- Submissions

Full Text
Examines in Marine Biology & Oceanography
Disinfection Effect of Moringa Oleifera Seed Extracts Against Bacteria Isolated from River Water
Muhammad Ali*1, Muhammad SA2, Idris SI3 and Ibrahim UI4
1 Department of Microbiology, Nigeria
2 Desert Research Monitoring and Control Centre, Nigeria
3 Department of Pharmaceutical Technology, Nigeria
4 Department of Vector and Parasitology, Nigeria
*Corresponding author: Muhammad Ali, Department of Microbiology, Nigeria
Submission: March 15, 2019;Published: April 25, 2019

ISSN 2578-031X Volume2 Issue5
Abstract
The traditional use of the Moringa Oleifera seeds for domestic household water treatment has been well known in some part of the world. The study was conducted to evaluate the disinfection effects of Moringa seeds extract against bacteria isolated from River Wudil, Kano State Nigeria. Two hundred and fifty millilitres (250mL) of water sample was collected in a sterile bottle from the bank of river. The bacteria were filtered from the water sample using Membrane filters (MF) method and inoculated onto the surface of nutrient agar. Identification of bacterial isolates was done by Gram staining, microbiological analysis and biochemical test. The Moringa seeds extract was screened for phytochemical screening and test for disinfection efficacy of the extract against bacteria isolated from river Wudil. The result of the study showed a total of 5 isolates obtained namely; Escherichia coli, salmonella typhi, Enterobacter, Klebsiella pneumoniae and Proteus mirabilis. The results of phytochemical screening of the extract indicated the presence of saponins, flavonoids, glycoside, anthraquinones, tannin and terpenoids. The finding of the study on the disinfection property of the extract demonstrated that the extract possessed disinfection effect against the isolate and the activity of the extract is dose dependent wise. Higher activity was shown by 100mg/L. The sensitivity of the isolate to the extract also differs. Statistical analysis of the result showed that Proteus mirabilis is more sensitive to the extract with average zone of inhibition of 13.75mm while Escherichia coli is the less sensitive (10.37mm) among the isolates. However, there is no statistical difference on the sensitivity of the isolates to the extract. Based on the finding of the study, Moringa seeds extract can be applied in water treatment as disinfection agent.
Keywords: Moringa Oleifera; Disinfection; Bacteria; Seeds extract; River water
Introduction
Drinking water has always been a major issue in many countries, especially developing countries such as Nigeria. In Nigeria, majority of the rural populace do not have access to potable water and therefore, depend on well, stream and river water for domestic use [1]. Contaminated water sources are vehicles for the transmission of waterborne diseases such as cholera, shigellosis, and Campylobacteria’s [2]. The World Health Organization (WHO) estimated that about 1.1 billion people globally drink unsafe water and most diarrheal diseases in the world (88%) are attributable to unsafe water, sanitation and hygiene. Approximately 3.1% of annual deaths (1.7 million) and 3.7% of the annual health burden world-wide (54.2 million) are attributable to unsafe water, sanitation and hygiene [3]. Major factors affecting microbiological quality of surface waters are discharges from sewage works and runoff from informal settlements. Indicator organisms are commonly used to assess the microbiological quality of surface waters and faecal coliforms are the most commonly used bacterial indicator of faecal pollution [4]. They are found in water that is contaminated with faecal wastes of human and animal origin. Nowadays, numerous drugs resistance has developed due to the indiscriminate use of synthetic antimicrobial drugs commonly used in the treatment of infectious diseases. In addition to this problem antibiotics are sometimes associated with adverse effects on the host including immune suppression, hypersensitivity and allergic reactions [5]. This situation forced scientists to search for new antimicrobial substances. Given the alarming incidence of antibiotic resistance in bacteria of medical importance, there is a constant need for new and effective therapeutic agents. Therefore, there is a need to develop alternative antimicrobial drugs for the treatment of infectious diseases from medicinal plants [5].
One of the greatest dangers to which ground water can be exposed to is contamination by pathogenic organisms. Disinfection is a chemical process for eliminating pathogenic microbes from an environment using chemical agents such halogens, phenols, alcohols, heavy metals, dyes, soap and detergents, ammonia compounds, hydrogen peroxide, and various alkalis and acids [6]. Most of the chemicals have the potential of forming carcinogenic and mutagenic disinfection by-products (DBPs) [7]. Disinfectants and their by-products may also be associated with increased risks of cardiovascular diseases, cancers, and birth defects [8]. These, and the high cost of chemicals, especially in developing countries where it needs to be imported, makes it imperative to look for cheaper alternatives that are also environmentally friendly. The traditional use of the M. Oleifera seeds for domestic household water treatment has been well known to certain rural areas in Africa [9]. In the West Asia, one of the best-known uses for Moringa is the use of powdered seeds to flocculate contaminants and purify drinking water [10]. The seeds of Moringa also exhibit antimicrobial properties [10,11]. It purifies water by removing microbes as a result of settling same as the colloids in properly coagulated and flocculated water is processed [12]. Additionally, these seeds are also expected to perform their actions directly on microorganisms and cause growth inhibition. The action of antimicrobial peptides produced by this plant parts are supposed to disrupt the cell membrane or inhibit necessary enzymes [13]. Studies by [14-17] identified the presence of an active antimicrobial agent in Moringa Oleifera seeds. The study was conducted to identify bacteria in River Wudil and test for disinfection effect of Moringa seeds extract against the faecal coliforms.
Materials and Methods
Study site
Wudil is one of the Local Government Council in Kano State, Nigeria. It is geographically located in the south-eastern part of Kano state along Maiduguri-Kano way with about 41 Kilometres from the State capital. It is located at Latitude 110 49’ 0” N and Longitude 80 51’ 0” E. It covers an area of about 362Km2 of land and population of about 185,189 according to 2006 census [18,19]. Wudil Local Government shares common boundaries with Warawa (North), Dawakin-kudu (West), Garko (South) and Gaya Local Government (East).
Collection of water sample
Two hundred and fifty millilitre (250mL) of water sample was collected in a sterile bottle from the bank of river Wudil in Wudil Local Government Area Kano State, Nigeria. The water sample was transported as soon as possible to Laboratory of Microbiology Department, Kano State University of Science and Technology Wudil for bacteriological analysis of the water.
Microbiological analysis of the water
Membrane filters (MF) method as described by Anas and Ali, [20] was adopted. During the process, 100mL of the water sample was filtered through sterile membrane which retained the bacteria on its surface. The membrane was removed aseptically and placed on a Nutrient agar (NA) as a basal medium and MacConkey agar as a differential medium to determine bacteria isolates. The plates were incubated at 37°C for 24 hours. Each colony was isolated in a pure form by sub culturing in fresh MacConkey agar plates for further studies and identification. Distinctive morphological properties of each pure culture such as colony form, elevation of colony and colony margin were observed.
Characterization of bacteria
Isolated colonies were confirmed by Gram staining and Biochemical (Indole, Methyl-red, Voges Proskauer and Citrate utilization) tests and each plate were graded as positive or negative for the test as according to Bergy’s manual of systemic determinative Bacteriology by Holt et al. [21]
Collection and identification of Moringa Oleifera seeds
Moringa Oleifera seeds used were purchased from Kano central market on 23th October 2018. Identification and authentication of the seeds was done at Herbarium in the Department of Plant Science, Bayero University Kano with the following voucher number BUKHAN 11 and voucher specimen were deposited there for reference. The seed wings were removed, and the kernels were air dried for two weeks, then grounded into a fine powder using sterile pestle and mortar under laboratory condition and stored in air tight container for further use.
Preparation of Moringa Oleifera seeds extract
Fifty grams (50g) of the seeds powder were extracted using 1000mL of 1 molar solution of sodium chloride by maceration for 2 days [22]. The resulting suspension was filtered through a muslin cloth and then using Whatman filter paper. The filtrate was concentrated in a water bath until a solid residue was obtained. The solid residue was diluted using 30% Dimethyl-suphoxide (DMSO) to produce a stock solution of 100mL, and from the stock solution, different concentrations (25,50 & 75mg/L) were prepared and stored further use.
Phytochemical screening of Moringa Oleifera seeds extract
Phytochemical screening of the seeds extract was conducted to determine the presence of bioactive components in the seeds extract of Moringa Oleifera. Presence of alkaloids, saponin, glycoside, tannin, flavonoids, steroids, terpenoids, anthraquinones and phenols was determined using procedures described by [23,24].
Antibacterial activity of the extracts
The antibacterial activity of the extract against the isolates was determined using agar well diffusion method as adopted by Gambo et al. [25] with little modifications. During the process, 0.1ml of standardize organism equivalent to 0.5 MacFarland standard, were introduced onto the surface of newly prepared Mueller Hinton agar in a sterile Petri dish and labelled accordingly. A sterile corkborer 6mm was used to produce five wells at equal distance in the inoculated agar. The wells were filled with 4 different concentrations of the extracts accordingly as 25,50,75 and 100mg/L. 5th well was filled with 50mg/L of the while the last well contain 50mg/mL of Amoxicillin (Chi pharmaceuticals) as positive control in the study. The inoculated agar plates could diffuse for a period of hour and incubated at 37°c for 24hours. After then, the diameter of the zones of inhibition around each well was measured to the nearest millimeter (mm) [26]. The experiment was conducted in triplicate for each organism.
Statistical analysis
The data of average zone of inhibition produced by the isolates against the seeds extract for each organism was analyzed using One-Way ANOVAs and the statistical program SPSS 21.0 (Statistical Package for the Social Sciences). Significance level for the differences was set at p<0.05.
Results
Identification of bacteria
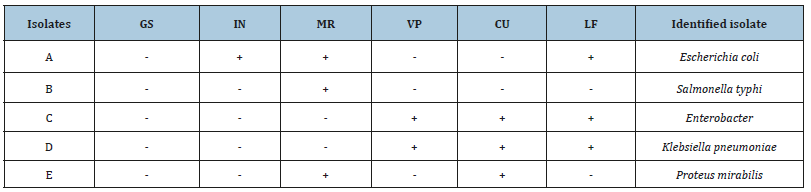
The biochemical characterization of the bacteria isolated from River Wudil is presented in Table below (Table 1). The results showed a total of 5 isolates obtained namely; Escherichia coli, salmonella typhi, Enterobacter, Klebsiella pneumoniae and Proteus mirabilis. Isolates were tested based on Gram staining, Lactose fermentation using MacConkey agar, Indole, Methyl red, Voges Proskauer and Citrate utilization test.
Table 1:Biochemical Characterization of the Bacteria Isolated from River Wudil.
Phytochemical screening
The phytochemical constituent of Moringa seeds extracts is presented in Table 2. The results indicate the presence of saponins, flavonoids, glycoside, anthraquinones, tannin and terpenoids.
Table 2:Phytochemical constituents of Moringa Oleifera seed extract.
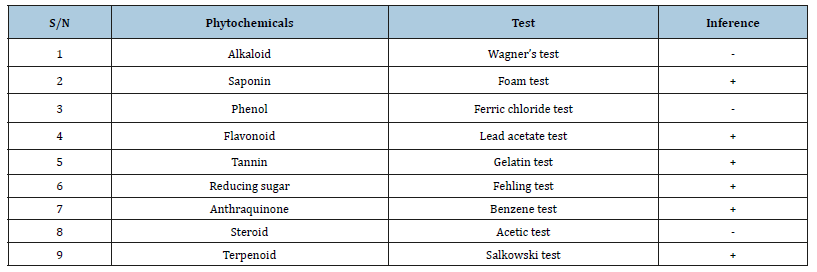
Key: +=Presence of phytochemical & -=Absence of phytochemical.
Antibacterial activity of the extracts
The antibacterial activity of different concentration of Moringa seeds extract against the isolates is presented in Table 3. The result showed that the extract is active against the isolates. However, the activity of the extract is dose dependant wise. Higher activity was shown by 100mg/L. The sensitivity of the isolate to the extract also differs. Proteus is more sensitive to the extract with average zone of inhibition of 13.75mm while E. coli is the less sensitive among the isolates with average zone of inhibition of 10.37mm. Zones of inhibition shown by the control ranged from 16.2-21.6mm.
Table 3:Antibacterial Activity of Moringa seeds Extract against bacteria isolated from River Wudil Concentration (mg/mL)/ Zone of inhibition (mm)
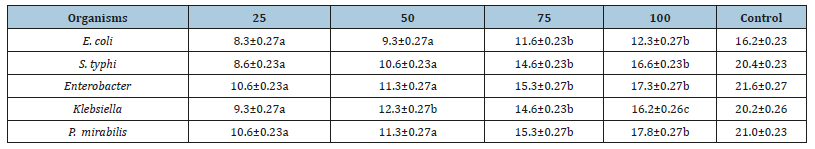
Key: Values having different superscript in the same row are considered significantly different at probability level of p<0.05.
Discussion
Bacteria such as coliforms are the most frequent bacteria in water responsible for water borne diseases such as cholera, dysentery, diarrhea, typhoid fever which is responsible for mortality across the world especially in Africa [27,28]. The finding of the present study revealed the presence of E. coli, salmonella typhi, Proteus mirabilis, Enterobacter sp and Klebsiella pneumoniae in River Wudil. Presence of fecal coliforms such as Escherichia coli, Enterobacter sp and Klebsiella pneumoniae indicates high pollution of the River. The poor microbiological quality might be due to contamination caused by human activities and livestock [29]. It is a common practice for people living along the river catchment to discharge their domestic and agricultural wastes as well as human wastes into rivers. In addition to using the river as a source of drinking water people use the source for bathing, washing of clothes and for recreational purposes such as swimming [30]. In addition to that wild and domestic animals seeking drinking water can also contaminate the water through direct defecation and urination [31]. The result of this study was in conformity with several results of similar findings which showed the presence of coliforms such as E. coli, salmonella typhi, Proteus mirabilis, Enterobacter sp and Klebsiella pneumoniae in most water sources [32-34]. The finding of this study supported that of Olorode et al. [35] who study the physicochemical and microbial analysis of some rivers in Rivers State, Niger-delta in Nigeria, and found the presence of bacteria such as Escherichia coli, Campylobacter, Pseudomonas, salmonella, Vibrio, Proteus, Shigella, Enterococcus sp. A study on Physico-Chemical and Microbiological Analysis of Well Water Samples in Settlements around Akperan Orshi College of Agriculture, Yandev in Benue State, Nigeria was conducted by Mwekaven et al. [36]. The results of microbiological analysis of the samples indicates that most of the wells were grossly contaminated with bacteria pathogens especially, Escherichia coli (100%), Proteus species (47%) and salmonella specie (7%). This result supported the present study. The phytochemical screening of Moringa seeds extracts indicates the presence of saponins, flavonoids, reducing sugar, glycoside, anthraquinones, tannin and terpenoids. Several studies conducted on phytochemical screening of Moringa Oleifera seed extract. Study conducted by Fahey [37] revealed that the Moringa Oleifera seed contain Alkaloids, Glycoside, Flavonoids and Saponins were present in the extract of Moringa Oleifera seed.
This finding supported the present study. The result of disinfection effect of Moringa seeds extract against the isolates showed that the extract possessed antibacterial agent. This is due to the presence of bioactive ingredients presence in the extract. Flavonoids have been shown to have antimicrobial activity in vitro [38]. Terpenoids are also known to possess antimicrobial [39]. Studies by [14-18] identified the presence of an active antimicrobial agent in seeds. Eilert et al [14] identified 4α-4-rhamnousyloxybenzyf- isothiocynate as an active antimicrobial agent in M. Oleifera. This is readily soluble to water at 1.3umol/l and is non-volatile. In a study using pure 4α-4- rhamnotyloxy-benzylsothiocynate isolated from defatted M. Oleifera seeds, the antimicrobial action of M. Oleifera was investigated on three bacteria species -Bacillus Subtilis, Serratia Marcescens and Mycobacterium Pheli. The result showed that B. Subtlis was completely inhibited by 56μmol/l and M. Pheli by 40μmol/l. the finding of this study was in conformity with that of Bukar et al. [18] who studied the antimicrobial activities of Moringa Seed Chloroform extract and Moringa Seed Ethanol extract. They found both to have inhibitory effects on the growth of E. coli and determined the Minimum Inhibitory Concentration (MIC) to be >4mg/ml. Thilza et al. [17] using extract from Moringa leaf stalk, found that at dilutions of 1000mg/ml, 700mg/ml, 400mg/ml, and 200mg/ml, only mild activity against E. coli and Enterobacter was noticed. They also found that the highest activity was produced by E. coli at 1000mg/ml which comparatively was less than that of the standard drug tetracycline (250mg/ml). Suarez et al. [15] had reported that Moringa seeds protein may be a viable alternative to chemicals commonly used as food preservatives or for water disinfection. The mode of attack of the Moringa seeds extract on the bacteria cell was explained as by rupturing the cell and damaging the intercellular components, when water dips in to the cell which causes it to swell more and burst leading to death [40].
Conclusion
The findings of the present study revealed the presence of E. coli, salmonella typhi, Proteus mirabilis, Enterobacter sp and Klebsiella pneumoniae in River Wudil which indicates high pollution of the River. The phytochemical screening of Moringa seeds extracts indicates the presence of saponins, flavonoids, reducing sugar, glycoside, anthraquinones, tannin and terpenoids. The result of disinfection effect of Moringa seeds extract against the isolates showed that the extract possessed antibacterial agent. This is due to the presence of bioactive ingredients such as alkaloid, flavonoids and terpenoids presence in the extract. Moringa seeds extract can be applied in water treatment as disinfection agent.
Acknowledgment
The authors wish to acknowledge to the technical staff of Microbiology Department of Kano University of Science and Technology Wudil for supply of the reagent and use of laboratory facilities.
References
- Shittu OB, Olaitan JO, Amusa TS (2008) Physico-chemical and bacteriological analyses of water used for drinking and swimming purposes in Abeokuta, Nigeria. African Journal of Biomedical Research 11: 285-290.
- Burgess JE, Pletschke BI (2010) Microbiological water quality assessment (Catchment to Tap). Water and Health 2.
- World Health Organization (2004) Guidelines for drinking water quality. 3rd edn, Switzerland, pp.16-89.
- Antony RM, Renuga FB (2012) Microbiological analysis of drinking water quality of ananthanar channel of kanyakumari district, Tamil Nadu, India. Ambi Agua Taubaté 7(2): 42-48.
- Ndabigengesere A, Narasia KB, Tolbot BG (1995) Active agents and mechanisms of coagulation of turbid waters using Moring Oleifera. Wat Res 29(2): 703-710.
- Devarakonda V, Moussa NA, VanBlaricum V, Ginsberge M, Hock V (2010) Kinetics of free chlorine decay in water distribution networks. The World Environmental and Water Resources Congress.
- Goveas JL, Sinha R, Krishnan ER, Patterson CL, Namboodiri V (2010) Bench-scale evaluation of peracetic acid and twin oxide as disinfectants in drinking water. The World Environmental and Water Resources Congress pp. 231983
- Arbuckle TE, Hrudey SE, Krasner SW, Nuckols JR, Richardson SD, et al. (2002) Assessing exposure in epidemiologic studies to disinfection byproducts in drinking water: Report from an international workshop. Environ Health Perspect 110(1): 53-60.
- Yarahmadi M, Hossieni M, Bina B, Mahmoudian MH, Naimabadie A (2009) Application of Moringa Oleifera seed extract and polyaluminum chloride in water treatment. World Appl Sci J 7(8): 962-967.
- Olsen A (1987) Low technology water purification by bentonite clay and Moringa Oleifera seed flocculation as performed in Sudanese villages: effects on Schistosoma mansoni cercariae. Water Research 21(5): 517- 522.
- Madsen M, Schlunt J, Omer EF (1987) Effect of water coagulation by seeds of Moringa Oleifera on bacteria contamination. J Trop Med Hyg 90(3): 101-109.
- Okuda T, Baes AU, Nishijima W, Okada M (2001) Isolation and characterization of coagulant extracted from Moringa Oleifera seed by salt solution. Wat Res 35(2): 405-410.
- Martins M, Castillo G, Dutka BJ (1997) Evaluation of drinking water treatment plant efficiency in microorganism removal by the coliphage, total coliform and h2s paper strip test. Water Science and Technology 3(35): 403-407.
- Eilert U, Wolters B, Nahrstedt A (1981) The antibiotic principle of seeds of Moringa Oleifera and Moringa stenopetala. Planta Med 42(1): 55-61.
- Suarez M, Entenza JM, Doerries C, Meyer E, Bourquin L, et al. (2003) Expression of a plant-derived peptide harboring water-cleaning and antimicrobial activities. Biotechnol Bioeng 81(1): 13-20.
- Fisch F, Suarez M, Mermoud N (2004) Flo antibacterial peptide from the tropical tree Moringa Oleifera: A template for novel antibacterial agents. Florian Fisch pp. 1-28.
- Thilza IB, Sanni S, Isah ZA, Sanni FS, Talle M, et al. (2010) In vitro antimicrobial activity of water extract of Moringa Oleifera leaf stalk on bacteria normally implicated in eye diseases. Academia arena 2(6): 80- 82.
- Bukar A, Uba S, Oyeyi TI (2010) Antimicrobial profile of Moringa Oleifera lam extracts against some food-borne microorganisms. Bayero Journal of Pure Applied Science.
- National Population Commission (2006) National population census result, Abuja, Nigeria.
- Asiya A, Ali M (2017) Biological control of water pollution at challawa industrial area kumbotso local government, kano using Moringa Oleifera seed extract. Greener Journal of Biological Sciences 7(6): 63-68.
- Holt JG, Krieg NR, Senath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology.
- Katayon S, Noor MM, Asma M, Ghani LA, Thamer AM, et al. (2006) Effects of storage conditions of Moringa Oleifera seeds on its performance in coagulation. Bioresour Technol 97(3): 1455-1460.
- Sofowora A (1993) Medicinal plants and traditional medicine in Africa. Nigeria, pp. 55-201.
- Tiwara R, Das K, Shrivastava DK (2010) Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. Journal of Medicinal Plants Research 4(2): 104-111.
- Gambo S, Shehu A, Labaran H, Ali M (2018) Phytochemical screening and antibacterial activity of vitex doniana stem bark extracts against staphylococcus aureus and pseudomonas aeruginosa isolated from infected wound. J Biotech Biores 1(3). JBB.000511.
- Ali M, Yahaya A, Zage AU, Yusuf ZM (2017) In-vitro antibacterial activity and phytochemical screening of psidium guajava on some enteric bacterial isolates of public health importance. Journal of Advances in Medical and Pharmaceutical Sciences 12(3): 1-7.
- Raji MIO, Ibrahim YKE (2011) Prevalence of water borne infection in north-western nigeria: a retroepective study. J pub Health pidimiol 3(8): 382-385.
- Adeyinka SY, Wasiu J, Akintayo CO (2014) Review on prevalence of water borne diseases in nigeria. J adv med life science 1(2): 1-3.
- Agbogu VN, Umoh VJ, Okuofu CA, Smith SI, Ameh JB (2006) Study of the bacteriological and physicochemical indicators of pollution of surface waters in zaria, nigeria. 5(9).
- Olabisi OE, Awomeso AJ, Adebayo OJ (2008) Assessment of bacteria pollution of shallow well water in Abeokuta, Southwestern Nigeria. Life Science Journal 5(1): 59-65.
- Ahiarakwem CA, Onyekuru SO (2011) A comparative assessment of the physico-chemical and microbial trends in Njaba river, niger delta basin, Southeastern nigeria. Journal of Water Resource and Protection 3(9): 686-693.
- Ndamatso MM, Idris S, Likita MB, Jimoh OT, Ajai AI et al. (2013) Physicochemical and Escherichia coli assessment of selected sachet water produce in some area of minna, niger state Nigeria. Int J Water Res Environ Eng 5(3): 134-140.
- Anake WU, Eremosole CO, Siyambola TO, Edebor OA, Adeniyi IO et al. (2013) Physicochemical and microbial assessment of different water resources in ota, ogun state, nigeria. Int J Current Res 5(7): 1797-1801.
- Aminu T, Amadi AN (2004) Bacteriological contamination of growndwater from zango local government area katsina state Northwestern nigeria. J Geoscience Geomatic 2(5): 186-195.
- Oluduro AO (2012) Evaluation of antimicrobial properties and nutritional potentials of Moringa Oleifera lam leaf in south western nigeria. Malaysian Journal of Microbiology 8(2): 59-67.
- Mwekaven SS, Aorkwagh MT, Gundu EG, Yange T (2017) Physicochemical and microbiological analysis of well water samples in settlements around akperan orshi college of agriculture, yandev. International Journal of Science and Technology 6(1): 642-649.
- Fahey JW (2005) Moringa Oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees for Life Journal 1(5).
- Rabi T, Bishayee A (2009) Terpenoids and breast cancer chemoprevention. Breast Cancer Res Treat 115(2): 223-239.
- Galeoti FE, Barile P, Curir M, Dolci, Lanzotti V (2008) Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem Lett 1(5): 44-48.
- Bichi MH, Agunwamba JC, Muyibi SA, Abdulkarim MI (2012) Effect of extraction method on the antimicrobial activity of Moringa Oleifera seeds extract. Journal of American Science 8(9).
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






