- Submissions

Full Text
Examines in Marine Biology & Oceanography
Vulnerability and Resilience of Tropical Coastal Ecosystems to Ocean Acidification
Daniel M Alongi*
Tropical Coastal and Mangrove Consultants, Australia
*Corresponding author: Alongi DM, Tropical Coastal and Mangrove Consultants, Australia
Submission: December 14, 2019;Published: January 08, 2020

ISSN 2578-031X Volume3 Issue3
Abstract
Ocean acidification leads to a wide variety of responses from tropical coastal ecosystems. Coral reefs are most vulnerable with most coral species exhibiting declining calcification rates with decreasing pH and carbonate chemistry parameters. Some corals show resilience to acidification likely due to active physiological regulation of their calcifying fluid. Other calcifying organisms, such as some foraminifera and coccolithophores, exhibit negative responses, whereas some symbiont-bearing calcifiers respond positively, to increasing acidification. Seagrasses and brown macroalgae thrive under acidified conditions, with increasing rates of primary productivity. Some tropical coastal fish species are resilient, and in some species, respond positively, to acidification. Some tropical species show complex, nonlinear responses to declining pH and carbonate chemistry. Factors that influence the ability of a species to adapt to and/or resist acidification include food supply, nutrient availability, temperature, diet, interactions with symbionts and other organisms and species and community diversity. Interactive effects of ocean acidification with other climate change parameters, such as elevated temperature, play an important but poorly understood role in determining the resilience and vulnerability of tropical coastal species, communities and ecosystems. Some short-lived species can undergo acclimation and/or adaptive evolution to increase fitness in the face of acidification. Biota living in tropical estuarine and nearshore environments, such as mangroves, seagrasses and intertidal and subtidal inshore benthos, are unlikely to be significantly affected by future acidification as such environments exhibit very wide variations in water and sediment pH and carbonate chemistry. Nearly all tropical coastal environments exhibit significant CO2 efflux to the atmosphere due to pCO2 and [CO32-] oversaturation caused by high rates of respiration and factors linked to fluvial discharge. Except for coral reefs, most calcifying organisms and upwelling regions, tropical estuarine and inshore ecosystems unaffected by eutrophication and other anthropogenic problems should be resilient to future acidification.
Keywords: Coastal; Corals; Coral reefs; Carbonate chemistry; Macrophytes; Mangroves; Ocean acidification; pH; Seagrasses; Tropics
Abbreviations: CO32-: Carbonate ion; NH3: Ammonia; HCO3-: Bicarbonate ion; CO2: Carbon dioxide; H+: Hydrogen ion; HNO3: Nitric acid; H2SO4: Sulfuric acid; ppmv: Part per million by volume; μatm: Microatmosphere; mol: Mole; μmol: Micromole (X 10-6 mole); mmol: Millimole (X 10-3 mole); Tmol: Teramole (1012 moles)
Introduction
The global ocean is currently being impacted by climate change induced by human-induced alteration to the planet. These anthropogenically-induced changes include rising sea surface temperatures and sea level, changes in precipitation, and increasing uptake of greenhouse gases [1]. The global ocean takes up approximately one third of the atmospheric carbon released from fossil fuel combustion, cement production and land-use change, with the subsequent hydrolysis of increasing amounts of CO2 in seawater increasing the hydrogen ion [H+] concentration thereby reducing the pH of ocean water and causing wholesale shifts in seawater carbonate chemistry [1]. This latter process is known as ocean acidification [2]. The average ocean surface water pH has declined since preindustrial times by approximately 0.1 units and is expected to decrease a further 0.3-0.4 units if atmospheric CO2 concentrations reach 800ppmv by later this century [1,2].
In the coastal ocean, acidification is a more complex process as carbonate chemistry is also expected to be strongly regulated by changes in biological activity related to the increase in anthropogenic delivery of nutrients by rivers, groundwater and eutrophication [3]. Land-use change such as deforestation and fossil fuel combustion also produce increased dissociation products of strong acids (HNO3 and H2SO4) and bases (NH3) to the coastal waters, causing decreases in surface water alkalinity, pH and Dissolved Inorganic Carbon (DIC). These anthropogenic inputs are more concentrated in the coastal zone [4]. River discharge to estuarine and coastal waters further reduces alkalinity as river waters, especially in the tropics, are typically more acidic than receiving waters. The oxidation of organic matter, especially in bottom sediments and wetland soils, can reduce pH and alter carbonate chemistry, producing an increase in partial pressure of CO2 (pCO2) as a result of mainly microbial respiration.
Eutrophication in estuarine and coastal waters is a strong amplifier of acidification [3,5-9]. The increased loading of nutrients into estuaries and shallow inshore waters causes the accumulation of algal biomass and subsequent decomposition of this organic material decreasing Dissolved Oxygen (DO) levels and contributing towards hypoxia [8,9]. Hypoxia will increase pCO2 values and upwelling processes can bring CO2-enriched water in contact with coastal waters, amplifying the effects of ocean acidification [5,6]. These impacts may have a significant impact on tropical marine life, although few data exist examining the co-occurrence of low dissolved oxygen concentrations and low pH [7].
This paper assesses the evidence for variable responses to the complex processes underlying acidification in the tropical coastal ocean. The focus is on tropical ecosystems because, as opposed to trends in high latitude regions, tropical coastal waters are being subjected to increasing inputs of organic matter derived from rapid changes in land-use and in and from estuaries with high concentrations of humans. Tropical seas are also facing altered carbonate chemistry which has important consequences for calcifying organisms, and for carbon storage and air-sea fluxes in the coastal zone [10]. Because tropical coastal organisms live closer to their physiological tolerance limits (e.g. temperature) they may be more susceptible to ocean acidification.
Negative Responses
Marine organisms of several taxonomic groups show decreased growth and physiological tolerance to ocean acidification [4,7]. Tropical hermatypic corals are among the most susceptible organisms to the effects of lower pH and [CO32-] ion concentration, with lower rates of calcification, the process involving the formation of calcium carbonate skeletons [11]. The precise mechanism(s) by which acidification affects coral calcification is not well understood. The formation and degradation of coral skeletons takes place in an Extracellular Calcifying Medium (ECM). The ECM is a semi-enclosed compartment of micro thickness that is sandwiched between the skeleton and the Calcifying Calicoblastic Epithelium (CCE), which spatially separates the ECM from direct contact with the surrounding environment [12]. The chemical composition of the ECM is widely held to be a critical factor controlling calcification [11,12]; it is widely accepted that corals increase pH and carbonate concentrations in the ECM to elevate the aragonite saturation state (Ωarag) to favour the formation of aragonite. Recently, it was found that pH, [Ca2+] and [CO32-] and thus [DIC] and Ωarag were elevated in the ECM compared to the surrounding seawater [13]. Thus, the physiological machinery of corals to calcify is spatially separated from the surrounding environment and is likely a critical step in the ability of corals and coral reef ecosystems to tolerate reduced seawater Ωarag that will occur under ocean acidification [14-20].
Table 1: Percentage decline in calcification of individual coral species to ocean acidification in experiments where corals were subjected to twice and three times pre-industrial atmospheric CO2. Calcification declines are relative to calcification at present-day pCO2.
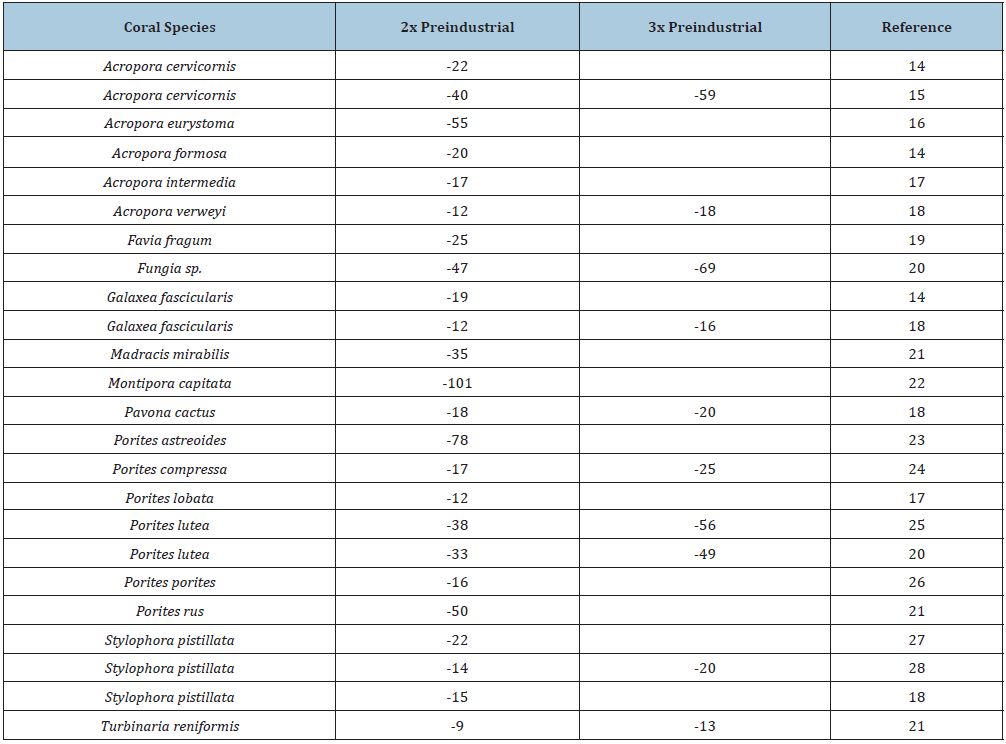
A wide range of experimental studies have shown that calcification rates in several species of hermatypic corals decline with ocean acidification, at both twice and three times (Table 1); [14-21] the preindustrial level of atmospheric CO2 [21-28]. The higher concentrations of pCO2 lead to a greater decline in calcification rates (Table1). Field studies have found similar results of the decline in calcification with increasing ocean acidification. For example, Albright et al. [29] experimentally subjected a coral reef community on One Tree Island in the Great Barrier Reef to increasing concentrations of pCO2. They found that the enhanced levels of pCO2 led to suppression of net community calcification.
Other calcifying organisms are negatively affected by acidification. The major calcifiers in the tropical ocean are photosynthetic calcareous algae (mostly coccolithophores), photosynthetic symbiont-bearing foraminifera and hermatypic corals which all show light-enhanced calcification (Table 2). Some species of molluscus, jellyfishes, fishes, echinoderms and pteropods, many prominent in tropical coastal waters, show a decline in calcification with increasing acidity [30-32]. Early calcifying stages of benthic molluscs, such as mussels and oysters, and echinoderms, show a strong negative response to increased seawater pCO2 and decreased pH, [CO32-] and calcium carbonate (CaCO3) saturation state. Although other benthic organisms such as crustaceans, cnidarians, sponges, bryozoans, annelids, brachiopods and tunicates possess portions of CaCO3 in their skeletons, nothing is known of the effect of acidification on these taxa. Decreased calcification presumably compromises the fitness of these organisms and possibly shifts the competitive advantage towards non-calcifying organisms, resulting in a shift in community organisation, structure and function. Other responses of marine fauna to ocean acidification include shell dissolution, reduction in shell mass, growth reductions, reduced metabolism, fertility and embryo development, increased mortality, reduced thermal tolerance, reduced food intake and increased ventilation [33-41].
Table 2: Percentage decline in calcification in some coccolithophore and foraminifera species to ocean acidification in experiments where species were subjected to twice and three times pre-industrial atmospheric CO2. Calcification declines are relative to calcification at present-day pCO2.
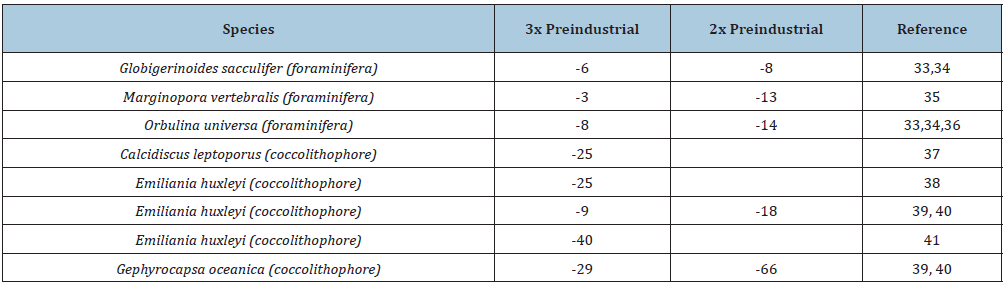
Non-calcifying organisms of several phyla appear to be negatively impacted by ocean acidification, including reef invertebrates and fish. Many species of tropical fish, for instance, exhibit behavioural impairment, lower reproduction and fertility, and impairment in the ability to detect prey. At CO2 seeps in Papua New Guinea. Munday et al. [42] found that while fish abundances did not appear to be affected closer to the seeps, fish exhibited abnormal behaviour such as still being able to be attracted to predator odour but being unable to distinguish between odours of different habitats. Similarly, Cripps et al. [43] found that elevated CO2 and reduced pH resulted in the common coral reef meso-predator, the brown dotty back (Pseudochromis fuscus), to shift its behaviour from preference to avoidance of the smell of prey, suggesting a dramatic shift in predator-prey interactions when coral reefs are exposed to acidifying conditions. Elevated CO2 and ocean acidification can have severe consequences for the physiology of tropical fish species [44]. Notable impacts include changes in neurosensory and behavioural ‘endpoints’, otolith growth, mitochondrial function and metabolic rates, despite some ability to compensate for or cope with altered environmental conditions [44].
Positive Responses
Not all estuarine and marine organisms exhibit a negative response to ocean acidification, as a variety of organisms of different phyla show positive responses to decreasing pH and changes in pCO2 and aragonite saturation state, including seagrasses and other macrophytes (such as brown macroalgae and kelp), sea anemones, fishes and most non-calcifying organisms. Some calcifying organisms exhibit positive responses to increasing pCO2. For example, when Fujita et al. [45] subjected three symbiont-bearing reef foraminifers (Baculogypsina sphaerulata, Calcarina gaudichaudii, Amphisorus hemprichii) to five pCO2 levels (260 to 970μatm) in culture, net calcification of B. sphaerulata and C. gaudichaudii increased under intermediate (580 and 770μatm) levels of pCO2 but decreased at 970μatm pCO2. Calcification of A. hemprichii, however, tended to decrease at elevated pCO2. Sensitivity of calcifying organisms to ocean acidification vary depending on individual species’ tolerances and the degree of changes in seawater carbonate chemistry [46].
Seagrasses, brown macroalgae and kelps exhibit positive responses to ocean acidification. Kelps grown under elevated pCO2 showed enhanced growth and iodine accumulation; not only was growth of the kelp, Saccharina japonica enhanced, but also in several other edible, cultured seaweeds [47]. As kelps are major iodine accumulators in the sea, these results imply that iodine levels in kelp-based coastal food webs will increase, causing changes in the biogeochemical cycles of iodine in the coastal zone. Tropical brown macroalgae thrive under increasing acidified conditions, as shown along natural CO2 gradients created by a volcanic seep in Papua New Guinea [48]. Along these gradients, species of the calcifying macroalgal genus, Padina spp., showed reductions in CaCO3 content but an increase in abundance with increasing acidified conditions closer to the seep. The success of these macroalgae may be partly explained by reduced sea urchin grazing pressure and significant increases in photosynthetic rates [48]. Generally, coralline macroalgae that deposit high-Mg calcite are most susceptible to high pCO2, but dolomite-depositing species can acclimate to such conditions [49]. Although CO2 is not likely to be limiting for photosynthesis for most macroalgae, the diffusive uptake of CO2 is less energetically expensive than active HCO3-uptake, and so macroalgae using HCO3-likely have a selective advantage over other photosynthetic organisms under acidifying conditions [50]. As acidified conditions become more intense such as at volcanic vents where pH can decline to 6.7, macroalgal communities shift in structure and composition, with non-calcifying species thriving while calcifiers are absent [51]. At CO2 seeps and vents, macroalgal communities are much more simplified, with a clear fall in species richness. In this sense, the ecosystems associated with these seeps and vents are not climate change winners. As many macroalgal species live close to their thermal limits, they will have to up-regulate the use of HCO3 to tolerate sublethal temperatures and to promote calcification over dissolution [52].
Tropical seagrasses respond positively to increased CO2 and decreased pH. The species, Cymodocea serrulata, Halodule uninervis and Thalassia hemprichii, exhibit increases in net productivity, maximum photosynthetic rates and efficiency, and an increase in the ratio of gross primary production to respiration (PG/R) with increasing levels of pCO2 [53]. Leaf growth rates in C. serrulata did not increase, but those in the other two species increased significantly with increasing pCO2 concentrations. An increase in pH of up to 0.38 units and aragonite saturation state increases of 2.9 are possible in the presence of tropical seagrass beds as opposed to their absence, with actual changes dependent on water residence time, tidal flushing and water depth [54]. Moreover, hermatypic coral calcification downstream of seagrasses has the potential to be about 18% greater than in habitats without seagrass, implying that coral reef resilience to ocean acidification can be enhanced by the presence of seagrass. Seagrass invertebrate communities, however, may not always benefit from the positive responses of seagrass to ocean acidification. At CO2 vents off the coast of Italy, invertebrate communities associated with the Mediterranean seagrass, Posidonia oceanica, show a decline in species richness along the CO2 gradient, but differences in community structure appear to be driven by indirect effects of acidification, such as changes to canopy structure and food availability [55]. However, despite the decline in number of species, abundance of invertebrates in acidified conditions was almost double that of control sites; many heavily calcified species thrived in the high CO2 environment.
Some tropical fishes show positive responses to enhanced levels of pCO2. Bignami et al. [56] raised larvae of the large, highly mobile, pelagic-spawning species, Rachycentron canadum, under acidified conditions. They found that the larvae exhibited resistance in growth, development, swimming ability and swimming activity at 800 and 2100μatmCO2. However, there was evidence of a significant increase in otolith size at the lowest pCO2 levels. Otoliths of this species showed increases not only otolith size but also in otolith density under acidifying conditions [57], suggesting a 50% increase in hearing range, which may alter the ability of this species’ larvae to survive in the environment. Larvae of the tropical orange clownfish, Amphiprion percula, when subjected to a range of acidifying conditions, showed no discernible effect on embryonic duration, egg survival, size at hatching and maximum swimming speed [58]. However, the growth rate of the larvae increased, and their size at settlement was 15-18% longer and 47-52% heavier in acidified seawater compared with controls. Thus, the growth and performance of larvae from benthic spawning marine fishes may be relatively unaffected or even enhanced under ocean acidification. It is clear, however, that few studies have been conducted on the effects of ocean acidification on marine fish; most such studies have been of short-term duration precluding predicting long-term impacts of ocean acidification on fish and fisheries [59].
Contrasting Responses
Not only do different species exhibit different responses to ocean acidification, but even the same species can show nonlinear responses, often responding differently to declining pH and changes in pCO2 and aragonite saturation state. Biological processes and biophysical feedbacks are often the primary drivers of local pH and carbonate chemical conditions [60]. Complex responses are not uncommon. The Caribbean corals, Siderastrea siderea, Pseudodiploria strigosa, Porites astreoides and Undaria tenuifolia, from the Belize Mesoamerican Barrier Reef exhibit nonlinear declines in calcification rate with increasing pCO2 [61]. S. siderea was the most resilient to both warming and acidification owing to its ability to maintain positive calcification in all treatments. Pseudodiploria strigosa and U. tenuifolia were the least resilient, and Porites astreoides was midway in the resilience spectrum among the four species. In two species of the calcareous tropical green algae, Halimeda opuntia and H. taenicola, of Palmyra Atoll in the central Pacific, responses to ocean acidification were species specific [62]. H. opuntia exhibited net dissolution and a 15% reduction in photosynthetic capacity, whereas H. taricola did not calcify but did not show any change in photo physiology. Similarly, two algal symbiont-bearing, reef-dwelling foraminifera, Amphisorus kudakajimensis and Calcarina gaudichaudii, exhibited contrasting responses to five increasing concentrations of pCO2 [63].
Net calcification of A. kudakajimensis was reduced under high pCO2, whereas calcification of C. gaudichaudii increased with increasing pCO2. The different responses were likely due to the different complexes of algal symbionts of both species. Carbonate ion and pCO2 were the carbonate species that most affected both foraminifera. The effects of ocean acidification on tropical fleshy and calcareous algae on Palmyra Atoll in the central Pacific were mixed, dependent on the individual species (Table 3); [64]. There were several negative, positive and no responses among species to ocean acidification. Acidification will likely reduce the ability of crustose coralline algae to calcify, but the results from these experiments [64] suggest that conditions may favor non-calcifying algae and shift relative dominance on coral reefs under projected acidification conditions to fleshy macroalgae.
Table 3: Ocean acidification effects on tropical benthic algae from Palmyra Atoll, central Pacific. +=positive effect, -=negative effect, 0=no effect. Data from Johnson et al. [64].
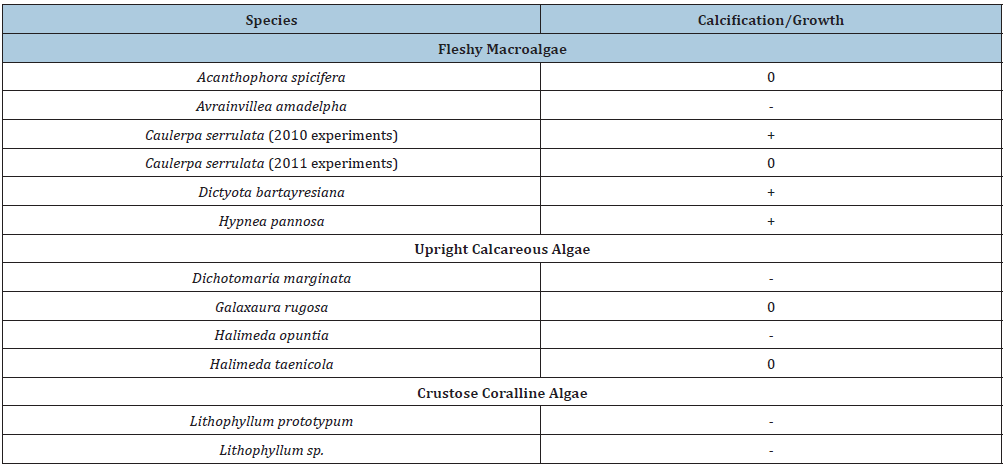
Experimental and field studies have suggested that there will be winners and losers as ocean acidification progresses in the future. At coral reefs acclimatized to elevated CO2 concentrations in proximity to seeps in Papua New Guinea, reductions in coral diversity, recruitment and abundances of structurally complex reef builders, and shifts in competitive interactions, were found between taxa [65]. Coral cover remained constant between pH 8.1 and 7.8 because massive Porites corals were dominant despite low calcification rates. Seagrasses had higher shoot densities and below-ground biomass in proximity to the intense seep locations, but they were less diverse. Other non-calcareous macroalgae showed a similar positive response. Large differences in sensitivity between species to declining pH resulted in complex changes, with a few taxa winning but most losing biodiversity, structural complexity and resilience [65]. Tropical plankton productivity, species diversity and abundances may decline with ocean acidification depending on phyla, with shifts in community composition favouring non-calcifiers and microbes [66].
However, some tropical plankton communities exhibit complex responses to ocean acidification. In waters from the tropical Atlantic, Indian and Pacific Oceans, the coccolithophore calcification to primary production ratio and cell-specific calcification were largely constant across a wide range of calcite saturation states (1.5-6.5), [HCO3-]/[H+] (0.08-0.24mol:μmol) ratios and pH (7.6-8.1) indicating that calcification by coccolithophore assemblages is independent of carbonate chemistry. At least in tropical oceans, coccolithophore calcification may not be declining in response to ocean acidification [67]. In the subtropical North Atlantic, colonies of the cyanobacterium, Trichodesmium, increased nitrogen fixation rates at pH 7.8 by 54% compared to present day seawater pH, whereas community assemblages dominated by Prochlorococcus and Synechococcus exhibited no clear response to changes in pH or/and pCO2. Responses of these three cyanobacteria genera may be indirect and controlled by other factors such as nutrients and temperature [68].
In the unicellular nitrogen-fixing cyanobacterium, Crocospaera watsonia, isolated from the western tropical Atlantic Ocean, the combined effects of light and CO2 resulted in complex responses with cyanobacteria in one set of treatments exhibiting no response, whereas other cultures grown under different light/CO2 conditions showed a significant increase in both CO2-fixation and N2-fixation rates. Overall, cellular nitrogen retention and CO2-fixation rates of C. watsonia appear to be positively affected by elevated light and pCO2 [69]. In Indian waters, tropical plankton communities exhibited mostly positive responses to ocean acidification. Along the Goa coast in the Arabian Sea, an upwelling-induced, highly productive region, growth of a diatom-dominated phytoplankton community increased under increasing levels of pCO2 [70]. Similarly, the growth of natural phytoplankton communities increased in response to increased pCO2 in the Godavari estuary; the community composition shifted from diatom to cyanobacteria dominance [71]. And in the coastal zone of the Bay of Bengal, diatom-dominated phytoplankton assemblages grew faster at increasing pCO2 concentrations [72,73]. However, responses were contrasting when other variables were introduced, such as light and nutrients.
The response of tropical zooplankton communities to ocean acidification is virtually unknown. At natural volcanic CO2 seep in Papua New Guinea, a three-fold reduction in the biomass of demersal zooplankton was observed compared with reef sites with ambient CO2 [74]. Abundances were reduced in most taxonomic groups, but there were no dramatic shifts in community composition or in fatty acid composition, implying that ocean acidification affected food quantity but not the quality for nocturnal plankton feeders. The reduction in zooplankton abundance may be partly attributable to changes in habitat from branching to massive bouldering corals which may offer less daytime shelter.
Resilience and Adaptation
Resilience is usually defined as the capacity of biological organisations (species, populations, communities and ecosystems) to absorb disturbance without shifting to an alternative state and losing services and function [75]. Resilience encompasses two separate processes: resistance-the magnitude of disturbance that causes a change in structure, and recovery-the speed of return to the original form or structure [76]. These processes are very different but are often not distinguished. Three broad ecological properties underlie resilience: diversity, connectivity and adaptive capacity [76]. The variety of responses to disturbance and the probability that species can compensate for one another increases with diversity. The capacity for recovery from disturbance is enhanced by connectivity among species, populations, communities and ecosystems. And adaptive capacity involves a combination of species range shifts, phenotypic plasticity and microevolution [76].
Resistance of reef calcifiers of the same species to ocean acidification may vary across distances. For instance, when the corals, Pocillopora damicornis and Porites sp. and two calcified algae, Porolithon onkodes and Halimeda macroloba were incubated at various pCO2 conditions in French Polynesia, Hawaii and Okinawa, both corals and H. macroloba were insensitive to the treatments at all locations. The effects of ocean acidification on P. onkodes varied among locations, with calcification of the species depressed in French Polynesia and Hawaii, but unaffected in Okinawa [77]. Resistance of reef calcifiers is a ‘constitutive character’ expressed across the Pacific [77].
Coral resistance and resilience to ocean acidification may have its basis in coral physiology. Some corals as well as coralline algae appear to be capable of maintaining constant rates of calcification by maintaining their carbonate chemical conditions, specifically aragonite saturation state, within the calcifying fluid [78]. The ability to maintain calcium ion concentrations [Ca2+] in the calcifying fluid plays a key role in the ability of corals to resist acidification [79]. In response to declining pH, the coral, Pocillopora damicornis, increased calcium ion concentrations within the calcifying fluid [Ca2+]CF to as much as 25% above that of seawater and maintained constant rates of calcification [79]. In contrast, the coral, Acropora youngei, showed less control over [Ca2+]CF and its calcification rates declined with lower pH. Physiologically, most corals up-regulate pH at their site of calcification such that internal changes are roughly one-half those in ambient seawater [80]. This pH-buffering capacity enables scleractinian corals to raise the saturation state of their calcifying fluid, increasing calcification rates with little additional energy [80]. However, this ability is species-specific and not common among calcifying organisms.
Several factors influence the ability of calcifying organisms to resist ocean acidification, including food supply, nutrient availability, temperature, diet, interactions with symbionts and other organisms and species and community diversity. Food supply has been found to confer resistance in corals, molluscs, crustaceans and echinoderms [81]. Nitrogen availability combined with changes in the diurnal cycle play a strong role in increasing resilience of marine diatoms to ocean acidification [82]. However, diet may not affect resilience to acidification in some species. For example, veligers of the slipper limpet, Crepidula onxy, exposed to different pH levels and fed the microalga, Isochrysis galbana, showed no changes in larval mortality due to pH or diet, but their interactions promoted earlier larval settlement. This slipper limpet, introduced to Hong Kong in the 1960s, appears to be resilient to changes in pH and decreased algal nutritional value [83].
Host-microbe interactions can also confer resilience to acidification. Coralline red algae (Corallinales, Rhodophyta) exposed to increasing pCO2 conditions exhibited increased photosynthetic activity and no loss of calcium carbonate biomass over time. The microbiome associated with these algae remained stable and healthy, but the microbial community in the water column changed with increasing pCO2 [84]. Thus, the stability of the algal microbiome is important for host resilience to acidification stress. Diversity can also improve resilience to ocean acidification. At volcanic seeps off Greece, Baggini et al. [85] performed an exclusion experiment to test effects of herbivory in benthic communities along a pCO2 gradient, as well as a manipulative experiment to examine how large herbivores affect the subtidal algal communities. These experiments showed that sea urchins and herbivorous fish dramatically reduced macroalgal biomass at both the control site and along the gradient despite reduced sea urchin densities near the seeps; abundances of herbivorous fish increased near the seeps [85].
A shift from sea urchins to fish showed that acidification caused community level changes but maintaining high functional redundancy improved resilience. Coastal biota, especially if diverse as in the tropics, may be more resistant to ocean acidification than expected considering variable responses and stability as conferred by high diversity [86]. Interactive effects of ocean acidification with other climate change parameters, such as elevated temperature, play an important role in determining the resilience of organisms, communities and ecosystems. For example, when gametes of the small giant clam, Tridacna maxima, were fertilized under ambient conditions and under conditions of high temperature and low pH, fertilization success was within previously reported levels under ambient conditions, but significantly reduced at elevated temperature itself and in combination with lower pH. Acidification alone however had no effect on fertilization success, indicating that reproductive success in the giant clam is resilient to ocean acidification but is strongly inhibited by elevated temperature [87].
Adaptation, which involves selection on genetic variation to peak fitness, may serve as a mechanism to resist ocean acidification. For instance, corals from a site with naturally lower seawater pH calcify faster and maintain growth better under simulated ocean acidification than corals from a higher pH site [88]. This ability was consistently linked to higher pH but lower DIC concentrations in the calcifying fluid, implying that these differences are the result of long-term acclimatization or adaptation to naturally lower pH. Thus, high pH up-regulation with moderate DIC up-regulation may promote resistance and adaptation of coral calcification to ocean acidification [88]. Acclimation, which involves phenotypically plastic responses in morphology, behaviour or physiology to maintain fitness, can also help to maintain an organism’s performance in an increasingly acidified ocean. In experiments with the coccolithophore, Emiliania huxleyi, under conditions of elevated temperatures and declining pH, growth rates were up to 16% higher in populations acclimatized to warmer temperatures at their upper thermal tolerance limit [89]. Particulate inorganic and organic carbon production by this species were respectively 101% and 55% higher under combined warming and acidification, suggesting that owing to adaptive evolution, this globally important and abundant species can resist acidifying conditions [89].
Adaptive evolution is a process that can involve genetic alterations that result in an increase in fitness in the face of environmental change, including ocean acidification. The coccolithophore, E. huxleyi, founded by single or multiple clones and exposed to increased CO2 over time, showed that around 500 generations later, this species exhibited higher growth rates under acidified conditions [90,91]. Calcification rates were lower under conditions of increased CO2 but were up to 50% higher in adapted compared with non-adapted cultures. Further, the expression levels of ten candidate genes putatively thought to be relevant to pH regulation, carbon transport, calcification and photosynthesis in this species exposed to both short term to acidification and in cultures after 500 generations of high CO2 adaptation revealed downregulation of candidate genes and up-regulation of pH regulation and carbon transport genes [92-100]. These results indicate that adaptive evolution helps to maintain fitness in the face of ocean acidification [90,91].
Coastal pH and CO2 Variability Promotes Ecosystem Resilience
It is questionable whether coastal environments, excluding coral reefs exposed to open ocean conditions, will be significantly affected by ocean acidification. Nearly all estuarine and nearshore waters in the tropics naturally exhibit very wide variations in salinity, pH and carbonate chemical parameters, especially pCO2 and [CO32-] (Table 4). Tropical estuarine and coastal waters are a strong source of CO2 emissions to the atmosphere due to pCO2 and [CO32-] oversaturation (Table 4); [101-120].
Table 4: Temporal and spatial variations in pH and pCO2 (μatm) concentrations, and in rates of CO2 fluxes (molm-2yr-1) in tropical estuarine and nearshore waters. Salinity is expressed as PSU (practical salinity units).
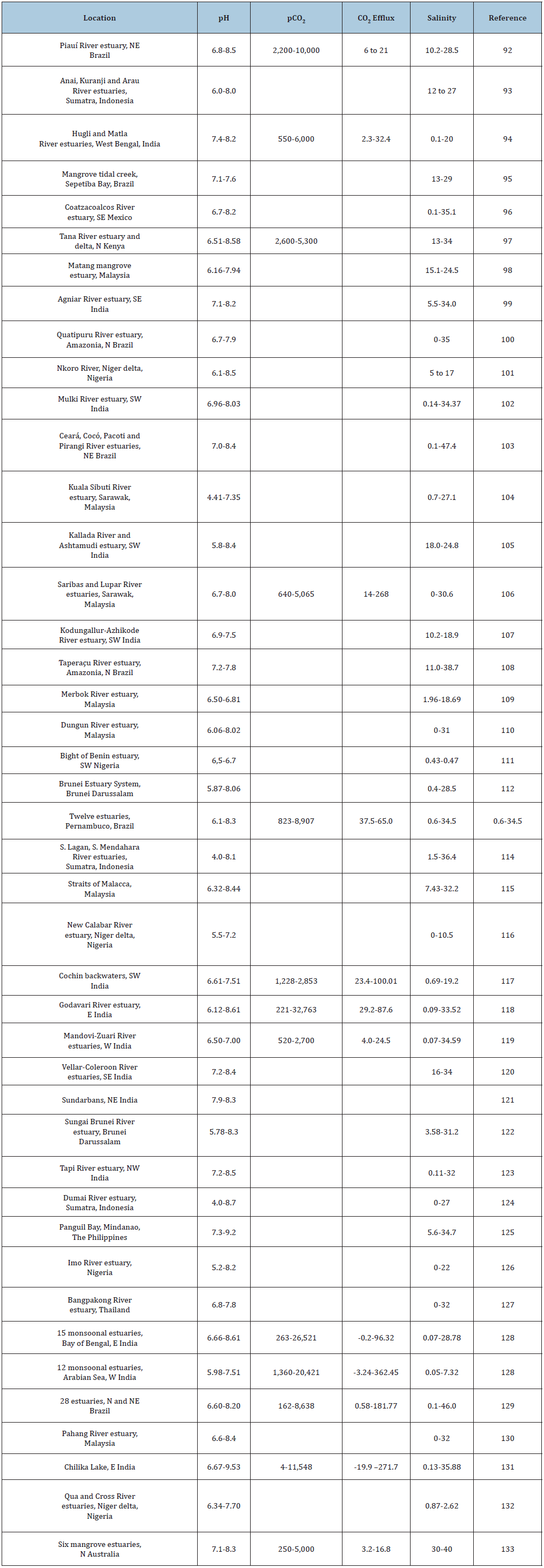
Evidence suggests that oversaturation and highly variable pH is the net result of high rates of (mostly) bacterial respiration, eutrophication and the influence of fluvial discharge, including export of alkalinity, organic matter and CO2, deposition of anthropogenic acids and bases, intense weathering, land-use change, acid sulphate soil discharge, and acidic groundwater [121-132] . Changes in water column alkalinity can also be large [133]. Duarte et al. [134] have argued that acidification is more of an open ocean process, and that coral reefs in the coastal zone may be resilient to some degree from acidification considering that even coral reef waters can range in pH from as low as 7.63 to as high as 8.4 (Table 2); [134]. Regulation of estuarine and coastal pH is complex compared with open ocean waters. Estuarine and nearshore environments that are metabolically intense increase aragonite saturation state due to high primary production; calcification is also regulated mainly by biological processes [135]. Further, impacts of ocean acidification must be considered with other climate change processes, such as rising sea surface temperatures, as it is likely that a combination of climate change factors will be the ultimate determinant of ecological change [136].
Mangroves and seagrasses will be the most resilient ecosystems to the effects of acidification. In the case of seagrasses, we have seen how individual species usually respond positively, or not as all, to lower pH. Seagrasses and other macrophytes have a capacity to modify pH within their canopy and within their habitat [137]. Within seagrass meadows, strong diel variability in pH, DIC and aragonite saturation state and O2 are driven by primary productivity; changes in carbonate chemistry are related to leaf surface area available for photosynthesis [137]. However, some organisms associated with seagrasses, such as leaf epiphytes, may not benefit from the buffering capacity of seagrasses if the meadows are declining for other reasons, such as eutrophication.
Mangrove ecosystems may prove to be the most resilient in the face of coastal acidification. The pH of mangrove soils is usually low, within the range of 4-7, especially in the forests of south and southeast Asia and Africa [138], as interstitial water is often acidic. Mangrove soils have low pH due to high rates of soil respiration, high concentrations of polyphenolic acids and the net effects of metabolic processes associated with the trees and their root systems [139]. Recently, it has been found that subsurface transport of groundwater derived from acidic soil waters plays a major role in carbon cycling in mangrove forests and their waterways, having important consequences for resilience to acidification [133,138,139]. Mangroves are apparent buffers of acidification in the tropical coastal zone [133]. An examination of carbon (DIC, dissolved CO2,) and alkalinity dynamics in six Australian mangrove tidal creeks revealed a mean export of DIC, whereas alkalinity fluxes ranged from an import of 1.2 mmol m-2 d-1 to an export of 117 mmol m-2 d-1. A net import of free CO2 of -11.4 mmol m-2 d-1 was measured, equivalent to one third of the estimated air-water CO2 flux of 33.1 mmol m-2 d-1 [133]. Upscaling these results globally, mangrove alkalinity exports (4.2Tmol yr-1) are equivalent to about 14% of global river or continental shelf benthic alkalinity fluxes. The effect of DIC and alkalinity exports creates a measurable increase in pH, implying that mangroves partly counteract acidification in adjacent tropical coastal waters. Mangroves may thus be one of the largest sources of alkalinity to the tropical coastal ocean, providing buffering against acidification.
Mangrove environments can assist in the survival of other tropical organisms, including some species of corals. In the US Virgin Islands, over thirty species of corals grow in association with mangroves, including two major reef-building corals, Colpophyllia natans and Diploria labyrinthiformis. These corals thrive in low light from mangrove shading and at higher temperatures than nearby reef corals [140]. A higher proportion of colonies of C. natans live shaded by mangroves, with no bleached colonies. And although fewer D. labyrinthiformis colonies shade beneath mangroves, more unshaded colonies bleach. Mangrove habitats can therefore be a refuge for diverse coral assemblages from climate change [140].
Conclusion
Tropical coastal ecosystems and their associated species assemblages can be impacted by ocean acidification in complex and very variable ways. Some ecosystems such as seagrass meadows, macrophyte assemblages and mangrove forests and their associated waterways are either positively affected or not affected by acidification. Several coastal fish species show positive responses to increasing pCO2 concentrations. Coral reefs are the most vulnerable ecosystems to ocean acidification, with many hermatypic corals exhibiting declining rates of calcification with decreasing pH. Other calcifying organisms are similarly affected, including some species of foraminifera and coccolithophores. Some tropical organisms exhibit complex, non-linear responses to ocean acidification, including some corals and fleshy and calcareous algae. Coral communities associated with CO2 seeps show large changes in community composition with some species responding positively, but most impacted negatively, along the CO2 gradient. All ecosystems and their associated species assemblages exhibit various degrees of resilience and adaptation as well as adaptive evolution. The naturally wide range of water column pH in estuarine and nearshore environments predisposes resilience of most tropical estuaries and adjacent coastal waters, especially estuaries and rivers inhabited by mangrove forests.
References
- Caldeira K, Wickett ME (2003) Oceanography: Anthropogenic carbon and ocean pH. Nature 425(6956): 365.
- Doney SC, Fabry VJ, Feely RA, Kleypas, JA (2009) Ocean acidification: The other CO2 Annu Rev Mar Sci 1: 169-192.
- Borges AV, Gypens N (2010) Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnol Oceanogr 55: 346-353.
- Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, et al. (2010) The effect of ocean acidification on calcifying organisms in marine ecosystems: An organism to ecosystem perspective. Annu Rev Ecol Evol Syst 41: 127-147.
- Malzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, et al. (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160(8): 1875-1888.
- Cai, WJ, Hu X, Huang WJ, Murrell MC, Lehrter JC, et al. (2011) Acidification of subsurface coastal waters enhanced by acidification. Nature Geosci 4: 766-770.
- Gobler CJ, Baumann H (2016) Hypoxia and acidification in ocean ecosystems: Coupled dynamics and effects of marine life. Biol Lett 12(5): 2050976.
- Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ (2014) Coastal ocean acidification: The other eutrophication problem. Estuar Coast Shelf Sci 148: 1-13.
- Hagens M, Slomp CP, Meysman FJR, Seitaj D, Harlay J, et al. (2015) Biogeochemical processes and buffering capacity concurrently affect acidification in a seasonally hypoxic coastal marine basin. Biogeosci 12: 1561-1583.
- Jennerjahn TC (2012) Biogeochemical response of tropical coastal systems to present and past environmental change. Earth Sci Rev 114(1-2): 19-41.
- Erez J, Reynaud S, Silverman J, Schneider K, Allemand D (2011) Coral calcification under ocean acidification and global change. In: Dubinsky Z, Stambler N (Eds.), Coral reefs: An ecosystem in transition. Netherlands, pp. 151-176.
- Jokiel PL (2013) Coral reef calcification: Carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification. Proc Biol Sci 280(1764): 20130031.
- Sevilgen DS, Venn AA, Hu MY, Tambutté, E, Beer D, et al. (2019) Full in vivo characterization of carbonate chemistry at the site of calcification in corals. Sci Adv 5(1): eaau7447.
- Chalker BE (1976) Calcium transport during skeleton genesis in hermatypic corals. Comp Biochem Physiol 54(4): 455-459.
- Renegar DA, Riegl BM (2005) Effect of nutrient enrichment and elevated CO2 partial pressure on growth rate of Atlantic Scleractinia coral Acropora cervicornis. Mar Ecol Prog Ser 293: 69-76.
- Schneider K, Erez J (2006) The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol Oceanogr 51: 1284-1293.
- Anthony K, Kline DI, Pulido DG, Guldberg HO (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA 105(45): 17442-17446.
- Marubini F, Ferrier PC, Cuif JP (2003) Suppression of growth in Scleractinia corals by decreasing ambient carbonate ion concentration: A cross-family comparison. Proc Biol Sci 270(1511): 179-184.
- Cohen AL, Corkle DC, Putron S, Gaetani GA, Rose KA (2009) Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: Insights into the biomineralization response to ocean acidification. Geochem Geophys Geosyst 10(7): 1-12.
- Hossain MMM, Ohde S (2004) Calcification of cultured Porites and Fungi under different aragonite saturation state of seawater at 250C. Proc 10th Int Coral Reef Symp 3: 597-606.
- Horst GP (2004) Effects of temperature and CO2 variation on calcification and photosynthesis of two branching reef corals. USA.
- Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, et al. (2009) Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: Mesocosm-scale experimental evidence. Biogeosci 6: 1811-1823.
- Albright R, Mason B, Langdon C (2008) Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs 27(3): 485-490.
- Marubini F, Barnett H, Langdon C, Atkinson MJ (2001) Dependence of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa. Mar Ecol Prog Ser 220: 153-162.
- Ohde S, Hossain MMM (2004) Effect of CaCO3 (aragonite) saturation state of seawater on calcification of Porites coral. Geochem J 38: 613-621.
- Marubini F, Thake B (1999) Bicarbonate addition promotes coral growth. Limnol Oceangr 44(3): 716-720.
- Tambutté É, Allemand D, Mueller E, Jaubert J (1996) A compartmental approach to the mechanism of calcification in hermatypic corals. J Exp Biol 199: 1029-1041.
- Gattusco JP, Frankignoulle M, Bourge I, Romaine S, Buddemeier RW (1998) Effect of calcium carbonate saturation of seawater on coral calcification. Global Planet Change 18(1-2): 37-46.
- Albright R, Takeshita Y, Koweek DA, Ninokawa A, Wolfe K, et al. (2018) Carbondioxide addition to coral reef waters supresses net community calcification. Nature 555: 516-519.
- Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, et al. (2013) Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Global Change Biol 19(6): 1884-1896.
- Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Ann NY Acad Sci 1134: 320-342.
- Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65(3): 414-432.
- Bijma J, Spero HJ, Lea DW (1999) Reassessing foraminiferal stable isotope geochemistry: Impact of the oceanic carbonate system (experimental results). Use of proxies in paleoceanography pp. 489-512.
- Bijma J, Honisch B, Zeebe RE (2002) The impact of the ocean carbonate chemistry on living foraminiferal shell weight: Comment on Carbonate ion concentration in glacial-age deep waters of the Caribbean Sea by WS Broecker and E Clark. Geochem Geophys Geosyst 3(11): 1064.
- Sinutok S, Hill R, Kühl M, Doblin MA, Ralph PJ (2014) Ocean acidification and warming alter photosynthesis and calcification of the symbiont-bearing foraminifera Marginopora vertebralis. Mar Biol 161: 2143-2154.
- Spero HJ, Bijma J, Lea DW, Bemis BE (1997) Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes. Nature 390: 497-500.
- Langer MR, Geisen M, Baumann KH, Klas J, Riebesell U, et al. (2006) Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem Geophys Geosyst 7(9): Q09006.
- Sciandra A, Harlay S, Lefèvre D, Lemée R, Rimmerlin P, et al. (2003) Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation. Mar Ecol Prog Ser 261: 111-122.
- Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, et al. (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407: 364-367.
- Zondervan I, Zeebe RE, Rost B, Riebesell U (2001) Decreasing marine biogenic calcification: A negative feedback on rising atmospheric pCO2. Global Biogeochem Cy 15(2): 507-516.
- Delille B, Harlay J, Zondervan I, Jacquet S, Chou L, et al. (2005) Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Global Biogeochem Cy 19: GB2023.
- Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE (2014) Behavioural impairment in reef fishes caused by ocean acidification at CO2 Nature Clim Change 4: 487-492.
- Cripps IL, Munday PL, Cormick MI (2011) Ocean acidification affects prey detection by a predatory reef fish. Plos One 6(7): e22736.
- Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307(9): R1061-R1084.
- Fujita K, Hikami M, Suzuki A, Kuroyanagi A, Sakai K, et al. (2011) Effects of ocean acidification on calcification of symbiont-bearing reef foraminifers. Biogeosci 8: 2089-2098.
- Beaufort L, Probert I, Thoron GT, Bendif EM, Pino RD, et al. (2011) Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature 476: 80-83.
- Xu D, Brennan G, Xu L, Zhang XW, Fan X, et al. (2018) Ocean acidification increases iodine accumulation in kelp-based coastal food webs. Global Change Biol 25(2): 629-639.
- Johnson VR, Russell BD, Fabricius KE, Brownlee C, Spencer HJM (2012) Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 Global Change Biol 18(9): 2792-2803.
- Hofman LC, Bischof K (2014) Ocean acidification effects on calcifying macroalgae. Aquat Biol 22: 261-279.
- Cornwall CE, Hepburn CD, Prichard D, Currie KI, Graw CM, et al. (2011) Carbon-use strategies in macroalgae: Differential responses to lowered pH and implications for ocean acidification. J Phycol 48(1): 137-144.
- Porzio L, Buia MC, Spencer HJM (2011) Effects of ocean acidification on macroalgal communities. J Exp Mar Biol Ecol 400(1-2): 278-287.
- Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biol 19(1): 103-132.
- Ow YX, Collier CJ, Uthicke S (2015) Responses of three tropical seagrass species to CO2 Mar Biol 162: 1005-1017.
- Unsworth RKF, Collier CJ, Henderson GM, McKenzie LJ (2012) Tropical seagrass meadows modify seawater carbon chemistry: Implications for coral reefs impacted by ocean acidification. Environ Res Lett 7(2).
- Garrard SL, Gambi MC, Scipione MB, Patti FP, Lorenti M, et al. (2014) Indirect effects may buffer negative responses of seagrass invertebrate communities to ocean acidification. J Exp Mar Biol Ecol 461: 31-38.
- Bignami S, Sponaugle S, Cowen RK (2013) Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Global Change Biol 19(4): 996-1006.
- Bignami S, Enochs IC, Manzello DP, Sponaugle S, Cowen RK (2013) Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc Nat Acad Sci USA 110(18): 7366-7370.
- Munday PL, Donelson JM, Dixson DL, Endo GGK (2009) Effects of ocean acidification on the early life history of a tropical marine fish. Proc Biol Sci 276(1671): 3275-3283.
- Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373: 295-302.
- Silbiger NJ, Sorte CJB (2018) Biophysical feedbacks mediate carbonate chemistry in coastal ecosystem across spatiotemporal gradients. Sci Rep 8(1): 796.
- Bove CB, Ries JB, Davies SW, Westfield IT, Umbanhowar J, et al. (2019) Common Caribbean corals exhibit highly variable responses to future acidification and warming. Proc Biol Sci 286(1900): 20182840.
- Price NN, Hamilton SL, Tootell JS, Smith JE (2011) Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda. Mar Prog Prog Ser 440: 67-78.
- Hikami M, Ushie H, Irie T, Fujita K, Kuroyanagi A, et al. (2011) Contrasting calcification responses to ocean acidification between two reef foraminifers harbouring different algal symbionts. Geophys Res Lett 38(19): L19601.
- Johnson MD, Price NN, Smith JE (2014) Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. Peer J 2: e411.
- Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, et al. (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change 1: 165-169.
- Nagelkerken I, Connell SD (2015) Global alteration of ocean ecosystem functioning due to increasing human CO2 Proc Nat Acad Sci USA 112(43): 13272-13277.
- Marañόn E, Balch WM, Cermeño P, González N, Sobrino C, et al. (2016) Coccolithophore calcification is independent of carbonate chemistry in the tropical ocean. Limnol Oceanogr 61(4): 1345-1357.
- Lomas MW, Hopkinson BM, Losh JL, Ryan DE, Shi DL, et al. (2012) Effect of ocean acidification on cyanobacteria in the subtropical North Atlantic. Aquat Microb Ecol 66: 211-222.
- Garcia NS, Fu FX, Breene CL, Yu EK, Bernhardt PW, et al. (2013) Combined effects of CO2 and light on large and small isolates of the unicellular N2-fixing cyanobacterium Crocosphaera watsonia from the western tropical Atlantic Ocean. Eur J Phycol 48: 128-139.
- Shaik AUR, Biswas H, Pal S (2017) Increased CO2 availability promotes growth of a tropical coastal diatom assemblage (Goa coast, Arabian Sea, India). Diatom Res 32(3): 325-339.
- Biswas H, Cros A, Yadav K, Ramama VV, Prasad VR, et al. (2011) The response of a natural phytoplankton community from the Godavari River Estuary to increasing CO2 concentration during the pre-monsoon period. J Exp Mar Biol Ecol 407(2): 284-293.
- Biswas H, Shaik AUR, Bandyopadhyay D, Chowdhury N (2017) CO2 induced growth response in a diatom dominated phytoplankton community from SW Bay of Bengal coastal water. Estuar Coast Shelf Sci 198(Part A 5): 29-42.
- Sahu BK, Pati P, Panigraphy RC (2018) Impact of climate change on marine plankton with special reference to Indian Seas. Indian J Geo Mar Sci 47(2): 259-268.
- Smith JN, Glenn D, Richter C, Cornils A, Spencer HJM, et al. (2016) Ocean acidification reduces demersal zooplankton that reside in tropical coral reefs. Nature Climate Change 6: 1124-1129.
- Côte IM, Darling ES (2010) Rethinking ecosystem resilience in the face of climate change. Plos Biol 8(7): e10000438.
- Bernhardt JR, Leslie HM (2013) Resilience to climate change in coastal marine ecosystems. Annu Rev Mar Sci 5: 371-392.
- Comeau S, Carpenter RC, Nojiri Y, Putnam HM, Sakai K, et al. (2014) Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification. Proc R Soc B 281(1790): 20141339.
- Cornwall CE, Comeau S, Carlo TM, Moore B, Alexis Q, et al. (2018) Resistance of corals and coralline algae to ocean acidification: Physiological control of calcification under natural pH variability. Proc Biol Sci 285(1884): 20181168.
- Carlo TM, Comeau S, Cornwall CE, Culloch MT (2018) Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proc Biol Sci 285(1878): 20180564.
- Culloch MT, Falter J, Trotter J, Montagna P (2012) Coral resilience to ocean acidification and global warming through pH up-regulation. Nature Climate Change 2: 623-627.
- Ramajo L, Pérez E, Hendriks IE, Marbá N, Krause D, et al. (2016) Food supply confers calcifiers resistance to ocean acidification. Sci Rept 6: 19374.
- Valenzuela JJ, Lomana ALG, Lee A, Armbrust EV, Orellana MV, et al. (2018) Ocean acidification conditions increase resilience of marine diatoms. Nature Comm 9: 2328.
- Maboloc EA, Chan KYK (2017) Resilience of the larval slipper limpet Crepidula onxy to direct and indirect-diet effects of ocean acidification. Sci Rept 7(1): 12062.
- Cavalcanti GS, Shukla P, Morris M, Ribeiro B, Foley M, et al. (2018) Rhodoliths holobionts in a changing ocean: Host-microbes interactions mediate coralline algae resilience under ocean acidification. BMC Genomics 19(1): 701.
- Baggini C, Issaris Y, Salomidi M, Spencer J (2015) Herbivore diversity improves benthic community resilience to ocean acidification. J Exp Mar Biol Ecol 469: 98-104.
- Hendriks IE, Duarte CM, Álverez M (2010) Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuar Coast Shelf Sci 86(2): 157-164.
- Armstrong EJ, Dubousquet V, Mills SC, Stillman JH (2020) Elevated temperature, but not acidification, reduced fertilization success in the small giant clam, Tridacna maxima. Mar Biol 167: 8.
- Schoepf V, Jury CP, Toonen RJ, Cullough MT (2017) Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc Biol Sci 284(1868): 20172117.
- Schlüter L, Lohbeck KT, Gutowska MA, Gröger JP, Riebesell U, et al. (2014) Adaptation of a globally important coccolithophore to ocean warming and acidification. Nature Climate Change 4: 1024.
- Lohbeck KT, Riebesell U, Reusch TBH (2012) Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geosci 5: 346-351.
- Lohbeck KT, Riebesell U, Reusch TBH (2014) Gene expression changes in the coccolithophore Emiliania huxleyi after 500 generations of selection to ocean acidification. Proc Biol Sci 281(1786): 20140003.
- Souza MFL, Gomes VR, Freitas SS, Andrade RCB, Knoppers B (2009) Net ecosystem metabolism and nonconservative fluxes of organic matter in a tropical mangrove estuary, Piauí River (NE of Brazil). Estuar Coasts 32: 111-122.
- Bach BA (2012) Concentration and specification of pollution-relevant trace metals in tropical, Humic river and coastal systems in East Sumatra. Germany.
- Akhand A, Chanda A, Manna S, Das S, Hazra S, et al. (2016) A comparison of CO2 dynamics and air water fluxes in a river-dominated estuary and a mangrove-dominated marine estuary. Geophys Res Lett 43: 11,726-11,735.
- Ovalle ARC, Rezende CE, Lacerda LD, Silva CAR (1990) Factors affecting the hydrochemistry of a mangrove tidal creek, Sepetiba Bay, Brazil. Estuar Coast Shelf Sci 31(5): 639-650.
- Hoz LR, Edwards AC, Romero PC, Jaime CM, Santoyo MER (2003) Physico-chemical seasonal variability of a tropical estuary: Major and minor elements in water and air. Environ Geol 44: 790-798.
- Bouillon S, Dehairs F, Schiettecatte LS, Borges AV (2007) Biogeochemistry of the Tana estuary and delta (northern Kenya). Limnol Oceanogr 52(1): 46-59.
- Ramarn T, Chong VC, Hanamura Y (2012) Population structure and reproduction of the mysid shrimp Acanthomysis thailandica (Crustacea: Mysidae) in a tropical mangrove estuary, Malaysia. Zool Studies 51(6): 768-782.
- Sukumaran M, Muthukumaravel K, Sivakami R (2013) Seasonal variations in physico-chemical characteristics of Agniar estuary, southeast coast of India. Asia Pacific J Res 2(8): 108-120.
- Pamplona FC, Paes ET, Nepomuceno A (2013) Nutrient fluctuations in the Quatipuru river: A macrotidal estuarine mangrove system in the Brazilian Amazonian basin. Estuar Coast Shelf Sci 133: 273-284.
- Abowei JFN (2010) Salinity, dissolved oxygen, pH and surface water temperature conditions in Nkoro River, Niger delta, Nigeria. Adv J Food Sci Tech 2(1): 36-40.
- Vjayakumar S, Rajesh KM, Mendon MR, Hariharan V (2000) Seasonal distribution and behaviour of nutrients with reference to tidal rhythm in the Mulki estuary, south-west coast of India. J Mar Biol Ass India 42(1&2): 21-31.
- Sousa BH, Becker H, Melo VMM (2016) Influence of river discharge on phytoplankton structure and nutrient concentrations in four tropical semiarid estuaries. Brazilian J Oceanogr 64(1): 37-48.
- Saifullah ASM, Hena MK, Idris MH, Rajaee AH, Johan I (2014) Seasonal variation of water characteristics in Kuala Sibuti River estuary in Miri, Sarawak, Malaysia. Malay J Sci 33: 9-22.
- Jennerjahn TC, Soman K, Ittekkot V, Nordhaus I, Sooraj S, et al. (2008) Effect of land use on the biogeochemistry of dissolved nutrients and suspended and sedimentary organic matter in the tropical Kallada River and Ashtamudi estuary, Kerala, India. Biogeochem 90: 29-47.
- Müller D, Warneke T, Rixen T, Müller M, Mujahid A, et al. (2016) Fate of terrestrial organic carbon and associated CO2 and CO emissions from two Southeast Asian estuaries. Biogeosci 13: 691-705.
- Jayachandran PR, Bijoy NS, Sreedevi OK (2012) Water quality variation and nutrient characteristics of Kodungallur-Azhikode estuary, Kerala, India. Indian J Geo-Mar Sci 41(2): 180-187.
- Magalhāes A, Pereira LCC, Costa RM (2015) Relationships between copepod community structure, rainfall regimes, and hydrological variables in a tropical mangrove estuary (Amazon coast, Brazil). Helgol Mar Res 69: 123-136.
- Maznah WO, Rahmah S, Lim CC, Lee WP, Fatema K, et al. (2016) Effects of tidal events on the composition and distribution of phytoplankton in Merbok River estuary, Kedah, Malaysia. Trop Ecol 57: 213-229.
- Tahir NM, Suratman S, Shazili NAM, Ariffin MM, Amin MSM, et al. (2008) Behaviour of water quality parameters during Ebb tide in Dungun River estuary, Terengganu. J Sustain Sci Manag 3: 1-10.
- Abdul WO, Adekoya EO, Ademolu KO, Omoniyi IT, Odulate DO, et al. (2016) The effects of environmental parameters on zooplankton assemblages in tropical coastal estuary, south-west, Nigeria. Egyptian J Aquat Res 42(3): 281-287.
- Majewska R, Adam A, Mohammad NN, Convey P, Stefano M, et al. (2017) Spatio-temporal variation in phytoplankton communities along a salinity and pH gradient in a tropical estuary Brunei, Borneo, South East Asia. Trop Ecol 58(2): 251-269.
- Noriega C, Araujo M, Lefèvre N, Flores M, Gaspar F, et al. (2015) Spatial and temporal variability of CO2 fluxes in tropical estuarine systems near areas of high population density in Brazil. Reg Environ Change 15: 619-630.
- Sanderson PG, Taylor DM (2003) Short-term water quality variability in two tropical estuaries, central Sumatra. Estuaries 26: 156-165.
- Looi LJ, Aris AZ, Johari WLW, Yusoff FM, Hashim Z (2013) Baseline metals pollution profile of tropical estuaries and coastal waters of the Straits of Malacca. Mar Pollut Bull 74(1): 471-476.
- Chindah AC (2004) Response of periphyton community to salinity gradient in tropical estuary, Niger delta. Pol J Ecol 52: 83-89.
- Gupta GVM, Thottathil SD, Balachandran KK, Madhu NV, Madeswaran NV et al. (2009) CO2 supersaturation and net heterotrophy in a tropical estuary (Cochin, India): Influence of anthropogenic effect. Ecosystems 12(12): 1145-1157.
- Sarma VVSS, Kumar NA, Prasad VR, Venkataramana V, Appalanaidu S, et al. (2011) High CO2 emissions from the tropical Godavari estuary (India) associated with monsoon river discharge. Geophys Res Lett 38(8): L08601.
- Sarma VVSS, Dileep Kumar M, Manerikar M (2001) Emission of carbon dioxide from a tropical estuarine system, Goa, India. Geophys Res Lett 28(7): 1239-1242.
- Senthilkumar B, Purvaja R, Ramesh R (2008) Seasonal and tidal dynamics of nutrients and chlorophyll a in a tropical mangrove estuary, southeast coast of India. Indian J Mar Sci 37: 132-140.
- Roychowdhury R, Vyas P, Zaman S, Roy A, Mitra A (2019) Surface water pH: A proxy to acidification of estuarine water of Indian Sundarbans. Int J Res Analyt Rev 6: 1530-1535.
- Marshall DJ, Santos JH, Leung KMY, Chak WH (2008) Correlations between gastropod shell dissolution and water chemical properties in a tropical estuary. Mar Environ Res 66(4): 422-429.
- Kumar JIN, George B, Kumar RN, Sajish PR, Viyol S (2009) Assessment of spatial and temporal fluctuations in water quality of a tropical permanent estuarine system-Tapi, west coast India. Appl Ecol Environ Res 7: 267-276.
- Alkhatib M, Jennerjahn TC, Samiaji J (2007) Biogeochemistry of the Dumai River estuary, Sumatra, Indonesia, a tropical black-water river. Limnol Oceanogr 52(6): 2410-2417.
- Canini ND, Metillo EB, Azanza RV (2013) Monsoon-influenced phytoplankton community structure in a Philippine mangrove estuary. Trop Ecol 54: 331-343.
- Akoma OC (2008) Phytoplankton and nutrient dynamics of a tropical estuarine system, Imo River estuary, Nigeria. African Res Rev 2(2): 253-264.
- Boonphakdee T, Fujiwara T (2008) Temporal variability of nutrient budgets in a tropical river estuary: The Bangpakong River estuary, Thailand. Environ Asia 1: 7-21.
- Sarma VVSS, Viswanadham R, Rao GD, Prasad VR, Kumar BSK, et al. (2012) Carbon dioxide emissions from Indian monsoonal estuaries. Geophys Res Lett 39(3): L03602.
- Noriega C, Araujo M (2014) Carbon dioxide emissions from estuaries of northern and northeastern Brazil. Sci Rept 4: 6164.
- Kamaruzzaman BY, Waznah SA, Nurulnadia MY (2011) Physico-chemical characteristics and dissolved trace metals in the Pahang River estuary, Malaysia. Oriental J Chem 27(2): 397-404.
- Muduli PR, Kanuri V, Robin RS, Kumar CB, Patra S (2012) Spatio-temporal variation of CO2 emission from Chilika Lake, a tropical coastal lagoon, on the east coast of India. Estuar Coast Shelf Sci 113: 305-313.
- Williams AB, Benson NU (2010) Inter seasonal hydrological characteristics and variabilities in surface water of tropical estuarine ecosystems within Niger delta, Nigeria. Environ Monit Assess 165(1-4): 399-406.
- Sippo JZ, Maher DT, Tait DR, Holloway C, Santos IR (2016) Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal transect. Global Biogeochem Cycles 30: 753-766.
- Duarte CM, Hendricks IE, Moore TS, Olsen YS, Steckbauer A, et al. (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar Coasts 36: 221-236.
- Hendriks IE, Duarte CM, Olsen YS, Steckbauer A, Ramajo L, et al. (2015) Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuar Coast Shelf Sci 152: A1-A8.
- Harvey BP, Jones GD, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3(4): 1016-1030.
- Hendriks IE, Olsen YS, Ramajo L, Basso L, Steckbauer A, et al. (2014) Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosci 11: 333-346.
- Alongi DM (2009) The energetics of mangrove forests. Springer, Dordrecht, pp.216
- Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6: 195-219.
- Yates KK, Rogers CS, Herlan JJ, Brooks GR, Smiley NA, et al. (2014) Diverse coral communities in mangrove habitats suggest a novel refuge from climate change. Biogeosci 11: 4321-4337.
© 2020 Alongi DM. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






