- Submissions

Full Text
Examines in Marine Biology & Oceanography
Population Dynamics of Copepods (Lernaea Cyprinacea Linnaeus, 1758) Tilapia Parasites from the Senegal River-Mauritania
Idoumou M1,2, Nizar S1, Mustapha A2, Sanaa Y1, Khadija El K1, Abdechahid L1 and Belghyti D1*
1Department of Biotechnology, Environment and Quality, Morocco
2Department of Production and Animal Health, Mauritania
*Corresponding author: Belghyti D, Department of Biotechnology, Kenitra, Morocco
Submission: June 14, 2019;Published: June 27, 2019

ISSN 2578-031X Volume3 Issue1
Abstract
Tilapia species play an important role in the fish farming sector in Sub-Saharan Africa. This region is increasingly characterized by climatic and ecological changes influencing the population dynamics of Tilapia. The objective of this study is to establish the effect of biotic factors (size and sex) on the parasitism of tilapia (Oreochromis niloticus Linnaeus, 1758) reared in an extensive breeding system in ISET-Rosso Mauritania lakes. All organs of the collected fish were examined. Examination of 556 males and 444 females of sampled fish made it possible to count 251 of Lernaea cyprinacea Linnaeus, 1758 and obtain the following parasite indices: 14.1%; 1.7 and 0.2 respectively for Prevalence, Intensity and Abundance. ANOVA shows that sex and height have no significant effect (p>0.05) on parasite load. This will push us to study other factors such as the effect of the season on parasitism. Ultimately, parasitic indices can alert fish farmers to the health risks that hover over their production. With this in mind, it will be possible to use antiparasitic to reduce prevalence and improve zoo technical performance.
Keywords: Fish farming; Tilapia; Parasitology; Copepods; Pest control; Rosso
Introduction
For a long time, humans have been exploiting fish resources to meet their needs mainly for food, as well as for fishing and aquaculture. The first aquaculture trials began before 4000 years ago in Egypt with the production of the famous tilapia fish [1]. It is a fish that lives in fresh water. It is of African origin and also populates the basins of Niger, Volta, Senegal and Congo. According to the FAO [1], in the Cichlid family, two species are mainly cultivated: the Mozambique Tilapia (Oreochromis mosambicus) and the Nile Tilapia (Oreochromis niloticus), which is raised in the ISET fish station. Rosso in Mauritania. Fish has always been an abundant and inexhaustible food. And the reality is completely different, we are faced with the fact that many stocks of freshwater fish are poorly known. In this case we proposed to study the effect of the biotic factors on tilapia parasitism that will be the size, the sex of the fish with an analysis of the prevalence, the intensity and the abundance parasites. Among the parasites determined, we opted for the analysis of the dynamics of populations of those most represented within the parasitic communities of the fish examined, such as: Lernaea cyprinacea Linnaeus, 1758 copepods parasitic fish Tilapia.
Study Area
Sampling was carried out at the Experimental Fish Culture Station of the Higher Institute of Technology Education (ISET), which is located on the banks of the Senegal River in the town of Rosso in the Trarza wilaya of southern Mauritania. It is located between latitude 16° 34’18.038 ‘’ N and longitude 15° 48’36.906 ‘W (Figure 1). Are climate being desert. The average temperature is 27.7 °C. The average annual rainfall is 224mm.
Material and Methods
The present study involved 1000 tilapia fish including 141 infested with Lernaea cyprinacea Linnaeus, 1758. In ISET lakes, Tilapia fish are caught line by hooks using wet bread. The sex of the fish was determined according to Shelton [2], they are transported to the laboratory to perform the parasitology examination.
Figure 1:Location of the study area.
Parasites research
In the laboratory, the fish are weighed, measured. Then, their different organs carefully removed and examined for parasites Figure 2. The gills were collected, placed in Petrie’s boxes containing water and examined for parasites. The parasites were collected and preserved in 40% formula.
Figure 2:Fish of the Nile Tilapia (Oreochromis niloticus) in the laboratory.

Study of parasites
We were interested in the morphology and biometry of parasites published in books and articles by comparing them with the parasites we observed Figure 3. This comparison concerns the external morphological characters of copepods such as antennas, antennules, head, thorax and abdomen [3,4].
Figure 3:Lernaea cyprinacea.

Statistical Analyzes
The data was entered on Excel and analyzed by SPSS version 22.0. Infection levels were expressed as prevalence, mean intensity, and abundance as defined by Bush, Lafferty, Lotz, and Shostak (1997). An ANOVA was done to test the effect of height, sex and season on the rate of infestation.
Result
(Table 1) The results show a prevalence of 16.4% higher in males than females while the infested fish are more numerous in females (Figure 2,3 & Table 2)
Table 1:Dynamics of parasitic copepod populations of Tilapia Nile (Oreochromis niloticus).
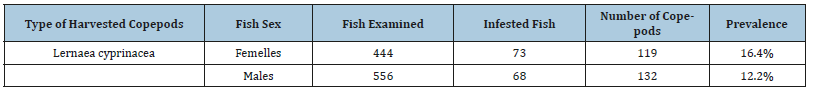
Table 2:Parasitic indices.

The parasite indices that were revealed in the results of this study show that parasite infestation reached 14.1%.
Infestation Studies
Figure (4-6).
Discussion
This parasite has been reported in Patagonia, a party of Argentina in common carp [5,6]. During this study, it was distributed over several areas of the body namely the mouth, skin and fins. The most severely infected areas were found near the base of the dorsal fins. The lessons found were similar in all fish. Parasitology revealed hyperplasia of the epithelium, abundant infiltration of inflammatory cells and fibrosis, not only in the epidermis, but also in the dermis and muscle tissue. This was confirmed by Plaul [7]. The infestation of fish with these copepods revealed that the number of parasites decreases with the size of the fish (p>0.05). On the other hand, parasitism evolves independently of sex (p>0.05). The recorded prevalence (14.1%) is lower than that determined by Mancini [8]. The frequency distribution shows the weak presence of Lernaea cyprinacea 251.
Figure 4:Distribution of Lernaea cyprinacea on different parts of the body of Tilapia of the Nile (Oreochromis niloticus) This graph shows that the region near the dorsal fin base was the most infested by these copepods which recorded the presence of 78 Lernaea cyprinacea.
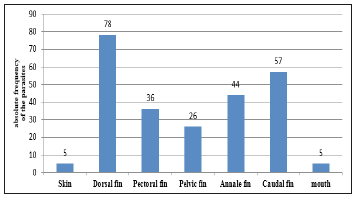
Figure 5:Variation in total length fish infestation of Nile tilapia (Oreochromis niloticus). These graphs show that the most infested fish are the youngest of which size varies between 15.5 and 19.5.
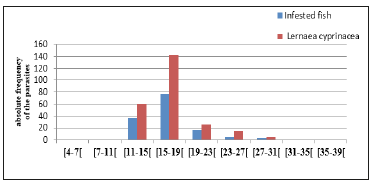
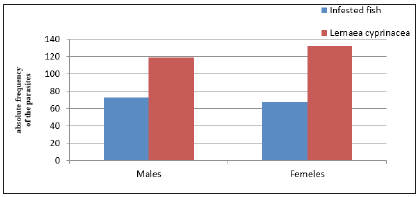
Figure 6:Variation of Lernaea cyprinacea infestation by sex of fish Nile Tilapia (Oreochromis niloticus) This graph shows that males are month infested during this study as females and Lernaea cyprinacea population was higher in females.
Conclusion
The study found that the infestation rate or parasite prevalence in tilapia fish (71.7%) is relatively high and the location of almost all parasitic copepods is at the level of the gills.
References
- FAO (2005-2017) Culture Aquatic Species Information Program Oreochromis niloticus. Culture Aquatic Species Fact Sheets. In: Rakocy JE (Ed.), AO Fisheries and Aquaculture Department. Rome, Italy.
- Shelton WL, Guerrero RD (1974) An aceto-carmine squash technique for sexing juvenile fishes. The Progressive Fish Culturist 36(1): 56.
- Loukili A (2010) Thesis: parasitological studies of European eel in the fresh and marine waters of the Gharb plain. PhD thesis, Ibn Tofail University, Morocco.
- Al-Nasiri, Ho JS, Mhaisen FT (2012) Pseudo lamproglena boxshalli sp. n. (Lernaeidae: Lamprogleninae) parasitic on gills of Cyprinion macrostomum (Teleostei: Cyprinidae) from the Tigris River, Iraq. Folia Parasitol (Praha) 59(4): 308-310.
- Waicheim A, Blasetti G, Cordero P, Rauque C, Viozzi G (2014) Macroparasites of the invasive fish, Cyprinus carpio, in Patagonia, Argentina. Comparative Parasitology 81(2): 270-275.
- Crichigno S, Cordero P, Blasetti G, Cussac V (2016) Dispersion of the invasive common carp Cyprinus carpio in southern South America: Changes and expectations, westward and southward. J Fish Biol 89(1): 403-416.
- Plaul SE, García Romero N, Barbeito CG (2010) Distribution of the exotic parasite, Lernaea cyprinacea (Copepoda, Lernaeidae) in Argentina. Bull Eur Ass Fish Pathol 30(2): 65.
- Mancini M, Bucco C, Salinas V, Larriestra A, Tanzola R, et al. (2008) Seasonal variation of parasitism in pejerrey odontesthes bonariensis (atheriniformes, atherinopsidae) from la viña reservoir (córdoba, argentina). Rev Bras Parasitol Vet 17(1): 28-32.
© 2019 Belghyti D. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






