- Submissions

Full Text
Examines in Marine Biology & Oceanography
Microbiological Evaluation of Scomberomorus brasiliensis (Carite) Muscle Tissue Purchased from Four Locations and Stored Under Three Temperatures
Derrick A Balladin*
University of Trinidad and Tobago, Trinidad W.I.
*Corresponding author: Derrick A Balladin, University of Trinidad and Tobago, Trinidad W.I.
Submission: July 23, 2020;Published: August 12, 2020

ISSN 2578-031X Volume3 Issue5
Abstract
In the Caribbean, fresh fish is a common food source. Fish including Carite (Scomberomorus brasiliensis) are sold daily on the highways by “highway fishmongers". To prevent deterioration, the fish may be sprinkled with a citrus-based juice or salt (NaCl) or kept chilled (10 °C). Although this strategy reduces bacterial growth on the outer regions (skin) of the fish, it is not clear whether this treatment also attenuates bacterial growth and proliferation in the muscles of the fish. This paper seeks to investigate the microbiological loads within the fillet fish muscle tissues and the frozen packages of the muscles of fish sold at supermarkets (single and chain operators). All assays show a constant value of the total aerobic bacteria throughout the specific purchased (single operators and chain supermarkets; and highway fishmongers) and different controlled storage conditions (-15 °C, 1 °C; and 29 °C). A one-way ANOVA test (α= 0.05) indicates no significant difference among the tissue of fish purchased from the four different locations, with a marked effect at the different storage temperatures. The mean Total Bacterial Count increased eight times when the storage temperature increased from -15° to 29 °C and four times when the storage temperature increased from 1° to 29 °C.
Keywords: Scomberomorus brasiliensis (Carite); Total bacterial count; Supermarket operators; Highway fishmongers
Introduction
For most Caribbean nations, the flesh of various fish species which inhabit the sea has become an important food source and better methods of preservation are making it possible for inland communities to be supplied with seafood. Since fish muscle is susceptible to both enzymic and bacterial decomposition, preservation is of the utmost importance. Tanner [1], suggested that preservation is influenced by sanitation which should work to ensure a product with no gross evidence of decomposition and free of undesirable bacteria. To this end, fishermen immediately ice or “lime” the fish after capture and careful handling of the commodity. Increased markets have led to the use of farther removed fishing grounds, necessitating longer trips to the market and longer storage periods. This has forced the adoption of more satisfactory methods of preservation. In Trinidad and Tobago, the safety and quality of fish sold is a cause for concern, in light of the unsanitary conditions under which fish are sold. This can inexorably result in food-borne disease outbreaks, further stressing the fragile health sector.
Efiuvwevwere [2], noted that fresh fish are highly susceptible to deterioration because of the highwater activity, neutral pH, high content of soluble nutrients, and rapid microbial multiplication in their habitat. Fish deterioration is influenced by many factors including the species type, the sanitary quality of the water from which the fish is harvested, and the methods of handling after capture. Generally, the flesh and internal organs of a healthy fresh fish are sterile, but the bacterial load is higher in the skin, gills, and intestine. There are several reviews [1-3]. Cited a strong correlation between bacteriology loading and fish spoilage. These studies suggest that most of the objectionable changes occurring during the storage of fish, except the rancid flavor due to fat oxidation so important in fish with high-fat content, resulting from bacterial activity.
The microbial flora of freshly caught fish
The habitat and incubation conditions dictate the number and type of bacteria isolated from fish muscle tissue. For example, marine fish from colder waters, which after being caught are stored in ice, have a resident flora which is psychrophilic (optimum growth temperature of 18 °C to 20 °C) in an environment of about 3% sodium chloride. However, during storage, the additional flora developing will be characteristic of a freshwater environment (not requiring sodium chloride to grow). Many studies have attempted to characterize the flora of fish [4]. While there are considerable variations in the details of the flora reported, the same relatively few genera predominate. Shewan [3], listed 9 out of 17 investigations (of "cold water" fish), for which the microflora comprised more than 80% (6 investigations), 65 to 67% (2 investigations), or 40% (1 investigation) of Gram-negative rods. Contrastingly, fish taken from warmer or tropical waters more often showed a predominance of Gram-positive bacteria.
Microbiological changes in chill-stored fish
Connell & Shewan [5] made many attempts to identify and list the "active spoilers" in seafood in lieu of quantifying microbiological activity by the total viable count method. When the total number of bacteria growing between 0 °C and 20 °C is monitored, it is found that there is a systematic increase in the Gram-negative genus, namely Pseudomonas. At the end of the edibility lifetime, this genus predominates and therefore, contributes significantly to the total viable count. Controlling the storage temperature seafood is of vital importance since bacterial growth and the biogenic amines production such as histamine are decreased by rapid chilling and appropriate cold storage temperature. The higher the storage temperature, the faster the seafood will deteriorate [6].
There are, however, two severe limitations to using this as a measure of spoilage, viz.: the initial count after catching can be variable and also apparently similar storage systems can give different results. These problems are heightened by the accumulation in stored fish of various chemical compounds which are the metabolic products of bacterial growth. Examples of these compounds are trimethylamine from trimethylamine oxide, various amines and ammonia from amino acids (these influence the concentration of the total volatile bases present); hydrogen sulphide, dimethyl sulphide, and methyl mercaptan from Sulphur containing amino-acids; lower fatty acids from sugars such as glucose and ribose; various carbonyl compounds from lipids, indole, skatole, tyrosine, putrescine, and cadaverine from protein (Hobbs [4]), as well as hypoxanthine and xanthine from nucleotide degradation, all of which contribute to the bitter off-flavor obtained during the sensory evaluation of the fish muscle tissue.
Tests designed to determine the bacteriological quality of fish rely on a determination of the size of the bacteriological population present and/or measure products of the metabolism of the microorganism. The entire subject of bacteriological spoilage of fish is complicated because the predominating microflora are not always the same, the types which grow depending on the variety of fish, its chemical composition, its storage temperature and whether the fish are whole (as in that obtained from highway fishmongers), eviscerated or filleted (as in the supermarket samples). This paper seeks to investigate the microbiological loads within the reportedly sterile fillet fish muscle tissues and the frozen packages of the muscles of fish sold at supermarkets (single and chain operators); highway fishmongers (who will ply their trade in the mornings and evenings); and fresh fish muscle tissue stored at -15 °C; 1 °C; and 29 °C. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Experimental
Materials
Tryptone, Soytone, Fast Green FCF and Ringer Solution Tablets (¼ strength) were all obtained from Oxoid Ltd. (Hampshire, UK.). The Stomacher® bags (30cm×18cm) were obtained from Seward Laboratory (Blackfriars, London). The Biohazard® bags (300cm×600cm) were obtained from American Hospital Supply Corp. (McGraw Park, IL.). The general-purpose Whirl Pak® bags (170g), and the 4-section compartmentalized petri dishes (100mm i.d. ×15cm) were obtained from Fisher Scientific Co. (Springfield, NJ.). All other materials used were of the analytical reagent grade and were purchased from BDH (Poole, UK.).
Fish source
Three whole Scomberomorus brasiliensis (Carite) fish samples from the locations listed below and the different storage conditions were purchased once a month over a 12-months period.
- Single supermarket operators - Refer to supermarkets with only one outlet, it is the view that single operators will have a slower turnover of their seafood commodities.
- Chain supermarket operators- Refer to supermarkets with many outlets; it is the view that chain operators will have a faster turnover of their seafood commodities.
- Highway fishmongers morning period (6:00am to 12:00 noon) refer to those fishmongers who will ply their trade on the major highways only during the stated time (Figure 1).
- Highway fishmongers evening period (12:01pm to 6:00pm) refer to those fishmongers who will ply their trade on the major highways only during the stated time (Figure 1).
- Fresh Fish muscle tissue [bought directly from the fishing boats (Carenage Fishing Port, Trinidad W.I.)] and stored at: -15 °C; 1 °C; and 29 °C.
Figure 1: Highway fishmongers morning and evening periods.

The whole fish were kept on ice until they arrived at the laboratory. Each fresh sample was in an early post-rigor condition (12 hours after netting them). All the fish samples were between 30-40cm in length. During the trip to the laboratory, each sample was placed in their respective coolers. The temperatures of these coolers were -15 °C; 1 °C; and 29 °C. The samples brought from the supermarkets or the fishmongers were stored at 100C during their trip back to the laboratory. Fish samples bought from the supermarkets and highway fishmongers were purchased on a monthly basis for a period of twelve months and analyzed immediately. Each month for a total of twelve months, the fish muscle tissue from each of the stated seven purchased or stored conditions was analyzed for Total Bacterial Count [resulted are quoted as Viable count (×106) per ml]. From each location or condition, the fish samples were processed in triplicate [7,8].
Microbiological analysis of fish muscle tissue
Preparation of the agar: Weighed quantities of Tryptone (15g/L); Soytone (5g/L); Sodium Chloride (5g/L); Fast Green FCF (0.25g/L); and Agar (15g/L) were made up to a total volume of 1L in deionized distilled water. This was sterilized by autoclaving (Market Forge Sterilmatic Sterilizer, Fisher Scientific Co.) at 121 °C for 15 minutes. The Violet Red Bile Agar needed no further sterilization. The agar preparation was poured into a 4-section compartmentalized petri dish and stored at 4 °C.
Preparation of inoculant: Samples from the pooled fish muscle fillets (150g) were stored in Whirl-Pak® bags at about 4 °C. Sub-samples (10g) of these were placed under aseptic conditions into a Stomacher bag containing 90ml Ringer Solution (¼ strength). This mixture was homogenized in this Stomacher bag (for 2 minutes) using the Stomacher Lab Blender 400, (Model BA 6021). This stock solution was serially diluted with Ringer solution (range 10-1 to 10-6). The various agars were inoculated by the Miles and Misra dropper technique (Miles, Misra, & Irwin, 1938) with the diluted solutions. The agar was incubated (Imperial II Lab line incubator) at 35-37 °C for 48 hours. The colonies formed after incubation were counted using the Darkfield Quebec Colony Counter (American Optical, Model 3327).
Result
Table-1
Table 1: Total bacterial count {Viable count (×106) per ml} determined in the muscle tissue of Scomberomorus brasiliensis (Carite) purchased from four (4) locations and stored under different conditions.

φ single operators
Refers to supermarkets with only one outlet, it is the view that single operators will have a slower turnover of their seafood commodities.
π chain operators
Refers to supermarkets with many outlets, it is the view that chain operators will have a faster turnover of their seafood commodities.
€ highway fishmongers morning period (6:00am to 12:00 noon)
Refers to those fishmongers who will ply their trade on the major highways only during the stated time period.
Ω highway fishmongers evening period (12:01pm to 6:00pm)
Refers to those fishmongers who will ply their trade on the major highways only during the stated time period.
Љ fresh fish muscle tissue
Bought directly from the fishing boats (Carenage Fishing Port, Trinidad, W.I.) and stored at -15 °C; 1 °C; and 29 °C.
Discussion
The results of the microbiological assays are shown in Figure 2-11. All assays show a constant value of the total aerobic bacteria throughout the specific purchased or storage conditions. The samples analyzed from the single operators and chain operators had a mean value of 4.06 ± 0.66 and 4.33 ± 0.94, Total Bacterial Count {Viable count (×106) per ml} respectively as shown in Table 1. A mean value of 4.40 ± 1.10 Total Bacterial Count {Viable count (×106) per ml} was obtained for fish samples obtained from highway fishmongers morning period (6:00am to 12:00 noon), while a mean value of 4.32 ± 1.03 Total Bacterial Count {Viable count (×106) per ml} was obtained for fish samples obtained from highway fishmongers evening period (12.01pm to 6:00pm). With respect to the fish muscle tissue stored at -15 °C; 1 °C; and 29 °C, the mean values for Total Bacterial Count {Viable count (×106) per mL} were 0.65 ± 0.25; 2.72 ± 0.30; and 8.71 ± 0.02, respectively.
Supermarket-single operator
Refers to supermarkets with only one outlet, it is the view that single operators will have a slower turnover of their seafood commodities. The range (Table 1) of the Total Bacterial Count was 3.18 to 5.27{Viable count (×106) per ml}. Figure 2 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
Figure 2: Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased the Supermarket-single operator over a twelve-month period.
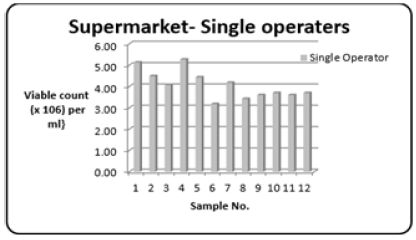
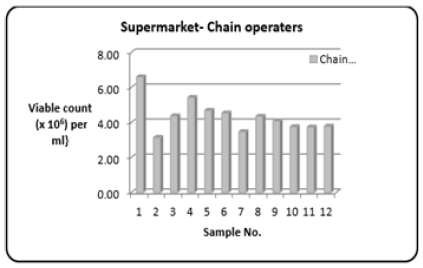
Figure 3: Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased the chain operators over a twelve-month period.
Supermarket-chain operator
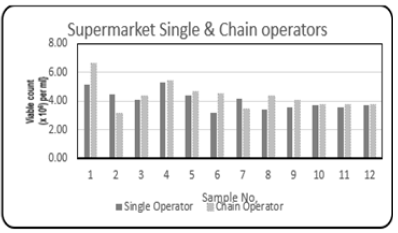
Figure 4: Comparison column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased the single and chain operators over a twelve-month period.
Refers to supermarkets with many outlets, it is the view that chain operators will have a faster turnover of their seafood commodities. The range (Table 1) of the Total Bacterial Count was 3.14 to 6.60 {Viable count (×106) per ml}. Figure 3 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
Comparison between the single and chain operators
Comparison between the Supermarket operators (Single and Chain) showed that on month #1, the levels of the Total Bacterial Count for the Chain operator was 6.60 {Viable count (×106) per ml} compared to the Single operator (5.15 /Viable count (×106) per ml}. Figure 4 shows the variations in the Total Bacterial Count {Viable count (×106) per ml} between the two supply chains. Factors contributing to the high microbiological loading observed may be due to the overstocking of the freezers in these supermarkets. Sometimes the packaged fish may be stored above the safe load line (the maximum level inside the freezer/display case to which product can be safely packed and remain frozen) of the display freezer. Over stocking the frozen commodities above the safe load line can result in thawing. Also, there are electricity fluctuations in the freezers, resulting in unregulated temperature variations.
Highway fishmongers (morning period 6:00am to 12:00 noon)
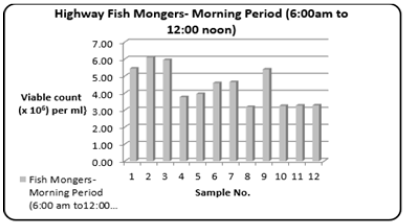
Refers to those fishmongers who will ply their trade on the major highways only during the stated time period. The range (Table 1) of the Total Bacterial Count was 3.18 to 6.09 {Viable count (×106) per ml}. Figure 5 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
Figure 5: Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased the highway fishmongers (morning period 6:00 am to 12:00 noon) over a twelve-month period.
Highway fishmongers (evening period 12:01pm to 6:00pm)
Figure 6: Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased the highway fishmongers (morning period 6:00 am to 12:00 noon) over a twelve-month period.
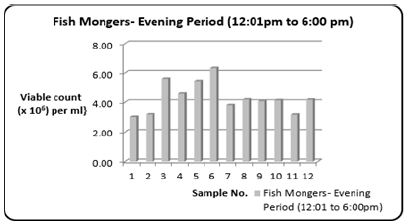
Refers to those fishmongers who will ply their trade on the major highways only during the stated time period. The range (Table 1) of the Total Bacterial Count was 3.03 to 6.34 {Viable count (×106) per ml}. Figure 6 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
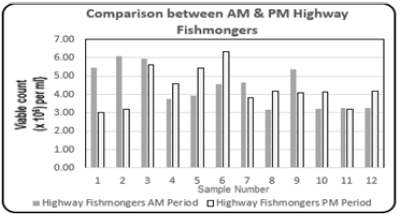
Comparison between the Highway fishmongers (morning period 6:00am to 12:00 noon, and evening period 12:01pm to 6:00pm): Comparison between the Highway fishmongers (am and pm) showed that on months 1,2, and 9, the levels of the Total Bacterial Count for the Chain operator were higher. Figure 7 shows the variations in the Total Bacterial Count {Viable count (×106) per ml} between the two fishmongers (am and pm). The high Total Bacterial Count observed at the sampling sites of the highway fishmongers may be due to the lack of proper sanitation and facilities such as toilets and hand washing amenities with running water, for all stakeholders. The whole and cut pieces of fish are exposed to diesel and gasoline vehicle emissions. Also, no proper solid and liquid waste disposal of gut and scales produced from the sale of the fish, this is a major source of the microbiological loading in and around the fishmonger’s booths. The fish is not displayed on ice, only sprinkled with water to keep the fish moist and keep the flies away. Other factors include the elements of the weather, roadside dust, 12 hours of continuous heat and exposure to the sun, and stray street dogs carrying harmful microorganisms which can be transmitted to the fish causing contamination to the quality and safety of the fish. The make-shift wooden display counter tops and cutting boards also contribute to the high bacteria loading seen in the results obtained for both morning and evening fishmongers, since these surfaces cannot be properly cleaned and sanitized.
Figure 7: Comparison column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased from the Highway fishmongers (morning and evening periods) over a twelve-month period.
Stored at -15 °C: Whole fish bought directly from the fishing boats (Carenage Fishing Port, Trinidad, W.I.) and stored at -15 °C. The range (Table 1) of the Total Bacterial Count was 0.30 to 0.9{Viable count (×106) per ml}. Figure 8 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
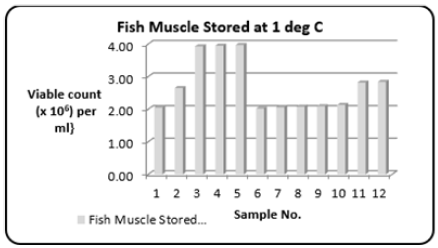
Stored at 1 °C: Whole fish bought directly from the fishing boats (Carenage Fishing Port, Trinidad, W.I.) and stored at 1 °C. The range (Table 1) of the Total Bacterial Count was 2.02 to 3.97{Viable count (×106) per ml}. Figure 9 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
Figure 8:Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fresh fish muscle tissue of Scomberomorus brasiliensis (Carite) stored at -15 °C. Over a twelve-month period.
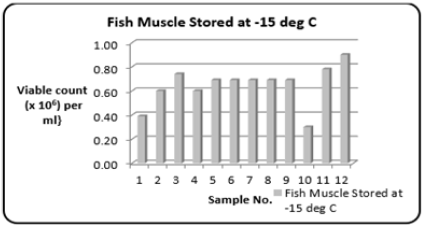
Figure 9: Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fresh fish muscle tissue of Scomberomorus brasiliensis (Carite) stored at 1 °C. Over a twelve-month period.
Stored at 29 °C: Whole fish bought directly from the fishing boats (Cocorite Fishing Port, Trinidad, W.I.) and stored at 29 °C. The range (Table 1) of the Total Bacterial Count was 8.35 to 8.91{Viable count (×106) per ml}. Figure 10 shows the variations in the Total Bacterial Count {Viable count (×106) per ml}.
Figure 10: Column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fresh fish muscle tissue of Scomberomorus brasiliensis (Carite) stored at 29 °C. Over a twelve-month period.

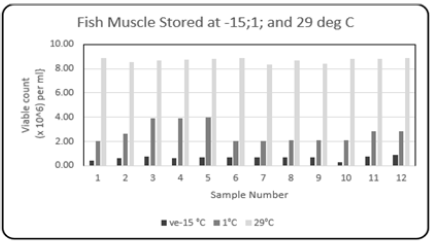
Comparison Total bacterial count {Viable count (×106) per ml} determined in the fresh fish muscle tissue stored at -15; 1; and 29 °C: Whole fish bought directly from the fishing boats (Carenage Fishing Port, Trinidad, W.I.) and stored at-15; 1; and 29 °C. The range (Table 1) of the Total Bacterial Count was 8.35 to 8.91{Viable count (×106) per ml}. Figure 11 shows the comparison variations in the Total Bacterial Count {Viable count (×106) per ml} at the three temperatures. The highest range occurred at the storage temperature of 29 °C. The odor was putrefying fish muscle tissue; all the textural profiles were destroyed. This was in keeping with the statement highlighted by [6], in the SIDC “Seafood Handbook-A Comsumer's Guide to the Purchase, Storage and Preparation of Seafood”- At high storage temperatures, the faster the fish will deteriorate. Controlling fish muscle tissue’s temperature is of principal importance since bacterial growth and the synthesis of biogenic amines such as histamine are decreased by rapid chilling and proper cold storage temperature. Maintaining the cold chain ‘from sea to kitchen’ by keeping fresh fish in clean ice, decreases the rate of bacterial spoilage and enzyme breakdown (uric acid cycle) which contributes to fish spoilage.
Figure 11: Comparison column bar chart showing total bacterial count {Viable count (×106) per ml} determined in the fresh fish muscle tissue of Scomberomorus brasiliensis (Carite) stored at -15; 1; and 29 °C, over a twelve-month period.
Statistical analysis: A one-way ANOVA test (α= 0.05) indicates no significant difference among the tissue of fish purchased from the four different locations. There was, however, a marked effect of storage temperature on the bacterial growth in Carite muscle tissue (Table 1). The mean population more than eight times when the storage temperature is increased from -15° to 29 °C, and four times when the storage temperature is increased from 10 to 29 °C.
Conclusion
The safety and quality of fish sold in Trinidad and Tobago are a cause for concern, in light of the unsanitary conditions under which fish are sold. This can inexorably result in food-borne disease outbreaks, further stressing the health sector. A value chain can both improve seafood quality and increased fish consumption. Also, educating the public through proper purchasing, handling, training, and storage protocols. A marked effect of storage temperature on the bacterial growth in Carite muscle tissue was observed. The mean population more than eight times when the storage temperature is increased from -15° to 29 °C, and four times when the storage temperature is increased from 1 °C to 29 °C. There was no significant difference among the tissue of fish purchased from the four different locations - Supermarket: Single and Chain; Highway fishmongers: morning and evening periods.
Factors contributing to the high microbiological loading observed may be due to the overstocking (stored above the safe load line) of the freezers in these supermarkets which can result in thawing. Also, there are electricity fluctuations in the freezers, resulting in unregulated temperature variations. The high Total Bacterial Count observed at the sampling sites of the highway fishmongers may be due to the lack of proper sanitation, toilets, and running water. The whole and cut pieces of fish are exposed to diesel and gasoline vehicle emissions. Also, no proper solid and liquid waste disposal of gut and scales is a major source of the microbiological loading in and around the fishmonger’s booths. The fish is not displayed on ice. Other factors include the elements of the weather, roadside dust, and 12 hours of continuous heat and exposure to the sun, and stray street dogs carrying harmful microorganisms. The make-shift wooden display counter tops and cutting boards also contribute to the high bacteria loading seen in the results obtained for both morning and evening fishmongers, since these surfaces cannot be properly cleaned and sanitized.
Although the edible portions of the fish muscle tissue are normally considered safe from microbiological contamination, the results show levels of microorganisms -Total bacterial count {Viable count (×106) per m} in the following order:
Fresh Fish muscle tissue stored at 29 °C > Highway Fishmongers > Single Operators > Fresh Fish muscle tissue stored at 1 °C > Fresh Fish muscle tissue stored at -15 °C.
Higher levels of microorganisms were observed in samples taken from the Highway Fishmongers Morning Period (6:00am to 12:00 noon), when compared to Highway Fishmongers Evening Period (12:01pm to 6:00pm), this may be due to improper transport conditions in the morning period, with slightly improved storage conditions in the evening period. At high storage temperatures, the faster the fish will deteriorate. Controlling fish muscle tissue’s temperature is of principal importance since bacterial growth and the synthesis of biogenic amines such as histamine are decreased by rapid chilling and proper cold storage temperature. Maintaining the cold chain ‘from sea to kitchen’ by keeping fresh fish in clean ice, decreases the rate of bacterial spoilage and enzyme breakdown (uric acid cycle) which contributes to fish spoilage. Safeguarding fish quality and safety can be achieved through the combined efforts of fisherfolk, and you the consumer.
References
- Tanner FW (1994) The microbiology of foods. Garrard Press, Illinois, USA.
- Efiuvwevwere BO, Iweanoge HA (1991) Microbiological and physico-chemical quality of various tissue types of fresh and potassium sorbate treated and untreated smoked mullet (Mugil cephalus). World Journal of Microbiology and Biotechnology 7(5): 562-566.
- Shewan J (1977) In the proceedings of the conference on handling, processing and marketing of tropical fish. Tropical Products Institute, London, pp. 51-56.
- Hobbs G (1987) Microbiology of fish. Food Microbiology 11: 199-226.
- Connell JJ, Shewan JM (1980) Fish microbiology. Advances in Fish Science and Technology, pp. 56-65.
- Marcelle C, Ramsaroop D, Nurse C (2013) Seafood Handbook-A Comsumer's Guide to the Purchase, Storage and Preparation of Seafood. Seafood Industry Development Company Ltd., Spain.
- Hobbs G (1983) Food microbiology. Food Microbiology 11: 217-227.
- Miles AA, Misra SS, Irwin JO (1938) Johyay C, (Ed.), Journal of Hygiene 38: 732-749.
© 2020 Derrick A Balladin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






