- Submissions

Full Text
Examines in Marine Biology & Oceanography
Fish Embryonic Stem Cells: Culture Types, Characterization and Applications: A Review
Lakhan LM, Chandan H* and Raju Ram
Fish Genetics and Biotechnology Division, India
*Corresponding author: Chandan H, Fish Genetics and Biotechnology Division, India
Submission: May 25, 2018;Published: December 19, 2018

ISSN 2578-031X Volume2 Issue4
Abstract
In last few years scientists observed the development of procedures and markers for embryonic stem cell characterization. These contain the identification of parameters for optimal chimera and fish homologs/paralogs of mammalian pluripotency genes and their formation. In this concern, fish models as lower vertebrates represent a significant reference to study the conserved mechanisms underlying self-renewal and differentiation process. Additionally, several fish germ cell cultures and transplantation have fascinated significant attention for germline transmission and surrogate production. For a Future prospectus, the analyses of transcriptomes and proteomes of fish stem cells of various origins will provide significant information for stem cell applications and research.
Keywords:Embryonic stern (ES) cell; Gene targeting; Germ cell; Zebra fish; Pluripotency; Characterization; Semi-cloning
Introduction
In last few years scientists observed the development of procedures and markers for embryonic stem cell characterization. These contain the identification of parameters for optimal chimera and fish homologs/paralogs of mammalian pluripotency genes and their formation. In this concern, fish models as lower vertebrates represent a significant reference to study the conserved mechanisms underlying self-renewal and differentiation process. Additionally, several fish germ cell cultures and transplantation have fascinated significant attention for germline transmission and surrogate production. Long-term undifferentiated cell culture systems derived from inner cell mass of developing embryos are called as embryonic stern (ES) cell culture systems (Evans and Kaufman, 1981; Martin, 1981). These cells are pluripotent in nature and can be induced to differentiate into cells of different lineage. Which is found in multicellular organisms. Stem cells manly divide into two broad categories, embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and second is adult stem cells, which are found in several tissues. In adult animals, stem cells and precursor cells act as a repair system for the body, replacing adult tissues. Self-renewal is the property of a stem cell which gives the ability to get divide and produce many identical daughter cells. Another attractive property of Stem cell is the Developmental potential which gives him the ability to produce specialized cells. The capability of self-renewal and differentiation are the unique properties of making stem cells to differ from other cells in the body. The establishment of the first embryonic stem (ES) cells in mice, but nowadays stem cells have been the focus of active research because of their enormous future for basic research, medicine and animal biotechnology. Work with fish stem cell culture has skilled 20 years. An overview on the history of stem cell research, will give the necessary knowledge and recent advances of fish stem cell cultures and biotechnology, various applications of stem cells such as the germ cell culture and transplantation, gene targeting and semi-cloning [1].
Types of Stem Cells
Stem cells are broadly classified into two major categories as:
a) Embryonic stem cell
Inner cell mass of a blastocyst is the source of Embryonic stem (ES). Zebrafish embryos reach the blastocyst stage within 2-2.5 hours post fertilization. ES cells are pluripotent (Potential to develop into any cells of body) and give growth during growth to all derivatives of the three primary germ layers: ectoderm, endoderm and mesoderm respectively. Fish are especially attractive models for developing ES cell technology for two reasons: Piscine species are of considerable interest for both basic studies in molecular, cellular, and developmental biology as well as for commercial interest. As model vertebrate organisms, zebrafish (Brachidanio rerio) and medaka (Oryzias latipes) are competitive with mouse for the analysis of gene functions relevant to humans. Fish have several technical advantages over other vertebrates such as high fecundity, large transparent embryos, and rapid development. These features simplify manipulation and allow phenotypic observations of markers during early development, especially in small aquarium fish such as zebrafish and medaka whose generation times are relatively short, about 2 to 3months. Conventional approaches to create transgenic fish involve the direct introduction of transgenes into germ cells or embryos or fertilized eggs.
b) Adult stem cell
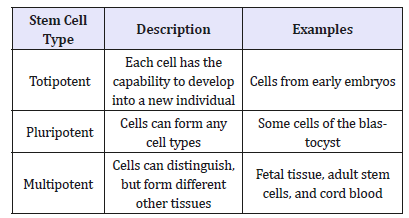
Adult stem cells which are also recognized as the somatic stem cells, are chiefly responsible for cells for the repairmen of damaged cells of the skin, blood and the intestinal lining. Stem cells are established throughout the body of an adult fish which maintain and repair the tissue in which they are found. Although Pluripotent adult stem cells are rare and generally lesser in number. Breakthrough in Stem cell Technology which have been used in treating several conditions including liver cirrhosis, chronic limb ischemia and end stage heart failure (Table 1) [2].
Table 1:Types of stem cells as potency
ES cell culture technology in fish, step by step
Collection of the fertilized eggs.

Blastula stage embryos are observed under a microscope, and embryos are immediately transferred to 35-mm small Petri dish.

A batch of 25-30 embryos are first disinfected with 70% ethanol and then wash five times with sterile phosphate-buffer saline (PBS, pH7.2) in the Petridis .

The inner cell mass is harvested by tearing the chorion with fine forceps, and the chorion and egg shells are removed.

Single cells are plated through gentle pipetting, and cells are transferred to a new gelatine-coated cell culture flask (25cm2) under ES cell conditioned medium (basic fibroblast growth factor, fish serum)

Maintenance of cells and characterization
Subculture
A. Subculture is done when confluency in the cells reaches 70-80 (about 10 days). Many mitotic figures should be noticeable throughout the flask. For each 25cm² of cells to be sub-cultured:
a) Take 2 ml of Trypsin/EDTA, 5 ml of Buffered Saline Solution, 4ml of Trypsin Neutralizing Solution (10% FBS-DPBS).
b) Rinse the flask with 5 ml of BSS and remove the BSS from the flask.
B. Then 2 ml of Trypsin/EDTA solution is added to the individually T25 flask. Permit the trypsinization to continue until nearly 90% of the cells have been detached from the surface. Hit the flask on the palm of the hand to detach the undetached cells. If only a few cells detach, wait 30 seconds and repeat this step again.
C. After cells have been detached, neutralize the trypsin in the flask with 4 ml of Trypsin Neutralizing Solution.
D. Transfer the detached cells to a sterile 15ml centrifuge tube and rinse the flask with a final 2ml of BSS. Add this rinse to the centrifuge tube [3].
Characterization
Several characterization methods are used to test the purity of the cell line, whether it is transformed or not. Apart from these the most critical aspect of cell line characterization is to know origin in terms of species.
a) Morphological characteristics
Stable growth, a round/polygonal shape, a small size, large nuclei and prominent nucleoli.
b) Cell surface marker
High alkaline phosphatase activity (a prominent marker of ES cells).
c) Expression of transcription factors
klf4, sox2, myc, ronin, sall4, tcf3 (tcf7l1) are characteristic of pluripotent stem cells.
In fishes oct4 and nanog have been used as markers for the transcription studies [4].
Applications of fish ES cells in biotechnology
A. Fish stem cells have proved its potential application in various biotechnologies.
a) Germ cell transplantation in fishes
b) Regenerative Medicine and Disease Therapeutics
c) Other applications like Production of transgenic fish and Production of Semi-cloned fish
Germ cell transplantation
Sperm or eggs produced by transplanted germ cells, are according to the host sex but independently of the sex of their origin. These pioneer experiments indicate the possibility of surrogate production of aquaculture brood stock by germ cell transplantation. This method might be extended to propagate/ reestablish a population of endangered species in conservation biology.
Regenerative medicine and disease therapeutics
ES cells have great promise for regenerative medicine, showing significant development in cell and gene therapy. Regenerative medicine deals with functional restoration of specific tissue and/ or organ of the patients suffering from chronic disease conditions or severe injuries. Progress from the use of both in vitro and in vivo regenerative medicine models already offers hope both for the facilitation of stem cell phenotyping in recursive gene expression profile models and for the use of stem cells as powerful new therapeutic reagents for cancer, stroke, Parkinson’s, and other challenging diseases.
Challenges
1. To control differentiation
2. Ethical issues
3. Alternative
There are five main parameters that control differentiation
a. Cell-cell interaction
b. Cell-matrix interaction
c. Cell shape and polarity
d. Oxygen tension
e. Soluble systemic factors
Alternative
The ethical problems specific to embryonic stem cell research can be avoided by reprogramming of somatic cells to produce induced pluripotent stem cells. The 2012 Nobel Prize was given to Shinya Yamanaka for developing iPSCs which he shared with John Guordan. He showed that by just expressing four transcription factors (OCt4, SOX2, KLF4, C/L myc) in any adult cells can be turned into iPSCs [5,6].
Conclusion
Stem cells offer great hope for curing most if not all diseases, but there is a reason to believe that these cells may themselves cause tumors. Ideally, rapid scientific advances in this new field of regenerative medicine will translate to new therapies and cures many diseases and injuries. In future, there might be new pharmaceutical compounds; those can activate tissue specific stem cells, promote stem cells to migrate to the side of tissue injury, and promote their differentiation to tissue specific cells.
References
- Alvarez MC, Bejar J, Chen S, Hong Y (2007) Fish ES cells and applications to biotechnology. Mar Biotechnol (NY) 9(2): 117-127.
- Lacerda SMSN, Batlouni SR, Assis LH, Resende FM, Campos SSM, et al. (2008) Germ cell transplantation in tilapia (Oreochromis niloticus). Cybium 32(2): 115-118.
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, et al. (2011) A more efficient method to generate integration-free human iPS cells. Nat Methods 8(5): 409-412
- Hong N, Li Z, Hong Y (2011) Fish stem cell cultures. Int J Biol Sci 7(4): 392-402.
- Goswami M, Lakra WS, Yadav K, Jena JK (2012) Development of an ESlike cell culture system (RESC) from rohu, Labeo rohita (Ham). Fish Physiol Biochem 38(6): 1775-1783.
- Inoue H, Nagata N, Kurokawa H, Yamanaka S (2014) IPS cells: A game changer for future medicine. EMBO J 33(5): 409-417.








.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)







