- Submissions

Full Text
Examines in Marine Biology & Oceanography
Morpho-Physiological, Blood Biochemistry And Health Status In Wild Yellow Snapper Lutjanus Argentiventris (Peters, 1869)
Juan Pablo Apún Molina2, Maximo García Marciano1, Leonardo Ibarra Castro3*, Juan Carlos Sainz Hernández2 and Apolinar Santamaría Miranda2,3*
1Programa de Maestría en Recursos Naturales y Medio Ambiente, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Mexico
2Instituto Politécnico Nacional, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Mexico
3University of Florida Whitney Laboratory for Marine Bioscience, USA
*Corresponding author: Leonardo Ibarra Castro and Apolinar Santamaria Miranda, University of Florida Whitney Laboratory for Marine Bioscience, USA
Apolinar Santamaria Miranda, Instituto Politécnico Nacional, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Mexico
Submission: May 20, 2021;Published: June 07, 2021

ISSN 2578-031X Volume4 Issue1
Abstract
Length-weight relationships, morpho-physiological index and blood biochemical parameters are frequently used to determine the health status of wild and cultured fish. Between December 2013 and July 2015, a total of 123 yellow snappers (L. argentiventris) were captured with gillnets in the Macapule Lagoon and blood samples were taken. All the blood biochemical parameter varied significantly among sampling seasons. The total proteins, triglycerides, and cholesterol values were significantly highs in December 2013 (cold season). We found significant differences intra and inter-correlations between morpho-physiological and blood biochemical parameters in bout seasons. The equation parameters (a & b) of the length-weight relationship in each of the seasons indicated that the growth of yellow snapper showed important variations over time between year 1 and year 2. Among the morpho-physiological index, only the Hepatosomatic Index (HIS) and Gastric Repletion Index (GRI) varied significantly among the seasons (P<0.01); and they were positively correlated with water temperature. In conclusion, the morpho-physiological and blood biochemical examinations determined for the first time for yellow snapper in Macapule Lagoon, showed a wide temporal variation and were related to environmental changes between seasons. This new data can be used as a reference to compare health conditions between wild and captive yellow snapper (L. argentiventris) and other fishes.
Keywords: Hematological variables; Biochemical variables; Warm water; Cold water; Environmental changes; Fish health
Abbreviations: pH: hydrogen Potential; Mg dL-1: Milligrams per deciliter; mL: Milliliters; g: Relative centrifugal force; log x+1: Logarithm of one to base ten; ANOVA: Variance Analysis; rs: Spearman’s correlation coefficient
Introduction
The worldwide marine environment is affected by global warming, as a result, we have climate change that affects physicochemical variables in the water and the internal fish physiology. Indeed, due to fish are ectothermic animals, variables as temperature, oxygen dissolved and pH, affect its physiology, metabolism and behavior (Pörtner 2008; Sampaio 2019). These physicochemical variables, singly, or combined, impose different morphophysiological stress in fish and impair their health Adams [1]. The different types of stress and health condition in fish have been evaluated using several approaches: e.g., hepatosomatic index, condition factor, gastric repletion index, natural season, origin, sex, size Cabrera [2] and reproductive status Martínez [3], Santamaría [4].
In addition, studies in stress and health condition in fish, also shown how variations in
environmental conditions can cause important internal physiologic modifications in blood
biochemistry and hematological status (Alvarado [5], Valenzuela [6], Pedro [7], Román [8]).
These variation can be observed in the total protein, cholesterol, triglycerides, and glucose levels in blood, which also have been used as physiological
indicators to infer the environmental effect on fish Quintana [9].
However, studies on how the warm and cold season in coastal
water can affect the natural hematological characteristics in wild
fish populations had not been explored.
In general, in fisheries and aquaculture field, the fish health
condition has been evaluated using fish growth as a principal
reference. Under this worldwide global warming situation (Alfonso
et al., 2020), water temperature changes would significantly impair
juvenile recruitment in the estuaries and commercial fishing may
be impaired shortly.
Belong to the Lutjanidae family, yellow snapper is one of the
most important fishery resources along the Mexican Pacific coast.
The commercial landings of this species have a total yield of 385.83
metric tons in 2014 (CONAPESCA 2016). Yellow snapper fishery is
mostly artisanal, given that the fish lives in the mangroves of coastal
lagoons during its juvenile phase (González [10]) and migrates
offshore as an adult, where it remains in reef zones down to 60m
depth (Fischer [11]). Research on yellow snapper has focused
mainly on determining its natural growth rate and age structure in
different coastal areas ([Rojas [12], Garcia [13], Ibañez [14], Gómez
[15], Ramírez [16]), as well as its feeding habits and reproductive
cycle (Santamaría [17], Piñón [18], Flores [19]). However, no
information is currently available about the health condition of
wild yellow snapper populations. Therefore, the objective of this
study was to evaluate the natural health condition in populations
of yellow snapper (L. argentiventris) inhabiting the coastal lagoon
of the Gulf of California, subject to a wide variation in temperatures
throughout the year. The new knowledge will be of great importance
to compare health condition between wild snapper or different
species of cultivated fish worldwide.
Material and Method
Site selected to collect fish and blood samples
Fish samples were collected in the Macapule Lagoon. This lagoon has fluctuations in temperature values, the maximum temperature in summer is 27 ºC and the minimum is 13 ºC during the winter. Coastal ecosystems like Macapule Lagoon support diverse and important fisheries in the Gulf of California and are reservoirs of great biological diversity. In addition, the Northern of Sinaloa State population has grown, and its urban development has increased the necessity for recreation areas, as well as the pollution, has substantially increased putting up the pressure on marine resources. Macapule lagoon is part of the San Ignacio- Navachiste system, located in the southern region of the Gulf of California 25º 21’ and 25º 24’ N; 108º 30’ and 108º 45’ W (Figure 1). In 2008, San Ignacio-Navachiste-Macapule lagoon system was declared an “Area of Reserve and Refuge for Migratory Birds and Wildlife - Gulf of California Islands” MEX-111 and 1826-RAMSAR, 79 87302, February 02, 2008. Due to its location, this lagoon has a high primary productive ambient (first links in the production chain) (Cota [20]. To see if the natural variation of physio-chemical variables as water temperature, dissolved oxygen, pH, and salinity an influence in health condition of yellow snapper (L. argentiventris) have, they were measured each time and in each place that we collected fish blood samples, using a YSI (55-12FT) probe and a portable refractometer (RHS-10ATC).
Figure 1:The study area. The Macapule Lagoon is located in the Gulf of California, Mexico.
Blood samples extractions and biological index
Yellow snappers were captured using a cast net (mesh size 17.8mm, net length 2.8m, maximum net diameter 4.0m). The fishing cruises were made at least 3 times per month or until getting 30 organisms by month. The syringes with anticoagulants were prepared before the fishing cruise trip. All the blood samples were drawn from the fish as soon as possible to avoid any type of fish stress. Briefly, blood samples of 1±0.1mL were extracted (only of fish larger than 10cm total length) by caudal puncture with a disposable plastic syringe containing 0.5mL of heparinized solution (Sigma-Aldrich) and place in ice until analysis. After one hour in ice, the blood samples were centrifuged at 9,500 g at 4 ºC for 10 minutes to separate the blood cells from the plasma. To determine total protein concentrations in the blood we follow Bradford’s method (1976), which is based on the reaction of the amino groups with the dye Coomassie Blue G-250. While triglycerides, glucose, and cholesterol concentrations were determined with colorimetric commercial kits Randox UK Molina [17], and the concentration was registered as mg dL-1. The measure of absorbance was determined with a microplate reader (Multiskan Go, Thermo Scientific UV, United States) and concentrations of triglycerides, glucose, and cholesterol were calculated from a standard solution of substrates.
After blood samples were taken, we registered the biological data from each fish collected. Briefly, total length was measured using an ictiometer (pentair®, 50cm) and the fish were weighed with a scale (Ohaus-202 Scout Pro). Organs as liver, stomach, and gutted weight were weighed and the data were used to calculate the morpho-physiological indices: 1) Fulton’s Condition Factor (CF): CF = Wt/Lt 3 * 100, where Wt = total weight and Lt = total length; 2) the Hepatosomatic Index (HSI): HSI = Wh/Wt*100, where Wh = liver weight and Wt = total weight of the individual; and 3) the Gastric Repletion Index (GRI): GRI = Ws/Wt *100, where Ws = stomach weight and Wt = total weight. In addition, The lengthweight relationship and the condition factor are parameters used to compare the condition of populations that inhabit aquatic systems with different degrees of anthropic intervention, to calculate lengthweight relationship, 123 unsexed fish were measured for their TL to the nearest 1mm and weighed to the nearest 1g. Parameters of the length-weight relationship of yellow snapper (L. argentiventris) were estimated using the equation: W = aLb, where W = weight of the fish (g), L = total length (cm), a = y-intercept or the initial growth coefficient, b = slope or the growth coefficient. On the other hand, the nutrient concentrations as nitrate, ammonium, and phosphate were determined from water samples taken at each site of fish blood samples collected. The water samples were taken using falcon tubes of 50mL and transported in a cooler with frozen gels. One time in the laboratory, the concentration of the nutrient was determined follow the method described by Strickland & Parsons (1972).
Statistical analysis
All data were subject to normality distribution Kolmogorov- Smirnov test using the Lilliefors (1967) approach and the equality of variances were tested using Bartlet test [21]. In the case of the normality test failed, the data were log-transformed (log x+1) e.g., morphophysiological index, whose results are expressed in percentages. Differences in the biochemical blood samples (total protein, triglycerides, cholesterol, and glucose) parameters and the morpho-physiological (CF, HSI, and GRI) between sampling months were evaluated using a Generalized Linear Model (GLM), with the parameters as dependent variables, month as the predictor variable, and the total length of fish as a covariance. While a oneway ANOVA was used to evaluate differences in physical-chemical parameters (temperature, dissolved oxygen, pH, and salinity) and nutrient concentrations between sampling months. A meta-analysis was develop using discriminant analyses based on Mahalanobis distances to identify differences in morpho-physiological and biochemical parameters between months, sampling was analyzed with analyses of variance after testing them for normality and homogeneity using Statistics® version 5.5.
Result and Discussion
Biochemical blood analyses
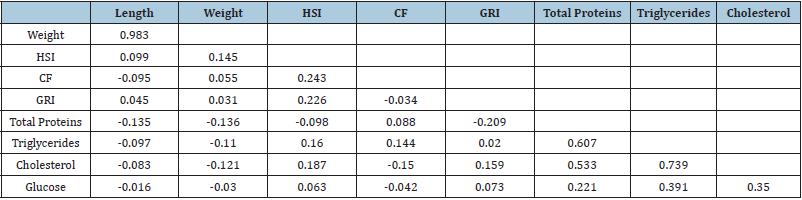
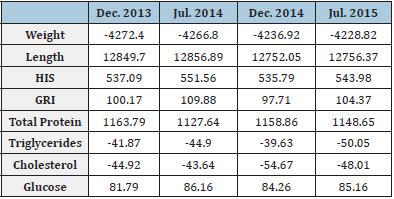
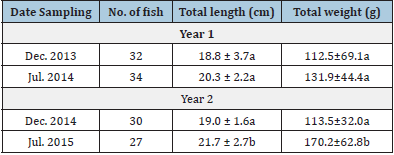
For blood samples extractions a total of 123 yellow snapper were sampled (December 2013, [n=32]; June 2014, [n=34]; December 2014 [n=30], and July 2015 [n=27]). All the blood biochemical parameters varied significantly among sampling months. The total proteins, triglycerides, and cholesterol values were significantly higher in December 2013 (ANOVA, F3,122 = 25.8, 29.3, and 47.8, respectively, P<0.01), while the highest glucose value was recorded in July 2014 (ANOVA, F3,122 = 4.5, P<0.01) (Table 1). We found significant differences intra and inter-correlations between morpho-physiological and biochemical blood biochemical parameters (Table 2). On the other hand, the total length was positively correlated with weight, while the CF also was positively correlated with the HSI, and so was the GRI with the HSI (Table 2). The total protein concentration in fish blood was the only blood biochemical parameter that correlated with one of the morphophysiological parameters (GRI). Total protein concentration was also positively correlated with Triglycerides Cholesterol, and Glucose biochemical parameters (Table 2). The blood total protein concentrations recorded in yellow snapper L. argentiventris were higher than those reported by Román [8] and García [22] for L. peru in Mexican coastal waters or for the serranids Acantopagrus latus and Epinephelus coioides (Akbary [23]). The variation recorded in blood total protein concentrations across the sampling months for this study might be due to changes in feeding behavior (about the type of prey consumed). In agreement with Table 2, it could be inferred that this yellow snapper population from Macupale Lagoon has not yet entered in the reproductive phase and all the protein they get is used for growth and welfare (García [24]) as has been suggested for other lutjanids species (García [22], Román [8]).
The levels of triglycerides and cholesterol are proportional at liver weight, as indicated by the positive correlation between cholesterol and HSI values (Table 3). Lipid concentrations decrease in the blood plasma after spawning and during periods of fasting, but after that, they increase periodically with feeding during the non-reproductive season (García [24]). The ranges of triglycerides and cholesterol levels in yellow snapper were very wide (Table 2), although the highest mean values were similar to those reported by Román [18] in L. peru. However, the concentrations recorded for L. argentiventris and L. peru are lower than those reported for other marine species, e.g., Acantopagrus latus (Akbary [23]) or Lates calcarifer (Satheesh [25]). In this study, we observe significant differences in the glucose concentration of the blood from fish obtained in July 2014 (Table 2) showing a direct relationship with dissolved oxygen concentration in the water (Table 1) and it could be that the organisms at the time of capture were more susceptible to acute stress after the capture (Molina [17]). Triglycerides and cholesterol levels are related to the age and reproductive stage in fish. For example, cholesterol concentrations increase with fish body size. The fish examined in the Macapule Lagoon, as well as those examined by Román [18], were juvenile organisms; therefore, the low concentrations recorded can be mainly attributed to their younger age. On the other hand, the triglycerides and cholesterol levels decreased steadily across the sampling (Table 2). The composition of the food ingested by yellow snapper has not been analyzed during this study; however, the higher levels of triglycerides and cholesterol recorded during the first two samplings (Table 2) can be attributed to a higher proportion of abdominal fat in the fish. However, it could be inferred that the population in the Macapule Lagoon has already started the reproductive phase and all stored nutrients are used for this new biological process.
Table 1: Blood biochemical parameters of Lutjanus argentiventris during the sampling months in Macapule Lagoon. Significantly different values (p<0.05) are different small letter.
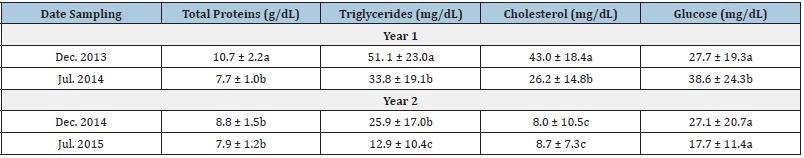
Table 2: Correlation coefficients (rs) among the morpho-physiological and blood biochemical parameters of Lutjanus argentiventris during the sampling months in Macapule Lagoon.
HSI = hepatosomatic index, CF = Fulton’s condition factor, GRI = gastric repletion index.
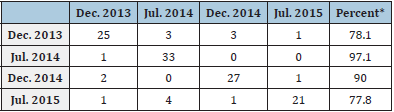
Table 3: Discriminant analysis classification showing the numbers and percentages of fish assigned to each sampling month (group).
Glucose levels may vary greatly due to changes in biological factors, such as the fish length, weight, age, nutritional status, reproduction, and stress level, or due to environmental variations related to changes in temperature and dissolved oxygen concentrations. The blood glucose concentrations of yellow snapper varied up to 46 % (Table 2) between sampling months. Nevertheless, these concentrations were lower than those reported for L. peru, Lates calcarifer, Mugil cephalus, or Chanos chanos. The biochemical parameters in the blood can vary greatly among fish species or between different climatic seasons (González [26], Castellanos [27], Bayir [28]). The multivariate analysis confirmed the great variation of these parameters in yellow snapper between different months and between years (Figure 2). The CF index was the only parameter rejected by the statistical model, due to its little variation (Table 2). However, although the health condition of yellow snapper was fairly constant over time, the fish biochemical parameters presented important changes, e.g., fish examined in December 2013 were characterized by higher blood total protein levels, while fish from July 2014 sample showed higher glucose levels (Table 2). On the other hand, yellow snapper examined in December 2014 had the lowest cholesterol concentrations than those sampled in July 2015, where triglyceride levels are more constant (Table 2).
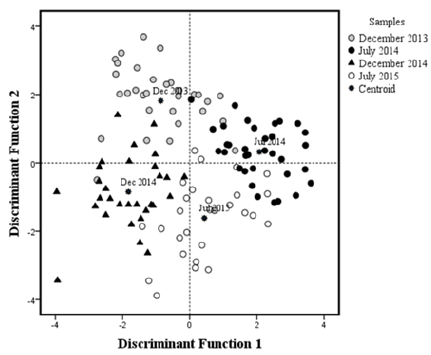
Figure 2:Graphic representation of the multivariate discriminant analysis of L. argentiventris samples from Macapule Lagoon. Each fish examined during the sampling months is represented by a symbol. Centroid = mean of the respective group.
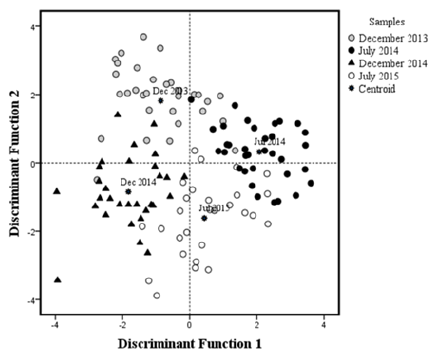
The meta-analysis result in a multivariate model constructed to find differences in morpho-physiological and biochemical variables of yellow snapper between sampling years, the first two discriminant variables explained 90.5% of the variance, contributing 52.3% (eigenvalue = 2.31) and 38.2% (eigenvalue = 1.69), respectively. The third discriminant variables explained only 9.5% of the total variance (eigenvalue = 0.42). A significant overall group effect was observed (Wilks’ lambda = 0.79, F325, P<0.001). Individual fish were evenly distributed along the two discriminant axes (Figure 2). Dimensionality tests showed that the two years (December 2013, 2014, and July 2014, 2015) in two years (groups) were significantly separated in both dimensions (χ2 = 294.51, df = 24, P<0.01) (Figure 2). Each fish was correctly assigned to one of the four groups with an accuracy of 86.2%. Seven fish from December 2013, three from December 2014, six from July 2015, and one fish from July 2014 were erroneously assigned to other groups (Table 3).
Biological index
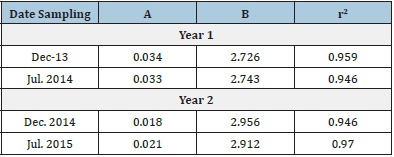
The equation parameters (a & b) of the length-weight relationship in each one of the samplings indicated that the growth of yellow snapper showed important variations over time between year 1 and year 2 (Table 4). For example, in December 2014 and July 2015, second year, the fish growth in length and weight was more homogeneous (b≈ 3, isometric growth), while in December 2013 and July 2014 first year (b≈ 2.73), the growth showed negative allometry (i.e., the fish grew faster in length than in weight).
Table 4: Length-weight relationships of Lutjanus argentiventris during the sampling months in Macapule Lagoon. Length-weight coefficients, y = a + b*x.
Among the morpho-physiological parameters (Table 5), only the HIS and GRI varied significantly among the sampling of the years mainly HIS and GRI (P<0.01); and they were positively correlated with water temperature (rs = 0.294 and 0.441, respectively, P<0.01). A significantly higher value of the HIS (1.7±0.4) was recorded in July 2014 (ANOVA, F3,122 = 9.28, P<0.01), while the GRI was significantly higher in July 2014 and 2015 (ANOVA, F3,122 = 21.1, P<0.01). The importance of each morpho-physiological or biochemical parameter in distinguishing between sampling months in two years (groups), was evaluated as the contribution of each variable to the total sum of Mahalanobis distances, indicating that the weight of the fish and the blood total protein concentration were the most important parameters to identify the December 2013 group (Table 6). The length of the fish, the HSI, the GRI and glucose values were the most important parameters to differentiate the July 2014 group, while the cholesterol concentration was the most important parameter for the December 2014 group. Finally, the triglycerides defined July 2015 group (Table 6) and only the CF index was rejected by the model due to its larger Wilks’ lambda P-value.<0.001.
Table 5: Morpho-physiological parameters of Lutjanus argentiventris during the sampling months in Macapule Lagoon. HSI = hepatosomatic index, CF = Fulton’s condition factor, GRI = gastric repletion index.
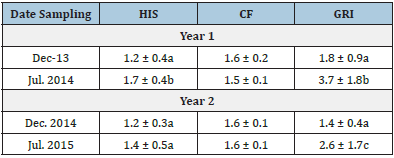
Table 6: Coefficients of discriminant functions of morphophysiological and biochemical variables that allow establishing differences (in bold) between sampling in two years (groups) of Lutjanus argentiventris in Macapule Lagoon.
In addition, the morpho-physiological indices have been considered, for a long time, an appropriate method to evaluate the physiological condition of fish (Tyler [29]). Variations in the GRI and HSI (Table 2) were related to significant temperature fluctuations during the sampling months. The temperature was considered an environmental parameter with important effects on the physiology and behavior of fish in the Macapule Lagoon. For example, the metabolic rate as well as several metabolic processes tend to increase as temperature increases( Vázquez [30]) The positive correlation between temperature and the HSI and the GRI indicated that feeding activity and storage of energy reserves in the liver of yellow snapper increased with higher water temperature. The increase in food uptake during the high-temperature months July 2014 and 2015, (Table 1) was also reflected in an increase in liver energy reserves (higher values of HSI), given that the liver is an important organ for energy storage in benthic and demersal fish species (Drazen [31]).
The CF did not vary significantly among the sampling months (Table 2), indicating that the adverse conditions that occurred during given sampling periods, such as in July 2014 when the oxygen concentration was very low and salinity very high, had no negative effect on the fish (Table 1). The CFs obtained during the sampling months were similar to those of L. argentimaculatus (Daliri [32]) and L. peru (Garduño [22]), but lower than those reported for L. malabaricus . Concerning the growth in yellow snapper, the b values were b<3 during the first two samplings, suggesting a negative allometric growth (Table 4). By contrast, b was ~3 in the last two samples, second-year indicating an isometric growth. Vázquez [30] and Gómez [11] recorded an isometric growth (b = 3.04 and 3.07, respectively) in yellow snapper from 21.5 to 58cm total length. Allometric growth, either positive (b > 3) or negative (b < 3), has been reported for many marine fish species. For example, Marzouqi [33] noted that allometric growth is a recurrent pattern among many carangid species. A study of 43 marine species of the families Carangidae, Lutjanidae, and Haemulidae from the central Mexican Pacific reported at least 26 allometric negative and 7 allometric positive length-weight relationships. Therefore, is possible that the growth for yellow snapper tends to be isometric in larger individuals. Species present an allometric growth pattern that could lead to important adaptive advantages associated with the use of benthic habitat Cifuentes [34].
Physico chemical variables
The physicochemical variables (Table 7) shown significant changes between the sampling, the concentrations of the three examined nutrients also showed significant variation between the years, such as Ammonium and Nitrates. As it generally happens, through the seasons we observed how at a lower temperature, higher dissolved oxygen was recorded in the sampling areas. Finally, the environmental parameters recorded in Macapule Lagoon showed a significant variation throughout the sampling months. This remarkable environmental variation can be due to the strong influence of the California Current on this coastal lagoon, causing important changes in the physicochemical parameters and nutrient concentrations Magaña [35], [36-40].
The maximum total length reported for yellow snapper from the Mexican Pacific coast ranges from 66 (Fischer [11], [41-43]) to 70cm Rojas [12], while the size of first sexual maturity has been estimated between 31.5 and 32.6cm (Piñón [18], Ramírez [16], [44,45]). The fish of the Macapule Lagoon measured between 18.8±3.7 and 21.7±2.7cm (Table 8), indicating that they were juvenile, approximately one-year-old organisms (Rojas [12], [46- 50]).
Table 7: Physico-chemical parameters and nutrient concentrations during the sampling months in Macapule Lagoon. PSU = practical salinity unit. Means not sharing the same superscripts are significantly different.
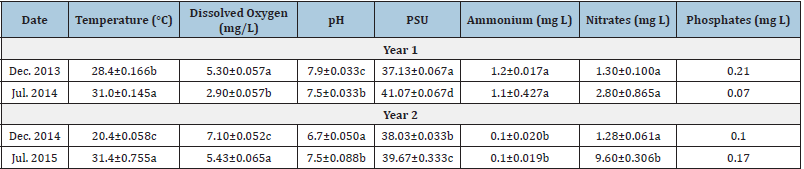
Table 8: Length and weight of Lutjanus argentiventris during the sampling months in Macapule Lagoon.
Conclusion
The morpho-physiological and biochemical parameters, determined for the first time for yellow snapper in Macapule Lagoon, showed a wide temporal variation. The variability in these parameters was related to environmental changes, such as fluctuations in water temperature, changes in feeding behavior, and the type of prey consumed over time. In further research, the blood biochemical parameters determined for the present research could be employed as a reference tool to compare the health condition between wild and captive yellow snapper.
Summary
The discovery in the blood biochemical parameters of Lutjanus argentiventris, could be employed as a useful tool to monitor the health condition in populations of fish.
Acknowledgement
INSTITUTO POLITECNICO NACIONAL financial support from scholarships from COFAA and E.D.I. and CONACYT. For the México government, received for J.P. Apún-Molina, A. Santamaria-Miranda and J.C. Sainz Hernandez.
Funding
This research was supported by grants from SIP-IPN 20140593, Financial support with the SEP-CONACyT Project: Estado de salud de la población silvestre de los pargos Lutjanus peru, Lutjanus guttatus y Lutjanus argentiventris en el Océano Pacifico Mexicano.
References
- Adams SM, Allen M, Ronald W (1993) A quantitative health assessment index for rapid evaluation of fish condition in the field. Trans Am Fish Soc 122: 63-73.
- Cabrera PY, Betancourt AC, González SG (2008) Indicadores morfológicos y reproductivos del pez Gambusia puncticulata (Poeciliidae) en sitios muy contaminados del Río Almendares. Cuba Rev Biol Trop 56(4): 1991-2004.
- Martínez AM, Herrera RA, Domínguez GF, Vázquez CEP, Villalejo M (2001) Ciclo reproductivo del pargo lunarejo Lutjanus guttatus (Steindachner 1869) en las costas de Guerrero, Mé Rev Biol Mar Oceanogr 36(1): 1-8.
- Santamaría MA, Garay JF, Villalejo FM, Herrera AA (2003) Desarrollo gonadal y ciclo reproductivo de Lutjanus peru (Pisces: Lutjanidae) en Guerrero, Mé Rev Biol Trop 51(2): 489-502.
- Alvarado H (1997) Efecto de tres concentraciones de calcio en el agua sobre algunos parámetros hematológicos de la trucha arcoí Veterinaria trop 22: 5-12.
- Valenzuela A, Oyarzún C, Silva V (2003) Células sanguíneas de Schroederichthys chilensis (Guichenot, 1848) (Elasmobranchii, Scyliorhinidae): Serie Blanca. Guayana 67(1): 130-136.
- Pedro D, Guijarro A, López PMA, Martínez RM, Delgado MJ (2005) Daily and seasonal variations in haematological and blood biochemical parameters in the tench, Tinca tinca Linnaeus, 1758. Aquacuc Res 36(12): 1185-1196.
- Román MA (2013) Valores hematológicos y bioquímica sanguínea de la población silvestre del huachinango Lutjanus peru (Nichols y Murphy, 1922) en el Pacífico Sur de Mé Tesis de maestría p. 75.
- Quintana CF (2002) Respuestas neuroendocrinas al estrés en peces teleó Rev Ictiol 10: 57-78.
- González AF, Agüero G, Campos G (1999) Ictiofauna asociada al manglar del Estero el Cochalito, Ensenada de La Paz, Baja California Sur, Mé Oceánides 14: 121-131.
- Fischer, Walter (1995) Guía FAO para la identificación de especies para los fines de la pesca. Pacífico centro-oriental. Vertebrados p. 647-1813.
- Rojas PA, Gutiérrez CF, Puentes V, Villa AA, Rubio EA (2004) Aspectos de la biología y dinámica poblacional del pargo coliamarillo Lutjanus argentiventris en el Parque Nacional Natural Gorgona, Colombia. Investig Mar 32(2): 23-36.
- García COE, Quiñónez VC, Morán AR, Valdez MC (2009) Age, growth, and age structure of yellowtail snapper off the coast of Mazatlán, Sinaloa, Mé N Am J Fish Manag 29: 223-230.
- Ibáñez AL, Espino BE, Gallardo CM (2012) Population connectivity among geographic variants within the Lutjanidae (Pisces) of the Mexican Pacific coast through fish scale shape recognition. Sci Mar 76: 667-675.
- Gómez BJL, Robles YA, Vega AJ (2013) Length-weight relationship and biological information of the yellow snapper Lutjanus argentiventris from a tropical estuary: Río Caté, Gulf of Montijo, Panama. J Appl Ichthyol 30(1): 227-229.
- Ramírez G, Ruiz S, González G, Cevallos VBP (2014) Biología reproductiva del pargo alazán, Lutjanus argentiventris (Pisces, Lutjanidae), en el Pacífico central mexicano. Cienc Mar 40(1): 33-44.
- Santamaría MA, Lozano SM, Moreno HMN, Molina JP (2005) Hábitos alimenticios del pargo amarillo Lutjanus argentiventris y del pargo rojo Lutjanus colorado (Pisces: Lutjanidae) en el Norte de Sinaloa, Mé Rev Biol Mar Oceanogr 40: 33-44.
- Piñón A, Amezcua F, Duncan N (2009) Reproductive cycle of female yellow snapper Lutjanus argentiventris (Pisces, Actinopterygii, Lutjanidae) in the SW Gulf of California: Gonadic stages, spawning seasonality and length at sexual maturity. J Appl Ichthyol 25(1): 18-25.
- Flores JR, Ávila CE, Haro HJ, Godínez DE (2014) Hábitos Alimentarios e interacciones tróficas de Anisotremus interruptus (Pisces: Haemulidae) y Lutjanus argentiventris (Pisces: Lutjanidae) en el Pacífico Central Mexicano. Lat Am J Aquat Res 42(1): 276-282.
- Cota SE, Noriega EA, Sánchez F, Gamboa D, Romero JJ, et al. (2007) The gulf of California: Review of ecosystem status and sustainability challenges. Prog Oceanogr 73(1): 1-26.
- Garduño DM, Unzueta BML, Hernández M, Lorán RM, Martínez FR (2010) Crecimiento de huachinangos juveniles silvestres (Lutjanus peru) en un encierro de engorda en Puerto Vicente Guerrero, Guerrero, Mé Ciencia Pesquera 18: 93-96.
- García PA (2008) Determinación bioquímica en plasma de la condición nutricional en reproductores de Lutjanus peru (Nichols y Murphy, 1922) bajo condiciones de cautiverio. Tesis de maestría p. 85.
- Akbary P (2014) Consideration of blood serum biochemical parameters of yellow fin sea bream (Acantopagrus latus Houttuyn, 1782) and orange-spotted grouper (Epinephelus coioides Hamilton, 1822). Adv Biol Chem 4(6): 407-413.
- García AV, Genes LF, Madariaga MD, Pardo CS (2007) Hematología y química sanguínea de juveniles de rubio (Salminus affinis Pisces: Characidae) del Río Sinú. Acta Biolo Colomb 12: 27-40.
- Satheesh P, Ananthan G, Kumar DS, Jagadeesan L (2012) Haematology and biochemical parameters of different feeding behaviour of teleost fishes from Vellar estuary, India. Comp Clin Path 21: 1187-1191.
- González P, Oyarzún C (2002) Variabilidad de índices biológicos en Pinguipes chilensis Valenciennes 1833 (Perciformes, Pinguipedidae): Están realmente correlacionados Gayana (Concepc.). 66: 249-253.
- Castellanos JA, Bustos MB, Arévalo GH, Mocha PE (2003) Valoración hematológica y química sanguínea del yamú Brycon siebenthalae, en tres etapas de cultivo. Orinoquia 7(1-2): 34-41.
- Bayir A, Sirkecioglu AN, Polat H, Aras NM (2007) Biochemical profile of blood Serum of Siraz Capoeta capoeta umbla. Comp Clin Path 16: 119-126.
- Tyler AV, Dunn RS (1976) Ration, growth, and measures of somatic and organ condition in relation to meal frequency in Winter flounder, Pseudopleuronectes americanus, with hypotheses regarding population homeostasis. JFRBC 33(1): 63-75.
- Vázquez JA, Lucano RG, Ramírez S (2009) Length-weight relationships for coastal fish species from the gillnet artisanal fishery in the central Mexican Pacific. J Appl Ichthyol 25(4): 497-498.
- Drazen JC (2002) A seasonal analysis of the nutritional condition of deep-sea macrourid fishes in the north-east Pacific. J Fish Biol 60(5): 1280-1295.
- Raeisi H, Daliri M, Paighambari SY, Shabani MJ, Bibak M, et al. (2011) Length-weight relationships, condition factors and relative weight of five fish species of Bushehr waters, Northern Persian Gulf. Afr J Biotechnol 82(10): 19181-19186.
- Marzouqi A, Jayabalan N, Nahdi A (2013) Length based growth and stock assessment of the longnose trevally Carangoides chrysophrys (Cuvier, 1833) from the Arabian sea coast of Oman. Indian J Fish 60: 1-6.
- Cifuentes R, González J, Montoya G, Jara A, Ortíz N, et al. (2012) Relación longitud-peso y factor de condición de los peces nativos del río San Pedro (cuenca del río Valdivia, Chile). Gayana 76(1): 86-100.
- Magaña ME (2004) Distribución de nutrientes y su efecto en el nivel trófico de la Laguna Macapule, Sinaloa. Tesis de maestría p. 105.
- Alfonso S, Gesto M, Sadoul B (2020) Temperature increase and its effects on fish stress physiology in the context of global warming. J Fish Biol.
- Apún JP, Santamaría MA, Luna GA, Gámez JC, Alcántar M, et al. (2015) Growth and metabolic responses of whiteleg shrimp Litopenaeus vannamei and Nile tilapia Oreochromis niloticus in polyculture fed with potential probiotic microorganisms on different schedules. Lat Am J Aquat Res 43(3): 435-445.
- Apún MJP, Robles RA, Alvarez RP, Santamaria MA, Arjona O, et al. (2017) Influence of stocking density and exposure to white spot syndrome virus in biological performance, metabolic, immune, and bioenergetics response of whiteleg shrimp Litopenaeus vannamei. Aquaculture 479: 528-537.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Castello F (1993) Acuicultura marina: Fundamentos biológicos y tecnológicos de la producció Universitat de Barcelona. p. 739.
- CONAPESCA (2014) Registro y estadística pesquera y acuí Consulta especifica por especies. Comisión Nacional de Acuicultura y Pesca. SAGARPA, México.
- Daliri M, Paighambari SY, Shabani MJ, Pouladi M, Davoodi R (2012) Length-weight and length-girth relationships, relative weight and relative condition factor of four commercial fish species of northern Persian gulf. Ann Res Rev Biol 2(1): 15-26.
- Godínez EJA, Maldonado M, López V, Carrillo M (2011) Ciclo reproductivo de la cabrilla sardinera Mycteroperca Rosacea en la Bahía de La Paz, Mé Cienc Mar 37(4): 425-441.
- Froesen R (2006) Cube law condition factor and weight-length relationships: History, meta-analysis and recommendations. J Appl Ichthyol 22(4): 241-253.
- Kori O, Ake JEG, Idoge E (2005) Hematological characteristics of the african snakehead, Parachanna obscura. Afr J Biotechnol 4(6): 527-530.
- Lilliefors HW (1967) On the Kolmogorov Smirnov test for normality with mean and variance unknown. J Am Stat Assoc 62(318): 399-402.
- Martínez LA, Diana CEU, Pérez AE, Raul A (2008) Dynamics of a prorocentrum minimum bloom along the northern coast of Sinaloa, Mexico. Cont Shelf Res 28(14): 1693-1701.
- Romestend B, Halsband E, Bragoni G, Knezevic G, Maric B, et al. (1982) Haematological study of erythrocytic constants in some marine and freshwater fishes. AGRIS 46: 147-56.
- Sánchez RI (2005) Hábitos alimenticios del pargo amarillo Lutjanus argentiventris (Peters, 1869) en la Bahía de la Paz, B. C. S., Mé Tesis de Maestría p. 123.
- Yousefi M, Abtahi B, Kenari AA (2012) Hematological, serum biochemical parameters, and physiological responses to acute stress of Beluga sturgeon (Huso huso, Linnaeus 1785) juveniles fed dietary nucleotide. Comp Clin Path 21(5): 1043-1048.
© 2021 Leonardo Ibarra Castro. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






