- Submissions

Full Text
Examines in Marine Biology & Oceanography
Biofilm Surrounded Hexachlorobenzene (HCB) Crystals and Wastewater Purification
Gero Benckiser*
Institute of Applied Microbiology, Interdisciplinary Research Center, Germany
*Corresponding author: Gero Benckiser, Institute of Applied Microbiology, Interdisciplinary Research Center, Germany
Submission: October 26, 2020;Published: December 18, 2020

ISSN 2578-031X Volume3 Issue5
Abstract
Recalcitrant aromatic compounds reach the aerobic, anaerobic run Wastewater Treatment Plant (WWTP) units. The outflow is discharged into receiving waters and transported into oceans. Recalcitrant aromatic compounds as Di-n-Butyl Phthalate (DBP) or HCB concerning is asked can they serve denitrifying and archaea in WWTPs and oceans as electron donators. A special research program, financed by the German Research Foundation, tried giving an answer by adding effluent of the experimental WWTP, Civil Engineering Department, University Stuttgart, Germany, to triplicated, 50ml of a chloride-free, nitrate mineral salt medium containing 200ml flasks for enriching denitrifying bacteria and archaea. On chloride-free, nitrate containing mineral salt medium agar plates which contained Inter alia DBP or HCB as sole carbon source pure cultures were isolated. The with DBP or HCB as pure cultures received strains B20b1 (DBP) and B8a (HCB) served as inoculum for studying in airtight closed, the nitrate mineral salt medium containing, autoclaved, and with about 107 cells of the denitrifying, on DBP or HCB agar growing isolates inoculated flasks degradation success. About DBP has been reported [1], about the U-14C-labeled HCB experiments under a to 10 or 19 Vol% O2 adjusted Helium (He) atmosphere is reported here. From the in hexane dissolved added U-14C-labeled HCB hexane evaporated during flask evacuation and afterwards crystalized HCB covered the bottle bottom. During the 5 months of incubation (30 ᵒC) the as Pseudomonas aeruginosa identified strain B 8a formed around the HCB crystals a biofilm and monthly controlled O2, CO2, N2O and N2 changes in the flask atmosphere, the from the originally added 156mg HCB as 14CO2 released 0.084 ± 0.3mg into the flask headspace, and the 13.26 ± 1.03mg accumulated U-14C-HCB in cells and biofilm of the 10% O2 flask variant signalized HCB interactions which are discussed.
Keywords: Tertiary wastewater treatment; Pseudomonas aeruginosa; Denitrifying wastewater effluent isolate; Hexachlorobenzene degradation; Biofilm formation
Introduction
Wastewater aeration is the first step in achieving optimal conversion of the WWTP reaching organic, NH2-substituted compounds into CO2 and NO3- by O2 respiration and nitrification. The pre-purified wastewater is as next step channeled through a non-aerated zone to reduce formed NO3- load in the first purification step largely to N2 with the not O2 respired, leftover organic compounds as carbon source [2]. The arising question is now are O2 respiration withstanding recalcitrant organic compounds suitable carbon sources for the denitrifying wastewater community? For giving an answer the Civil Engineering Department of the University Stuttgart, Germany, initiated a special, by the German Research Foundation financed research program in which the soil microbiology unit of the Soil Science Department, University Stuttgart-Hohenheim, has tested whether compounds as DBP or HCB are suitable electron donators for nitrate respiration. If not, such compounds accumulate in the forming sewage sludge or are discharged into the receiving water by finally reaching the oceans. After volume reduction in digesters the sewage sludge is deposited in dumps, spread in often soil nutrient buffer capacity surpassing amounts together with animal slurries from industrial livestock farming on a limited land area, or burnt [3].
Already the Romans realized around 100 years before our calculation of time that wastewater can create problems and connected households to a sewer network and forced by a growing population and industrialization the German capital Berlin hired 14.364 Hectares (Ha) land in 1913 and installed a sewage farm on which Berlin’s wastewater was spread (1988 still to 34%). In 1926 Berlin’s industry contributed to the discharged wastewater on the sewage farm with 7.3% and from the continuously with nutrients enriched sewage farm heath menacing amounts of side drained NO3- reached the glacial valley wells, groundwater, and surface water (riverbank filtrate), Berlin’s drinking water resources. Such contaminations are meanwhile worldwide observed and Civil Engineering Department of the University Stuttgart, Germany, initiated in the 1970ies a wastewater onsite treatment improving research program with the aim to get new insights in wastewater purification [4]. Concomitantly the U.S. Environmental Protection Agency (USEPA) and other administrations issued detailed guidance for onsite wastewater treatment systems (OWTSs; Design Manual: Onsite Wastewater Treatment and Disposal Systems, USEPA, 1980) and we started with lyophilized effluent of the experimental wastewater treatment plant (Civil Engineering department) by adding the lyophilized effluent powder in a concentration of 0.2% to an artificial, nitrate containing mineral salt medium agar for enriching eon recalcitrant organic compounds pre-adapted denitrifying bacteria strains. Textbooks at the time of experimentation stated that aromatic nuclei are preferred (exclusively) aerobically cleaved, but instead we were successful in bringing the on di-n-butyl phthalate agar growing strain B20b1 and the on HCB agar growing strain B8a in pure cultures [1,5].
The experiments with strain B20b1, deposited at the Sweden type culture collection are described [1] that with (U-14C)-labelled HCB here. Promising to start with (U-14C)-labelled HCB experiments was a reported degradation experiment with hexachlorocyclohexane [6]. A job offers from the International Rice Research Institute, Los Banos, Philippines, at the end of my doctorate (early 1980) to study the phenomenon of iron toxicity with rice prevented to publish the findings with HCB isolate strain B8a earlier, also the circumstance that in spite of made arrangements after my returning to the Stuttgart department the strain was lost. In the 40 years to today transformation experiments with relatively recalcitrant aromatic compounds, even with HCB and under denitrifying conditions, have been repeatedly reported the redox potential cascade in biofilms is much better understood [7-20]. Meanwhile is known that a self-produced, by an extracellular matrix surrounded biofilm makes in hostile environments microorganism communities more resistant and all that encourages to re-discuss the observations made with the wastewater concentrate isolate strain B 8a and HCB and to speculate about oceans and sewage sludge problem solutions.
Material and Method
Denitrifying bacteria enrichment
About 100 liters effluent of the experimental wastewater plant Civil Engineering Department, University Stuttgart, Germany, condensed in a double-walled steel container, through to -15 ᵒC cooled methanol flow. The condensed effluent was lyophilized, and the obtained concentrate was characterized by determining the ignition loss (ash content), the trace elements with a flame atomic absorption spectrometry (FAAS; Perkin-Elmer Model 303; [21]) and the fatty acids with a Flame Ionization Detector (FID) in a HP 6890 gas chromatograph [22] (carrier gas N2 30ml sec-1; HP-88 INNOWAX detector, carrier gas H2, 40ml min-1, make-up gas air 450ml min-1; Agilent 5183-4647-columns: Model Nr. 112-88A7, 100m x 0.25mm ID, 0.2µm, Model Nr. 19091N-133, 30m x 0.25mm ID, 0.25µm; column injector, detector temperature 250 °C). The such characterized effluent powder was in a 0.2% (w/v) concentration added to a chloride free, nitrate containing mineral salt medium and to agar plates for preselecting and enriching denitrifying, on not easily degradable compounds pre-adapted bacteria (Na2HPO4, KH2PO4, KNO3, NH4OH, MgSO4 x 7H2O, CaSO4, Na2MoO4 x 2H2O, Co(CH3CO2)2 x 4H2O, Mn(CH3COO)2, C6H8O7·nFe·nH3N g L-1, 11.2, 0.8, 6.0, 1.0, 0.2, 0.002, 0.001, 0.001,0.001, 0.005g L-1 dist. water, 15g agar-agar L-1, pH 7.4, respectively, [23,24]). During incubation in an anaerobic jar under a 9:1 N2-CO2 atmosphere up growing colonies were fractionated streaked out on fresh agar plates which instead of effluent powder contained the frequently used non-ionic emulsifier polyethylene sorbitane monooleate (Tween 80), phthalate based aromatic structures, trichlortoluene, 1,2, 1,3, 1,4 dichlorobenzene, 1,2,4 trichlorobenzene, y-hexachlorocyclohexane, or HCB as sole carbon source. Up growing colonies were sub-cultivated until morphologically uniform shaped cell structures became visible under the microscope.
Identification of the on HCB containing agar plates isolated strain B 8a
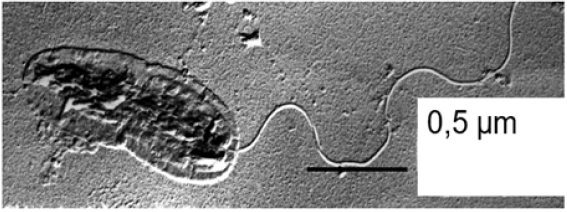
Under the microscope as morphologically uniform shaped strain B 8a cells cell was for describing morphology and flagellation type sputtered with Pt/Ir 45ᵒ for making the cells visible under the transmission electron microscope Siemens 80 kV: (Figure 1). For getting some genomic information DNA was extracted with Na-dodecyl sulfate and with lysozyme the DNA-strands separated. The DNA guanine, cytosine Mol% was estimated in a temperature-controlled cuvette by the melting point method (Tm-53.9 x 2.44; DNA absorbs UV light of 260nm, [25]). As further identification criteria the metabolic potential: growth at 4 and 41 ᵒC, oxidation of gluconate, aromatic ring cleavage, gelatinase, lecithinase, amylase activity, levane formation, the capability to gain energy with trehalose, inositol, geraniol, or acetamide and the strain B 8a properties of secondary taxonomic value as gaining energy with sugars (D-fructose, D-glucose, L-arabinose, 2-ketoglutarate, starch), alcohols (ethanol, adonitol, sorbitol, glycerol), organic acids (propionate, butyrate, myristic acid), aromatic, cyclic (chinate, protocatechuate, benzoate, p-, m-OH benzoate, benzole, phenylacetate, anthranilate, benzylamine, Hippurate), and N containing compounds (glycine, ß-alanine, arginine, amino valerate, keratin, betaine of strain B 8a were tested and the antibiotic sensibility by measuring by the antibiotics: gentamycin, kanamycin, neomycin, novobiocin, paromomycin, rifamide, rifampicin, rifamycin, sulphatriad, cephalexin, cephalotin, erythromycin, nitrofurantoin, ampicillin, bacitracin, methicillin, oxacillin, penicillin g, polymyxin, b colistin sulfate, fusidin acid, doxycycline, tetracycline, carbenicillin, chloramphenicol, and clindamycin caused inhibition zones [26-28].
Figure 1:TEM picture of the 80.000 times magnified denitrifying strain B 8a with polar flagellation and identified according to Stanier et al. [44] as Pseudomonas aeruginosa.
(U-14C)-labelled HCB experiment
For giving an answer on the question: can denitrifying bacteria grow with an organic compounds HCB as electron donator 50ml of a chloride-free mineral base medium was enriched with 700µg ml-1 NO3--N and filled in triplicated 200ml Erlenmeyer flasks. After autoclaving (10min, 121 ᵒC) the flasks were air-tight closed with rubber stopper and with sterile syringes in 2.5ml hexane dissolved 7.5mg cold and 0.3mg (U-14C)-labelled HCB and about 107, denitrifying, 3 times in sterile water pre-washed strain B 8a cells (Figure 1) added. Afterwards the flask atmosphere was 5 times replaced by filtered helium (purity 99.9, Messer Griesheim) and adjusted to an O2 (v/v) concentration of 10 or 19%. All not-inoculated and HCB-free control flasks and those which were inoculated and contained (U-14C)-labelled HCB were incubated in an anaerobic jar under a 9:1 N2-CO2 atmosphere as recommended for food preserving for 5 months. During flask evacuation the HCB dissolvent hexane evaporated, and HCB crystals covered the flask bottom (Figure 2). In the flask atmosphere monthly with the Gas Chromatograph (GC), model F22, equipped with a Thermal Conductivity (TCD) to an Electron Capture (ECD) connected detector (Perkin-Elmer, Überlingen, Germany) the CO2, N2O, O2 and N2 changes were controlled. The gas samples passed after injection a Porapak-Q packed, stainless-steel column and for separating O2, N2 a 5a molecular sieve packed column (helium flow 20ml min-1, column temperature, 54 ᵒC, injector, 54 ᵒC, detector 150 ᵒC, and filament 300 ᵒC). The peak areas were integrated with the Perkin-Elmer GC system SIP-1.
With help of a vacuum pump the flask atmosphere was sucked after the 5 months of incubation through 3 in line connected washing filters and the CO2 collected in 70ml of a 0.1N NaOH. In 1,2,4 trimethyl benzene sinicillator (Packard Insta Solve, nr 6003183), into which 0.1N NaOH proportions were transferred the 14CO2 was quantified with a Packard Tri Carb Liquid scintillation spectrometer (Model 3380). Since the liquid flask phase could not be alkalized out of NO3--N interference reasons, the assumed HCB conversion products in the mineral salt medium were extracted 3 times with 50ml hexane. Afterwards the hexane (150ml) was passed through a 2µ sterile filter for separating liquid phase and the grown bacteria, biofilm biomass (Figure 2A). The 14C share on the with 50ml hexane rewashed filters was converted into CO2 with an incinerator Packard Tri Carb sample oxidizer (Model 306) that was transferred into carbosorb scintillator (Packard) and after injection into a Packard Tri Carb Liquid scintillation spectrometer (Model 3380) quantified [29,30]. The 14C share in liquid flask phase was quantified by adding 10ml 0.1N NaOH to 20ml of the 150ml hexane extract. Aliquots of the into the eventually HCB conversion products containing NaOH phase were injected into the Packard Tri Carb Liquid scintillation spectrometer (Model 3380) and quantified. From the inoculated variants the 14C radioactivity, measured in the controls, was subtracted and 14C in biofilm, cells, the liquid phase and the14CO2 in 150ml flask atmosphere in mg, Eventually liberated chloride from the HCB crystals was analyzed titrimetrically [31].
All used chemicals were of analytical grade and purchased from Merck-Schuchardt, Germany. The from Messer Griesheim, Germany, purchased He had a purity of 99.998% and the by the Hoechst company provided (U-14C)-labelled HCB a specific activity of 9.0Ci g-1 and a radiochemical purity >> 99%. The water of the 50ml culture medium could have retained after evacuation hexane in an amount of maximum 0.5 mg (25 ᵒC) [32].
Result
Strain B 8a formed a green-blue phenazine pigment as Pseudomonas aeruginosa strains deposited in type culture collections was, monotrich, polar flagellated (Figure 1) and had a Guanine, Cytosine (GC) Mol% of 66.8 [33-36]. These of primary taxonomic value differentiating characteristics supplemented the tested physiological capabilities and antibiotic sensibilities of strain B 8a that grew at 41 ᵒC not at 4 ᵒC, could oxidize gluconate, cleave ortho-protochatechuate and other aromatic ring structures, and showed a positive lecithinase, esterase reaction, but a negative amylase and variable gelatinase reaction. Tolerated was cetrimide and energy could be gained with geraniol and acetamide, D-glucose, 2-ketogluconate, ethanol, adonitol, glycerine, propionate, butyrate, myristic acid, polyethylenesorbitanemonooleate (Tween 80), chinate, protochatechuate, benzoate, p-OH benzoate, anthranilate, glycine, ß-alanine, arginine, aminovalerate, and betaine. On the with Oxoid disks in a concentration of 10, 300, 30, 30, 300, 10, 300, and 100µg offered antibiotics gentamycin, doxycycline, rifampicin neomycin, sulphatriad, colistinsulfate, polymyxin B, and carbenicillin reacted strain B 8a with a bacteria-free areola of 14, 6, 6, 4, 8, 4, 6, 9mm, respectively. Based on all these tests strain B 8a (Figure 1) belongs very likely to the P aeruginosa family.
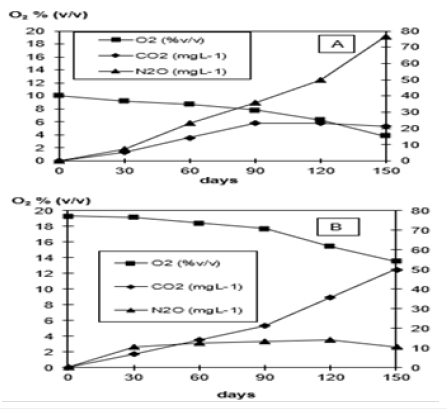
Whether strain B 8a (Figure 1) would be able to use the 6-fold chlorinated HCB as carbon source as tested in a (14U)-labelled HCB experiment showed at first that strain B 8a started to form a biofilm around the (14U)-labelled HCB crystals around 2 months after incubation (Figure 2). The monthly in the flask atmosphere measured O2 decreases and CO2, N2O and N2 releases into the O2/helium atmosphere (Figure 3), the 14CO2, released in significantly lower concentration, and the 14C in the liquid mineral salt, NO3- containing medium phase and in the P aeruginosa cells and biofilm (Table 1) signalized interaction between HCB and strain B 8a. The GC measured O2 concentration in the flask atmosphere of the 10% O2 variant decreased from about 10 to 4% and in the 19% O2 variant from 19 to 14%. With O2 decrease in the flask atmosphere of the 10% O2 variant N2O increase to almost 80mg L-1, in the 19% O2 variant to around 10mg N2O L-1 (Figure 3A). Reverse was the observation with CO2 that accumulated in the flask atmosphere of the 19% O2 variant to about 50mg CO2 L-1, in the 10% O2 variant to only 20mg CO2 L-1, whereas the 14CO2 release into the flask atmosphere of 10% 19% O2 variant was with 0.072 and 0.060mg almost similar (Table 1). About the CO2 release differences could be speculated that they may rely on not evaporated metabolized hexane but more likely on metabolized dead strain B 8a cells, used by the survivor cells as carbon source and that better at a higher O2 availability [37,38]. The addition of wastewater concentrates and a higher O2 availability was biofilm formation and HCB accumulation concerned rather preventing than supporting (Table 1). The high Zn concentration of 1740.4µg g-1, combined with 113.1, 102.6, 9.5, 2.3 Cu, Ni, Pb, Cd µg g-1 (0.3, 0.03, 0.03, 0.002, 0.001% of the ash content) in the effluent concentrate and the nearly 77% lower nitrate conversion in the 19% O2 partial pressure variant than in the 10% O2 (v/v) variant could be an explanation (Table 1; [39,40]).
Figure 2:Strain B 8a biofilm after 5 months of incubation in a nitrate and HCB containing mineral salt medium (A,B,C) and D [16].
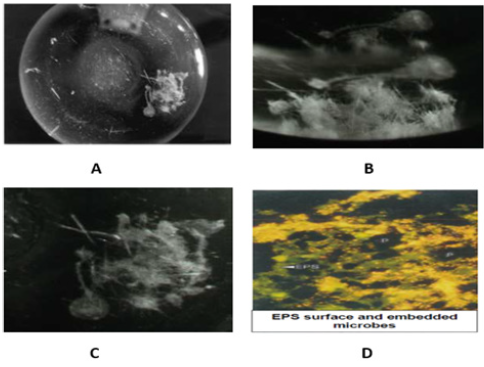
Table 1:U-14C-hexachlrobenzene transformation in presence of Pseudomonas aeruginosa B 8a in a chloride-free mineral
salt medium (pH 7.4) after a 5-month incubation (30 oC) at 2different O2 partial pressures® and with and without 40g
L-1 concentrate of the wastewater treatment plant effluent, model plant Civil Engineer Department, University Stuttgart,
Germany (U).
A. Values are the mean of 3 parallels minus controls
B. Original HCB content was 7.5mg cold, 0.3mg (U-14C)-labelled HCB per flask.
C. The used wastewater concentrate added to separate flasks with cold (U-14C)-HCB contained 388.2mg chloride.
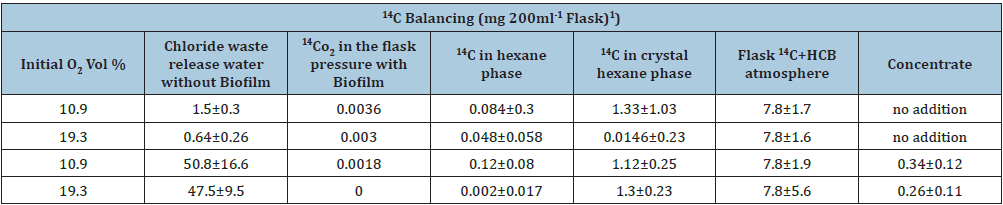
Figure 3: O2 decrease and CO2, N2 and N2 release at 2 different O2 partial pressures into the helium atmosphere during a 5-month incubation of strain B 8a, identified as Pseudomonas aeruginosa, in presence of HCB as only carbon source.
Discussion
From the estimated to 1.2 × 1030 prokaryotic cells on Earth roughly 4 × 1029 survive in deep oceanic sub-surfaces, 5 × 1028 in upper oceanic sediments, 3 × 1029 in deep continental sub-surfaces, and 3 × 1029 in ocean surface microlayers, the phyllo- and atmosphere, the groundwater, in solid soil particles surrounding water films, and in, on human, animal bodies [17]. For guaranteeing a better surviving prokaryotes have invented the capability to conserve energy by O2 respiration, anaerobic respiration, and fermentation and to form phenanzines and biofilms [41,42]. Most of these capabilities P. aeruginosa strains have for their disposal. They can conserve energy by O2 and NO3- respiration and have a remarkable adaptability on pollutants and antibiotics through phenanzine and biofilm self-production (Table 1; Figure 2) [27,43-46]. Biofilms consist out of nucleic acids, polysaccharides, lipids, proteins, outer membrane proteins and to approximately 30% the proteins go back on biofilm attached microbes [47,48]. A polymeric substance (EPS) layer surrounds a biofilm that consists to about 97% out of water, extracellular DNA (eDNA), gel-forming polysaccharides, proteins, insoluble amyloids, and cellulose. EPS facilitates the liquid transport into the biofilm and the escaping from the biofilm [37,49]. Inside of biofilms living pro- and eukaryotic Geno- and phenotypes sustain a wide range of physiological states (redox potentials) and distinct metabolic pathways [50-53]. Possess biofilm inhabiting or on the EPS layer attached planktonic cells as strain B 8a (Figure 1) fimbriae, pili, or a flagella the escaping from the biofilm into a growth and surviving better supporting outside environment is easier, but for escaping a DNA-specific endonuclease End A that degrades eDNA is needed [54-56].
Into mature, in average 210μm thick biofilm oxygen penetrates to a depth of about 50μm and large anoxic biofilm region beyond the O2 zone narrows the growth rate of their residing bacteria, archaea, fungi, protozoa compared with logarithmic growing planktonic cells drastically to 0.02h−1 [57,58]. Thus, biofilm surround or attached planktonic cells will first enter into logarithmic growth when they have left the biofilm interior or EPS layer attached position. The biofilm interior inhabiting microbial cell types are clustered in micro-colonies and surrounded by a mechanically stabilized, specific living environment (Figure 2) that after having perceived an eDNA constituted signal starts developing a higher resistance against bactericidal antimicrobials [59-62]. The EPS surface-adherent eDNA binds aminoglycosides, acidifies the system, and helps inducing an on genes instructions based PhoPQ and PmrAB two-component regulatory system. Now an aminoglycoside antibiotic resistant P. aeruginosa phenotype can develop [63,64]. In the transition from a planktonic into an interior biofilm lifestyle involved is the messenger molecule c-di-GMP that could have helped strain B8a to metamorphose and to attach after a longer adaptation phase to the HCB crystals and slowly to start with interactions (Figure 1-3, Table 1 & 2); [65]. Metamorphosing concerned P. aeruginosa strains possess several advantages. They are able to gain energy by oxygen and nitrate respiration and possess the capability to produce small pyocyanin molecules, phenazines. Pyocyanin is an osmotic pressure cheater and drives extracellular-matrix mediated biofilm expansion. Phenazines can alternate to oxygen act as electron acceptors [66]. With the wealth of microbial energy conserving surviving strategies equipped P. aeruginosa strains can survive in water saturated soil pores, sewage sludge flocs, in ocean sediments, and as shown in biofilm structures (Figure 2). In an arable fields to that 14C labelled HCB were added from the HCB only 1 remaining % could be found after soil extraction and may indicate that bacteria as the P. aeruginosa strain B8a may have played a role [11].
Table 2:Characterization of the lyophilized wastewater concentrate, experimental wastewater treatment plant effluent,
Civil Engineer Department, University Stuttgart, Germany
A. Caprinate (6.2%), laurate (2.8%), myristinic acid (5.9%), palmitic acid (20.1%), palmitoleic acid (20.6%), stearic acid
(17.0%), öleic acid (22.4%), linoleic acid (4.3%), arachidic acid (0.3%).
B. Zn, Cu, Ni, Pb, Cd 0.3, 0.03, 0.03, 0.002, 0.001%, respectively, the relatively high Zn% can cause toxic effects.

The micro scaled chemical gradients in biofilms and the genotypic variation of embedded and micro-clustered bacteria, archaea, fungi, algae in the biofilm interior make a biofilm to a predestined environment for selection, mutation, stochastic gene expression, to an exhibited, on varying conditions adapting environment. A multicellular In situ biofilm structure with attached planctomycetes is meanwhile seen as emergent form of bacterial life, a surviving strategy that completely differs from that of free-living bacteria. The laser-based imaging techniques, a variety of sensor types, and other physical, chemical, biological techniques opened a quite comprehensive insight in the biofilm organization, and we understand reasonably how the microbial community inside of a biofilm interacts [67-70]. Apparently nitrate and pyocyanin relieve the biofilm external and internal digestion and all mentioned biofilm properties make biofilms to one of the oldest, ubiquitously dynamic protection system and to the most successful form of life (Figure 2; Table 1) [20]. The biofilm inherent microscale chemical, biological gradients may support an attacking of recalcitrant organic substances as HCB as (Table 1) and the findings of others let assume [30,38]. The oxygen limitation in biofilms to which also P aeruginosa strain B8a was exposed in the described experiment, underlines the finding to such conditions and tobramycin, ciprofloxacin, carbenicillin, ceftazidime, chloramphenicol, tetracycline exposed bacteria rather tolerate such inhibiting compounds, perhaps also because a gene and phenazine controlled MexGHI-OpmD efflux pump can transport growth inhibiting toxins out of the biofilm [71-75]. Specifically, Gram-negative, denitrifying, redox-cycling skilled bacteria as the P. aeruginosa strain B8a may in presence of nitrate and supported by self-produced antibiotic active phenazine be enabled to metamorphose from a wild-type into a self-resistant biofilm inhabitant that is then able to attach after a certain adaptation phase on HCB crystals (Figure 2; Table 1).
Biofilm formation at high nutrient availability as conceivable in ocean sediments, in water retaining soil pores, in sewage sludge flocs of wastewater treatment plants, in water films around solid particles can be material destructive [76]. Biofouling, bio-corrosion is the term and the microbial activity beyond is identified as a costly problem for the public but a beneficial one for the biocides, cleaners, and antifouling materials producing industry. Early biofouling warning system are required for saving costly countermeasures, particularly in our industry like organized wastewater treatment plants [77-80]. Because biofouling is today majorly indirectly diagnosed and cured with biocides biofilm formation inside of sewage sludge flocs must be understood, the more restricted, huge amounts of sewage sludge producing WWTPs retention times in particular in steadily growing cities with concentrating administrations and industries at and the produced sewage sludge overburden deposition dumps and arable land on that in soil buffer capacity surpassing amounts industrially produced animal wastes and sewage sludge residues are spread [80-83]. Conceptualized for rural regions are wastewater flooded constructed wetlands in which anaerobic zones are expanding, a biofilm pore, void, channel arrangement develops that favours O2 respiration, NO3- respiration, and fermentation switching and a co-metabolic attacking of recalcitrant molecules as HCB may start (Figure 3; Table 1) [84,85].
For minimizing environmental pollution out of overproduced sewage sludge, animal wastes, and plant residues a relatively stable, carbon, nitrogen phosphorus, trace elements, recalcitrant organics, toxins, and other waste components integrating, less volume biochar can be fabricated by the pyrolysis/carbonization technique, but such an approach is only interim solution [66]. The biochar product can be considered as a kind of slow-release fertilizer and may find in agriculture a field of application. Biochar fabrication opens for science and engineering a multidisciplinary study field and contributes to a less alarming storage situation. Into an oligotrophic bulk soil incorporated biochar provides the mostly starving microbial community sufficient adaptation time to start transforming the slow out of biochar flowing ingredients. As observable in the few millimeters thick rhizo-, drill spheres (earthworm burrow walls) the out of biochar flowing nutrients will enhance metabolism and a co-metabolizing pollutants will set in [85-90]. Such a system differs from the situation in wastewater treatment plants microbial community forced in restricted retention times to convert as much as possible from the nutrient rich inflows. Microbes are generally patchy distributed in bulk soils but also in sewage flocs and long-term forced to switch between starving and soil nutrient satiety surpassing soil conditions, e.g., near decaying cadavers (Bruyn 2016 [78]). In the neighborhood of incorporated biochar, it is conceivable that out of biochar diffusing compounds as the plasticizer di-n-butyl phthalate, trichloroethylene, or phenolic structures as HCB are co-attacked, co-metabolized by the mostly hidden working soil microbial capabilities [91]. In this context biochar amendment in agriculture is problem solving when the sewage sludge concerned pyrolysis technique is further developed and reasonably understood [92-99]. A biochar-based agriculture and wastewater treatment integrating approach is a promising interim solution for a mass-production orientated economy which must less trust in a worldwide waste distribution. Understanding the biofilm formation around the HCB crystals better may provide valuable hints towards the hidden working soil microbial world with its mineralizing capabilities of manmade wastes.
Conclusion
In soils and WWTPs hidden is a high degradation potential of manmade organic compounds. However, longer adaptation times are required for being successful in reducing the incoming recalcitrant organic compound load. In particular recalcitrant organic compounds as HCB afford longer retention times and sewage sludge flocs, soil pores, ocean sediments and biofilms are inspiring environments for recalcitrant organic compounds accumulation and degradation. Favorable seem to be reduced oxygen, not-aerated denitrifying conditions and aerobic, anaerobic interface environment as biofilms. Biochar fabricated out of sewage sludge by the pyrolysis/carbonization technique may provide a worthwhile interim solution for managing in particular WWTPs in enlarging cities and reducing pollution.
Acknowledgement
During writing I benefitted a lot from the biofilm research work of Hans-Curt Flemming, who also researched in the program, initiated by the Civil Engineering Department, University Stuttgart, Germany.
Compliance with Ethical Standards
Declaration
I declare that I have no conflict of interest and the using the wastewater concentrate, gained from the wastewater of Civil Engineering Department, University Stuttgart, Germany, was approved at the time of experimentation by the Department Head Prof Hunken.
Further
I declare that in the submitted study human participants, human data nor human tissue are involved and I state “Not applicable” in this section.
References
- Benckiser G, Ottow JCG (1982) Metabolism of the plasticizer di-n-butylphthalate by Pseudomonas pseudoalcaligenes under anaerobic conditions, with nitrate as the only electron acceptor. Appl Environ Microbiol 44(3): 576-578.
- Benckiser G, Eilts R, Linn A, Lorch HJ, Sumer E, et al. (1996) N2O emissions from different cropping systems and from aerated, nitrifying and denitrifying tanks of a municipal wastewater treatment plant. Biol Fertil Soils 23: 257-265.
- Cooper PF (2000) Historical aspects of wastewater treatment. In: Lens P, Zeeman G, Lettinga G (Eds.), Decentralised sanitation and reuse: Concepts, systems and implementation. 4(2).
- Hunken KH, Sekoulov ID, Bardtke D (1971) Tertiary treatment of biologically treated wastewater by means of an algal filter. Water Res 5(7): 453-457.
- Evans WC (1977) Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature 270(5632): 17-22.
- Hsu TS, Bartha K (1979) Accelerated mineralization of two organophosphate insecticides in the rhizosphere. Appl Environ Microbiol 37(1): 36-41.
- Wang Z, Ma X, Yao Z, Quanheng Y, Zhao W, et al. (2018) Study of the pyrolysis of municipal sludge in N2/CO2 App Therm Eng 128: 662-671.
- Fathepure BZ, Tiedje JM, Boyd SA (1988) Reductive dechlorination of hexachlorobenzene to tri- and dichlorobenzenes in anaerobic sewage sludge. Appl Environ Microbiol 54(2): 327-330.
- Fiedler H, Hub M, Willner S, Hutzinger O (1995) Stoffbericht Hexachlorbenzol (HCB). Germany.
- Adrian L, Szewzyk U, Wecke J, Görisch H (2000) Bacterial dehalorespiration with chlorinated benzenes. Nature 408 (6812): 580-583.
- Brahushi F, Dörfler U, Schroll R, Munch JC (2004) Stimulation of reductive dechlorination of hexachlorobenzene in soil by inducing the native microbial activity. Chemosphere 55(11): 1477-1484.
- Bhatt P, Kumar SM, Mudliar S, Chakrabarti T (2007) Biodegradation of chlorinated compounds-A Review. Critical Rev Environ Sci Technol 37(2): 165-198.
- Wang Y, Kern SE, Newman DK (2010) Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J Bacteriol 192(1): 365-369.
- Hreiz R, Latifi MA, Roche N (2015) Optimal design and operation of activated sludge processes: State-of-the-art. Chem Eng J 281: 900-920.
- Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nature Rev Microbiol 6(3): 199-210.
- Flemming HC, Wingender J, Szewzyk U, Peter S, Scott A, et al. (2016) Biofilms: An emergent form of bacterial life. Nature Rev Microbiol 14(9): 563-575.
- Flemming HC, Wuertz S (2019) Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol 17(4): 247-260.
- Price WA, Dietrich LEP, Newman DK (2007) Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa J Bacteriol 189: 6372-6381.
- Neczaj E, Fijałkowski K, Grobelak A, Anna G, Krzysztof F, et al. (2017) Sewage sludge disposal strategies for sustainable development. Environ Res 156: 39-46.
- Lin YC, Sekedat MD, Cornell WC (2018) Phenazines regulate nap-dependent denitrification in Pseudomonas aeruginosa J Bacteriol 200(9): e00031-00081.
- Waite RD, Papakonstantinopoulou A, Littler E, Curtis MA (2005) Transcriptome analysis of Pseudomonas aeruginosa growth: Comparison of gene expression in planktonic cultures and developing and mature biofilms. J Bacteriol 187(18): 6571-6576.
- Elefsiniotis P, Wareham DG (2007) Utilization patterns of volatile fatty acids in the denitrification reaction. Enzyme Microb Technol 41(1-2): 92-97.
- Fabig W, Ottow JCG (1979) Isolierung und Identifizierung neuer, denitrifizierender Bakterien aus einer Modellklaranlage mit anaeroben Festbettreaktoren. Arch Hydrobiol 85: 372-391.
- Fabig W, Ottow JCG, Muller F (1980) Failure of denitrifying bacteria to utilize benzoic acid under anaerobic conditions with nitrate as only terminal electron acceptor. Eur J Appl Microbiol Biotechnol 9: 133-135.
- Matin A, Little CD, Fraley CD, Keyhan M (1995) Use of starvation promotors to limit growth and select for trichloroethylene and phenol transformation activity in recombinant Escherichia coli. Appl Environ Microbio 61(9): 3323-3328.
- Kehrein P, Loosdrecht M, Osseweijer P, Marianna G, John P, et al. (2020) A critical review of resource recovery from municipal wastewater treatment plants-market supply potentials, technologies and bottlenecks. Environ Sci Water Res Technol 6(4): 877-910.
- Spoering AL, Lewis K (2001) Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 183(23): 6746-6751.
- Yan J, Nadell CD, Stone HA, Ned S, Bonnie L (2017) Extracellular-matrix-mediated osmotic pressure drives Vibrio cholerae biofilm expansion and cheater exclusion. Nature Com 8(1): 327.
- Engst R, Fritsche W, Knoll R, Kujawa M, Straube G, et al. (1979) Interim results of studies of microbial isomerization of gamma-hexachlorcyclohexane. Bull Envronm Contam Toxicol 22(4-5): 699-707.
- Payne DE, Boles BR (2016) Emerging interactions between matrix components during biofilm development. Curr Genet 62(1): 137-141.
- Danneberg E (1958) Mercurimetrie zur Chloridbestimmung. Anal Chim Acta 18: 315-316.
- Marti E, Huerta B, Rodríguez MS, Damià B, Juan J, et al. (2014) Characterization of ciprofloxacin-resistant isolates from a wastewater treatment plant and its receiving river. Water Res 61: 67-76.
- Cezairliyan B, Vinayavekhin N, Grenfell LD, Grace J, Alan S, et al. (2013) Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis PLoS Pathog 9(1): e1003101.
- Schlatter DC, Reardon CL, Johnson MJ, Erin B, Kendall K, et al. (2019) Mining the drilosphere: Bacterial communities and denitrifier abundance in a no-till wheat cropping system. Front Microbiol 10: 1339.
- Sakhtah H, Koyama L, Zhang Y, Diana K, Blanche L, et al. (2016) The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. PNAS 113(25): E3538-E3547.
- Van EJ, Bartels TJ (1968) Paraffin oxidation in Pseudomonas aeruginosa I. Induction of paraffin oxidation. J Bacteriol 96(3): 706-712.
- Tiunov A, Scheu S (1999) Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol Biochem 31(14): 2039-2048.
- Bruyn DJ (2016) The dead and their microbes. Microbe 11(3): 119-124.
- Balemans S, Vlaeminck SE, Torfs E, Leonie H, Laura Z, et al. (2020) The impact of local hydrodynamics on high-rate activated sludge flocculation in laboratory and full-scale reactors. Processes 8(2): 131.
- Klein G (2003) Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int J Food Microbiol 88(2-3): 123-131.
- Benckiser G (1997) Organic inputs and soil metabolism. In: Benckiser G (Ed.), Fauna in soil ecosystems. New York, USA, pp. 7-62.
- Glasser NR, Kern SE, Newman DK (2014) Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol Microbiol 92(2): 399-412.
- Eschbach M, Schreibe K, Trunk K, Jan B, Dieter J, et al. (2004) Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J Bacteriol 186(14): 4596-4604.
- Stanier RY, Palleroni NJ, Doudoroff M (1966) The aerobic Pseudomonads: A taxonomic study. J Gen Microbial 43(2): 159-271.
- Königs AM, Flemming HC, Wingender J (2015) Nanosilver induces a non-culturable but metabolically active state in Pseudomonas aeruginosa. Front Microbiol 6: 395.
- Yayan J, Ghebremedhin B, Rasche K (2015) Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. PLoS One 10(10): e0139836.
- Davies DG (2011) Biofilm Dispersion. In: Flemming HC, Wingender J, Szewzyk U (Eds.), Biofilm Highlights, Pp. 1-26.
- Jagnow G, Haider K, Ellwardt PC (1977) Anaerobic dechlorination and degradation of hexachlorocyclohexane isomers by anaerobic and facultative anaerobic bacteria. Arch Microbiol 115(3): 285-292.
- Toyofuku M, Roschitzki B, Riedel K, Eberl L (2012) Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J Proteome Res 11(10): 4906-4915.
- Verslyppe B, Smet W, Baets B, Paul D, Peter D (2014) Strain Info introduces electronic passports for microorganisms. Syst Appl Microbiol 37(1): 42-50.
- Folsom JP, Richards L, Pitts B, Frank R, Garth D, et al. (2010) Physiology of Pseudomonas aeruginosa in biofilms as revealed by transcriptome analysis. BMC Microbiol 10: 294.
- Carsten M (2011) Competition, communication, cooperation: Molecular crosstalk in multi-species biofilms. In: Flemming HC, Wingender J, Szewzyk U (Eds.), Biofilm Highlights, pp. 29-40.
- Dietrich LEP, Okegbe C, Price WA, Hassan S, Ryan C, et al. (2013) Bacterial community morphogenesis is intimately linked to the intracellular redox state. J Bacteriol 195: 1371-1380.
- Persat A, Nadell CD, Kim MK, Francois I, Albert S, et al. (2005) The mechanical world of bacteria. Cell 161(5): 988-997.
- Beiter K, Wartha F, Albiger B, Staffan N, Arturo Z, et al. (2006) An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Cell 16(4): 401-407.
- Cherny KE, Sauer K (2019) Pseudomonas aeruginosa requires the DNA-specific endonuclease End A to degrade eDNA to disperse from the biofilm. Journal of Bacteriology.
- Borriello G, Werner E, Roe F, Aana M, Garth D, et al. (2004) Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemotherapy 48(7): 2659-2664.
- Schobert M, Tielen P (2010) Contribution of oxygen-limiting conditions to persistent infection of Pseudomonas aeruginosa. Future Microbiol 5(4): 603-621.
- Gilan I, Sivan A (2013) Extracellular DNA plays an important structural role in the biofilm of the plastic degrading actinomycete Rhodococcus ruber. Adv Microbiol 3(8): 543-551.
- Okshevsky M, Meyer RL (2013) The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol 41(3): 341-352.
- Das T, Kutty SK, Tavallaie R, Amaye I, Janjira P, et al. (2015) Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation. Sci Rep 5: 8398.
- Wilton M, Charron ML, Moore R, Lewenza S (2016) Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 60(1): 544-553.
- Bhagirath AY, Li Y, Patidar R, Katherine Y, Xiaoxue M, et al. (2019) Two component regulatory systems and antibiotic resistance in Gram-Negative Int J Mol Sci 20(7): 1781.
- Liu XM, Sheng GP, Luo HW, Feng Z, Juan X, et al. (2010) Contribution of extracellular polymeric substances (EPS) to the sludge aggregation. Environ Sci Technol 44(11): 4355-4360.
- Liu C, Sun D, Zhu J, Jiawen L, Weijie L (2020) The regulation of bacterial biofilm formation by cAMP-CZP: A mini review. Front Microbiol 11: 802.
- Jennings L, Storek KM, Ledvina HE, Charlène C, Lindsey S, et al. (2015) Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. PNAS 112(36): 11353-11358.
- Friedman L, Kolter R (2004) Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol 186(14): 4457-4465.
- Neu TR, Lawrence JR (2014) Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol 23(4): 233-242.
- Flemming HC (2016) EPS-then and now. Microorganisms 4(4): 41.
- Wang Y, Liu L, He J (2019) Biofilms: The microbial “protective clothing” in extreme environments. Int J Mol Sci 20(14): 3423.
- Ramos JL (2004) Pseudomonas biosynthesis of macromolecules and molecular metabolism. Newyork, USA.
- Høiby N, Bjarnsholt T, Givskov M, Søren M, Oana C (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35(4): 322-332.
- Morita Y, Tomida J, Kawamura Y (2013) Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol 4: 422.
- Chatterjee M, Anju CP, Biswas L, Anil K, Gopi C, et al. (2016) Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol 306(1): 48-58.
- Flemming HC (2011) Microbial biofouling: Unsolved problems, insufficient approaches, and possible solutions. In: Flemming HC, Wingender J, Szewzyk U (Eds.), Biofilm Highlights, pp. 81-109.
- Molina MA, Pérez CAA, Leiva CA (2020) Characterization of filamentous flocs to predict sedimentation parameters using image analysis. J Sensors.
- Benckiser G (2019) Plastics, Micro- and nanomaterials, and virus-soil microbe-plant interactions in the environment. Plant Nanobionics, pp. 83-101.
- Bruyn DJ (2016) The dead and their microbes. Microbe 11: 119-124.
- Fey A, Benckiser G, Ottow JCG (1999) Emissions of nitrous oxide from a constructed wetland using a ground filter and macrophytes in waste-water purification of a dairy farm. Biol Fertil Soils 29: 354-359.
- Palansooriya KN, Fung WJT, Hashimoto Y (2019) Response of microbial communities to biochar‑amended soils: A critical review. Biochar 1: 3-22.
- Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41(1): 100-180.
- Williams T (1985) Oligotrophy in soil: Fact or fiction. In: Fletcher M, Floodgate G (Eds.) Bacteria in the natural environment: The effect of nutrient conditions. London, UK, Pp. 81-110.
- Walton BT, Anderson TA (1990) Microbial degradation of trichlorethylene in the rhizosphere: Potential applications for biological remediation of waste sites. Appl Environ Microbiol 56(4): 1012-1016.
- Soerensen J (1997) The rhizosphere as a habitat for soil microorganisms. In: van Elsas JD, Trevors IT, Wellington EMH (Eds.), New York, USA.
- Schink B (2002) Anaerobic digestion: Concepts, limits and perspectives. Water Sci Technol 45(10): 1-8.
- Lee HH, Molla MN, Cantor CR, Collins JJ (2010) Bacterial charity work leads to population-wide resistance. Nature 467: 82-85.
- Gao X, Wu Z, Liu R, Jiayun W, Qiaoying Z, et al. (2019) Rhizosphere bacterial community characteristics over different years of sugarcane ratooning in consecutive monoculture. Biomed Res Int 2019: 4943150.
- Benckiser G, Santiago S, Neue HU (1984) Effect of fertilization on exudation, dehydrogenase activity, iron-reducing populations and Fe2+ formation in the rhizosphere of rice (Oryza sativa L.) in relation to iron toxicity. Plant and Soil 79: 305-316.
- Fathepure BZ, Nengu JP, Boyd SA (1987) Anaerobic bacteria that dechlorinate perhloroethene. Appl Environ Microbiol 53(11): 2671-2674.
- Flemming HC, Tamachkiarowa A, Klahre J, Schmitt J (1998) Monitoring of fouling and biofouling in technical systems. Water Sci Technol 38(8-9): 291-298.
- Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3(2): 208-218.
- Auliffe C (1966) Solubility in water of paraffin, cycloparaffin, olefin, acetylene, cycloolefin, and aromatic hydrocarbons. J Phys Chem 70(4): 1267-1275.
- Petroff CP, Patt HH, Nair PP (1965) A rapid method for dissolving tissue for liquid scintillation counting. Int J Appl Rad Isotop 16(10): 599-601.
- Rahmasary AN, Robert S, Chang IS, Jing W, Jeryang P, et al. (2019) Overcoming the challenges of water, waste and climate change in Asian cities. Environ Manage 63: 520-535.
- Schiessl KT, Hu F, Jo J, Sakila Z, Bryan W, et al. (2019) Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa Nature Com 10: 762.
- Simoska O, Sans M, Fitzpatrick MD, Christopher M, Livia S, et al. (2019) Real-time electrochemical detection of Pseudomonas aeruginosa phenazine metabolites using transparent carbon ultramicroelectrode arrays. ACS Sens 4(1): 170-179.
- Teklehaimanot GZ, Kamikam I, Coetzee MAA, Momba MNB (2015) Population growth and its impact on the design capacity and performance of the wastewater treatment plants in Sedibeng and Soshanguve, South Africa. Environ Manage 56: 984-997.
- Walsh A (1955) The application of atomic absorption spectra to chemical analysis. Spectrochim Acta 7: 108-117.
- Wichern M, Gehring T, Lübken M (2011) Modelling of biological systems in wastewater treatment. Treatise Water Sci 4: 231-263.
© 2020 Gero Benckiser. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






