- Submissions

Full Text
Examines in Marine Biology & Oceanography
Anaerobic Biodegradation of High Strength Real Wastewater Stream Generated from Manufacturing of Solvent Black 46
Prablin Kaur ME*
The Maharaja Sayajirao university of Baroda, India
*Corresponding author: Prablin Kaur ME, Faculty of Technology and engineering, The Maharaja Sayajirao university of Baroda, India
Submission: January 24, 2018;Published: September 12, 2018

ISSN 2578-031X Volume2 Issue3
Abstract
An anaerobic sludge blanket wastewater treatment system uses an anaerobic process by forming a blanket of granular sludge which suspends in the tank whereas wastewater flows upwards through the blanket and is degraded by the anaerobic microorganisms. The upward flow combined witch the settling action of gravity suspends the blanket with the aid of flocculants while the blanket begins to reach maturity at around three months. Small sludge granules begin to form whose surface area is covered in aggregations of bacteria. The performance of Up flow Anaerobic Sludge Blanket (UASB) treating real wastewater generated from manufacturing of Solvent Black 46 (SB46) was investigated. Specifically, it was determined whether UASB could be used as treatment system at an existing dyestuffs production plant. Accordingly, UASB was developed with a reactor volume 25L was being operated in different phases. Consistent Chemical Oxygen Demand (COD) removal efficiency of around 90% was achieved at an organic loading rate (OLR) of 2kg COD/m3 d at inlet COD of 20000mgs/L. Effect of varying OLR on COD removal efficiency and methane production was evaluated by feeding wastewater at COD of 10000mg/L. The COD removal efficiency of >90% at OLR of 2 gradually decreased to ~80% at OLR of 5kg COD/m3d. Methane production was 320-343L/kg COD removed at OLR 2kg COD/m3 d decreased to 147-200L/kg COD removed at OLR of 5kgCOD/m3 d. Decolonization of wastewater led to generation of aromatic amines indicated by formation of a new peak at 286 nm in UV-Vis spectrum. Inlet pH of feed was in the range 6-6.3 which increased to 8.5-8.6 indicating degradation of acetate in the wastewater. Results of this study suggest that anaerobic treatment can be employed advantageously for SB46 wastewater, resulting in excellent COD removal without any energy input and producing useful methane.
Keywords:Up flow anaerobic sludge blanket; Treatment of wastewater; Solvent black effluent; COD; BOD; Methane production
Abbreviations: UASB: Up flow Anaerobic Sludge Blanket; COD: Chemical Oxygen Demand; HRT: High solids Retention Time; DHS: Down-Flow Hanging Sponge; GLS: Gas-Liquid-Solid
Introduction
Biological treatment of wastewater basically reduces the pollutant concentration through microbial coagulation and removal of nonsettleable organic colloidal solids. Organic matter is biologically stabilized so that no further oxygen demand is exerted by it. The biological treatment requires contact of the biomass with the substrate. Various advances and improvements in anaerobic reactors to achieve variations in contact time and method of contact have resulted in development of a suspended growth systems, attached growth or fixed film systems or combinations thereof. Although anaerobic systems for waste treatment have been used since late 19th century, they were considered to have limited treatment efficiencies and were too slow to serve the needs of a quickly expanding wastewater volume, especially in industrialized and densely populated areas. At present aerobic treatment is the most commonly used process to reduce the organic pollution level of both domestic and industrial wastewaters. Aerobic techniques, such as activated sludge process, trickling filters, oxidation ponds and aerated lagoons, with intense mixing devices, have been successfully installed for domestic wastewater as well as industrial wastewater treatment. Anaerobic digestion systems have undergone modifications in the last two decades, mainly because of the energy crisis. Major developments have been made about anaerobic metabolism, physiological interactions among different microbial species, effects of toxic compounds and biomass accumulation. Recent developments however, have demonstrated that anaerobic processes might be an economically attractive alternative for the treatment of different types of industrial wastewaters. One of the more interesting new processes is the UASB process, which was developed by Letting and his coworkers in Holland in the early 1970’s.
The key to the process was the discovery that anaerobic sludge inherently has superior flocculation and settling characteristics, provided the physical and chemical conditions for sludge flocculation are favourable. When these conditions are met, a High solids Retention Time (at high HRT loadings) can be achieved, with separation of the gas from the sludge solids. The UASB reactor is one of the reactors types with high loading capacity. It differs from other processes by the simplicity of its design. UASB process is a combination of physical and biological processes. The main feature of physical process is separation of solids and gases from the liquid and that of biological process is degradation of decomposable organic matter under anaerobic conditions. No separate settler with sludge return pump is required, as in the anaerobic contact process. There is no loss of reactor volume through filter or carrier material, as in the case with the anaerobic filter and fixed film reactor types, and there is no need for high rate effluent recirculation and concomitant pumping energy, as in the case with fluidized bed reactor. Anaerobic sludge inherently possesses good settling properties, provided the sludge is not exposed to heavy mechanical agitation. For this reason, mechanical mixing is generally omitted in UAS Reactors. At high organic loading rates, the biogas production guarantees enough contact between substrate and biomass. Regarding the dynamic behaviour of the water phase UASB reactor approaches the completely mixed reactor. For achieving the required enough contact between sludge and wastewater, the UASB-system relies on the agitation brought about by the natural gas production and on an even feed inlet distribution at the bottom of the reactor.
Dyestuff Waste Water
Dyestuffs sector is one of the core chemical industries in India and second highest export segment in chemical industry. Increased demand for Dye products, their production, and the use of synthetic dyes together contributed to dye wastewater, becoming one of the major sources of severe pollution now a days. Dyes are mostly introduced into the environment through industrial effluents. Dye leads to severe number of environmental & health hazards 10,000 different dyes and their pigments are used industrially, and over 7x105 tons of synthetic dyes are annually produced worldwide. Azo dyes are the largest group of dyes used in the textile industry. They have become a concern in wastewater treatment because their colour and potential toxicity to animals and human [1]. Treatment of dye effluents has been one of the major concerns to the environment. Various types of treatment can be used to treat dye wastewater. Physical processes like adsorption [2-5], and membrane filtration, chemical processes like coagulation-Flocculation [6-8] Ozonation Electrochemical process like Electro coagulation [9]. The treatment mentioned above lead to effective decolourization, but their application is restricted due to various reasons; Physical process lead to phase transfer of dyes from one phase to another without its destruction and possess high maintenance cost. Chemical process is generally non-selective due to high cost of chemicals and sludge production which is difficult to be disposed. Since, Biological treatment is less expensive, less energy consumption, and requiring lesser amount of chemicals, it can be efficiently used as an alternative to the above-mentioned treatments [10].
In a study performed by Tawfik et al. 2014, colour and COD removal efficiencies of 64±17 and 65±6 % were obtained, respectively in UASB that was used to treat reactive dyes wastewater in an Up-Flow Anaerobic Sludge Blanket (UASB) coupled with Down-Flow Hanging Sponge (DHS) [11]. Murali et al. 2013 obtained 94% colour removal and 69% COD removal in UASB that was used to treat 50mg/L Methyl orange for 35 days [1]. reported 99.8 and 61% colour and COD removal efficiencies, respectively, when using sequential anaerobic-aerobic system to treat Azo dye Reactive Black 5 dye.
Existing ETP of dyestuff industry was unable to treat SB46 wastewater efficiently. As the SB46 wastewater contains high concentration of acetates yielding high oxygen demand, which can be easily degraded by methanogens. Also, there is no so source of sulphate or is present in much less amount, so this gives a favourable situation for anaerobic bacteria. If the sulphate concentration is more it will lead to production of Hydrogen Sulphide (H2S) rather than methane. Hence, it gives favourable condition for anaerobic bacteria, where COD is high due to high amount of acetates and low concentration of sulphate. In the light of these facts, UASB was designed for the decolourization and anaerobic degradation of mother liquor generated from the manufacturing of SB46.
Experimental Study of Solvent Black 46 (SB46)
SB46 is a dye soluble in organic solvent. Molecular Weight and Molecular formula are shown in Table 1 and Molecular structure of SB46 is shown in Figure 1.
Table 1:Characteristics of SB46.
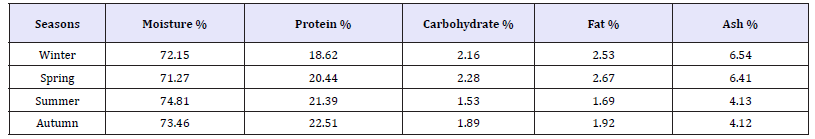
Figure 1:Molecular structure of SB46.
Source of solvent black 46 (SB 46) effluent
The samples of mother liquor of solvent black 46 (SB46) was collected from dyestuffs industry located in GIDC Ankleshwar. The characteristics of the mother liquor of SB46 are shown in the Table 2.
Table 2:Characteristics of mother liquor of SB46.
Analytics methods
The characteristics of mother liquor of SB46 such as pH, COD, Biochemical Oxygen Demand (BOD), Chloride, Conductivity, Ammoniacal nitrogen, Total Dissolved Solids (TDS), Sulphate were determined according to the Standard Methods for Examination of water and wastewater (APHA 22nd edition). Volatile Fatty Acid/Alkalinity (VFA/ALK) ratio was determined using Kapp’s Method [12]. Colour was determined using SHIMADZU UV- 1800 spectrophotometer where UV-VIS spectrum of mother liquor of SB46 shows 3 peaks: peak in visible region at 580nm, indicates crystal violet and peak in UV region at 286nm was due to unreacted raw material used for Metanil yellow and, 346nm indicates metanil yellow.
The colour removal was calculated by change in absorbance of peak at 580nm as for following formula: % Colour removal

At represents absorbance at t=t min.
Experimental set up of UASB
UASB reactor was designed and fabricated using polyacrylic material with an influent distributor and six outlets with an effective volume of 25L with L*W*H = 150*150*1150(mm). Gas-Liquid-Solid (GLS) separator was situated in the upper portion of the reactor to prevent the loss of sludge and for easy release of the biogas produced by UASB reactor. UASB related to the pumps and proper collection containers with proper leak proof pipelines as shown in Figure 2. The reactor was inoculated with 10L of sludge collected from septic tank. Total volatile solids of inoculum sludge were 44.24g/L [13].
Figure 2:Schematic Diagram of UASB Set up.

Two pumps were used as shown in Figure 2: Pump 1 is the peristatic pump for influent flow rate adjustments and pump 2 is the submersible pump for recycling the effluent internally to maintain up flow velocity 0.5- 0.6m/h inside the reactor. Flow rates in pump 1 were adjusted based on the desired flow rates. Influent was introduced from the bottom of the UASB reactor with the help of pump 1.
The temperature during the study period was maintained to (27-35 °C). To maintain the temperature of the reactor in winters [14], UASB effluent was heated with the help of magnetic stirrer with heating coil. Collection and recirculation of heated effluent was carried out by maintaining up flow velocity in the range of 0.5-0.6m/h.
Biogas produced from the reactor was passed through the solution of KOH to dissolve the amount of CO2 with KOH. Hence, obtaining the amount of Methane [15], collected in rubber bladder. The amount of Methane in the bladder was measured using water displacement method.
Operation of UASB
The current study was carried out in different phases. pH of the feed was 4-5 which was adjusted around 6.5 using NaOH. Initially in phase 1, the study was carried out by directly using mother liquor of SB46 wastewater sample having COD=22000-29000mg/L. This resulted in lowered efficiency of the reactor and methanogens stopped working. Therefore, phase 2 was carried out by diluting the wastewater sample using tap water, started with COD=1000mg/L to acclimatize the microorganisms in the sludge and was done until reactor achieved steady state condition i.e. (93-95 % COD removal for minimum 2-3HRT) [16]. The feed was supplemented incrementally to the reactor. The feed COD was increased gradually from 1000mg/L to 18000mg/L simultaneously with increase in OLR from 0.1 to 3.37kg COD/ m3 d. Afterwards, Phase 3, where OLR= 2kg COD/m3 d was kept constant with gradually increasing feed COD from 10000 to 20000mg/L and Phase 4, where COD= 10000mg/L was kept constant with gradually increasing OLR from 2 to 5kg COD/m3d, were carried out. (Reason for considering COD=10000mg/L only is because the mother liquor SB46 in industry when combines with washing water yields COD around=10000mg/L) [17]. Therefore, samples were diluted to obtain the feed COD=10000mg/L. At the end of the study, combined wastewater i.e. mother liquor of SB46 and washing water was treated in the UASB having COD around 10000mg/L at OLR=4kg COD/m3d. COD: N: P ratio was maintained 100: 0.5: 0.1.
Result and Discussion
Initially in phase 2, acclimatization was carried out by diluting SB46 wastewater to obtain a feed COD=1000mg/L. When COD removal≥90 % was achieved for 2-3 HRT, COD of the feed was increased gradually from 1000mg/L to 13000mg/L simultaneously with increase in OLR from 0.1 to 3.37kg COD/m3d, which resulted in % COD removal>90%, methane production was in the range of 312-346L/kg COD removed and VFA/ALK ratio was consistent<0.1, when operated up to COD=13000mg/L and OLR=3.37kg COD/m3d [18]. When the feed COD was further increased to 18000 mg/L at same OLR i.e. 3.37kg COD/m3d, performance of reactor suddenly lowered down and COD removal efficiency decreased to around 60% as shown in Figure 3, methane production also reduced to the range of 248-260L/kg COD removed as shown in Figure 4, and VFA/ALK ratio increased to >0.4. as shown in Figure 5
Figure 3:Inlet COD, Outlet COD, and % COD Removal from SB46 wastewater during Phase.
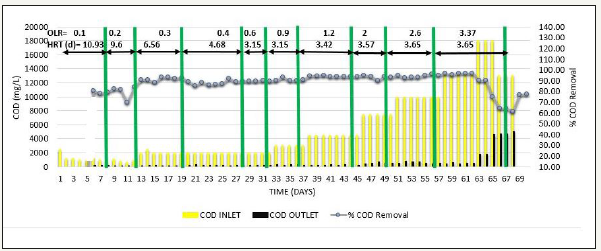
Figure 4:Inlet COD, Outlet COD, and VFA/ALK ratio from SB46 wastewater during Phase 2.
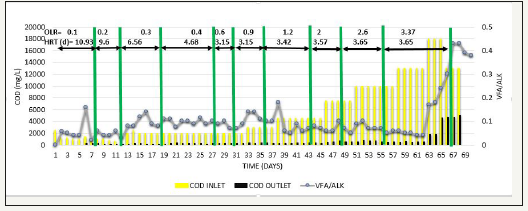
Figure 5:Inlet COD, Outlet COD, & Methane Production from SB46 wastewater during Phase 2.
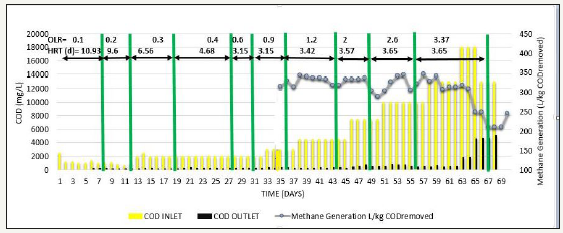
When COD removal efficiency was reduced, feed COD was again brought back to COD=13000 mg/L, but the system did not recover as shown in Figure 3, in fact COD removal decreased further. It shows that, biological activity of UASB was disturbed. Therefore, COD removal efficiency could not be recovered. Moreover, due to the sudden rise in feed COD and OLR, some loss of sludge also occurred. Hence, to overcome the above-mentioned disturbance, new 2L of sludge of septic tank was appended [19,20]. It seems that the above-mentioned disturbance may be because of simultaneous increase in OLR as well as feed COD. Therefore, to determine the cause for sudden changes in the reactor, either it was feed COD or increase in OLR, phase 3 or phase 4 were carried out.
Figure 6:Inlet COD, Outlet COD, and % COD Removal from SB46 wastewater during Phase 3.
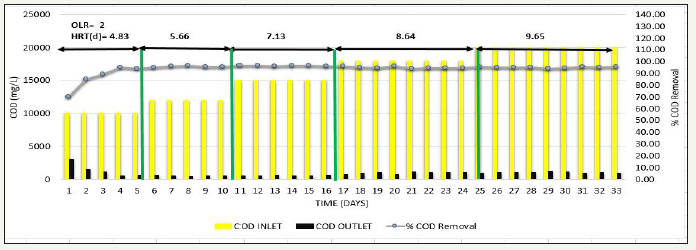
Figure 7:Inlet COD, Outlet COD, and VFA/ALK ratio from SB46 wastewater during Phase.
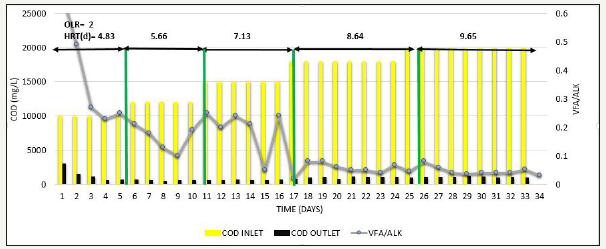
Figure 8:Inlet COD, Outlet COD, and Methane Production from SB46 wastewater during Phase 3.
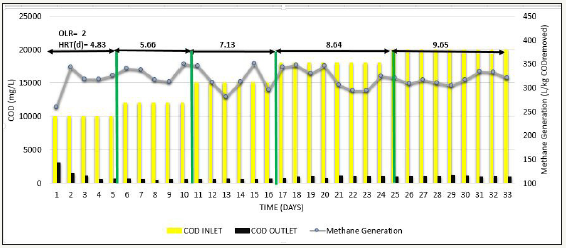
After appending 2L of sludge, Phase 3 was carried out where feed COD was gradually increased from 10000mg/L to 20000mg/L simultaneously at constant OLR=2kg COD/m3 d to see the effect of feed COD [20]. Consistent COD removal efficiency of >90% was observed as shown in Figure 6, VFA/ ALK ratio observed was <0.1as shown in Figure 7, and methane production was also consistent in the range of 320-343L/kg COD removed as shown in Figure 8, indicating that at low OLR i.e. 2kg COD/m3d, even COD up to 20000 mg/L can be treated efficiently and COD removal observed was significant.
Next, to determine the effect of increase in OLR, phase 4 was carried out, where OLR was gradually increased from 2kg COD/m3d to 5kg COD/ m3d at constant feed COD=10000mg/L. Whenever the OLR was increased, % COD removal slight decreased to around 80% in initial stage of each OLR, but afterwards steady results >90% were obtained, methane production was in the range of 280-325L/kg COD removed VFA/ALK ratio was also <0.15. This has been observed when operated up to OLR=3.6kg COD/ m3d. Further increase in OLR to 4-5kg COD/m3d, COD removal efficiency gradually reduced from 90% to around 80% as shown in Figure 9, Methane production gradually starts decreased to the range of 147-200 L/kg COD removed as shown in Figure 10, and VFA/ALK ratio increased to >0.2 as shown in Figure 11.
Figure 9:Inlet COD, Outlet COD, and % COD Removal from SB46 wastewater during Phase 4.
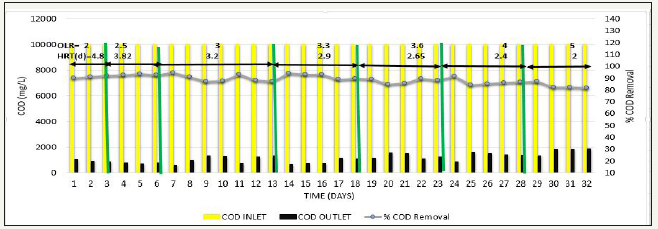
Figure 10:Inlet COD, Outlet COD, and VFA/ALK ratio from SB46 wastewater during Phase 4.
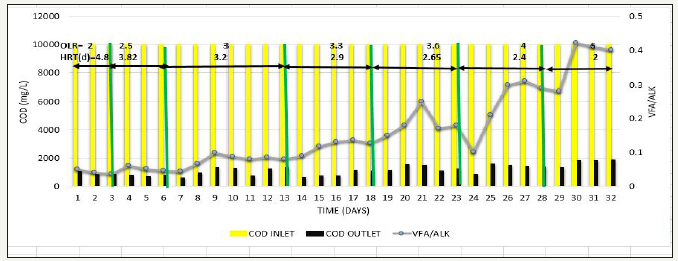
Figure 11:Inlet COD, Outlet COD, and Methane Production from SB46 wastewater during Phase.
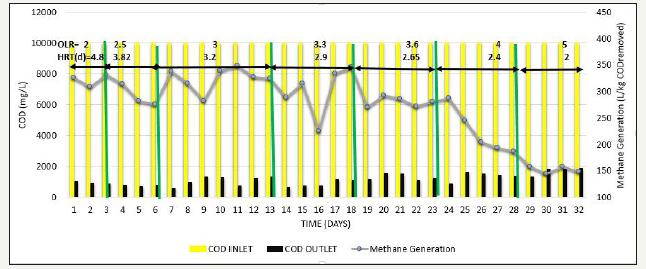
Chellipan, studied that when pharmaceutical wastewater containing macrolide antibiotics was treated in UASB, having COD around 7.5g/L at OLR 1.86kg COD/m3 d, COD reduction was around 70-75%. When OLR was increased to 2.41kg COD/m3d, COD removal efficiency decreased gradually to 45%. Despite poor performance at high OLR, COD removal efficiency was recovered to previous levels when OLR was reduced to 1.86kgCOD/m3d and pharmaceutical wastewater has low concentration of total VFA concentration (avg. 300mg/L) was present in effluent when operated at OLR in the range of 0.43-1.5kg COD/m3d. Further increase in OLR at 3.37kg COD/m3d resulted in higher VFA concentration in the range of 3300mg/L, indicating the reduction in acetate degradation. Mendoza (1998) studied that when UASB was fed with coffee wastewater having COD concentration 1-6g/L at OLR 1.89kg COD/m3 d, COD removal efficiency of 77% was attained. When OLR of reactor was increased rapidly reaching value of 2.36kg COD/m3d resulting in dropped COD removal efficiency to 25%, which forced to a reactor shutdown for 7 days. After then, reactor was resumed with low OLR 1.78kg COD/m3d for same COD, resulted in average COD removal efficiency of 63%, indicating that when operated at optimum OLR for COD, results observed are significant. Gao et al. [12] stated that volumetric biogas production increased with increasing OLR but afterwards began to decrease due to deterioration of COD removal efficiency during treatment of distiller’s grains wastewater in UASB.
Torkian 2003, studied that UASB treating slaughterhouse effluent produced up to 300L/kg COD removed at OLR 27kg COD/m3d. Methane production rate seemed to decline below 200L at high OLR up to 39.5 3.37kg COD/m3d. Hampannavar [13], studied that methane production with COD loading rate and was maximum of 3.8L/Ld. at OLR 16 kg COD/m3 d for treatment of sugar industry wastewater in UASB reactor. When OLR was increased from 16 to 24kg COD/m3 d, Methane production decreased from 3.8L/Ld. to 2.6L/Ld. This sudden decrease in methane production was due to VFA accumulation as acetate which inhibited biomass resulting in lower COD removal and ratio of VFA/ALK varied between 0.19-0.33 during treatment of sugar industry wastewater in UASB at OLR up to 16kg COD/ m3 d. When OLR was further increased to 24kg COD/m3 d, VFA/ALK ratio reached value of 0.7 indicating system instability. This can be attributed to insufficient alkalinity generation in reactor.
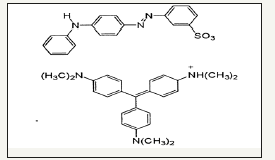
SB46 is a dye made up of crystal violet which is not an azo dye and Metanil Yellow (C18H14N3SO3Na) as shown in Figure 12 is an azo dye compound having N=N bonds is mainly responsible for colorization in SB 46 wastewater. SB46 wastewater had a peak in visible region at 580nm which was due to crystal violet & peak in UV region at 286 nm was due to unreacted raw materials used for production of metanil yellow and 346nm was due to metanil yellow. SB46 wastewater was treated in UASB, the degradation proceeded, disappearance of absorbace peak at 346nm, reflected signal of almost complete decolorization & breakdown of azo bond cleavages of Metanil yellow in SB46 and 580nm which seems to be because of anaerobic degradation of crystal violet as shown in Figure 13. Intermediates used for metanil yellow were examined and its UV-VIS spectra was taken as shown in Figure 14. It seems new peak appeared in outlet at absorbance of 286nm reflecting signal of formation of intermediates of metanil Yellow i.e. pure diphenyl amine (DPA) & Metanillic acid (MA) which can be confirmed by Figure 14. This can be said because of occurrence of the peak of mixture of intermediates i.e. DPA+MA on the absorbance value close to the absorbance value of the peak in the outlet. That is why it seems that metanil yellow part is reduced by breakdown of cleavage of azo bond forming aromatic amines at 286nm in the outlet.
Figure 12:Molecular structure of metanil yellow.

Figure 13:UV-VIS spectrum of SB46 wastewater before and after treatment.
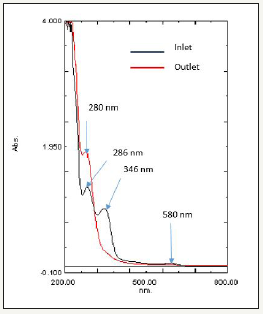
Figure 14:UV-VIS spectrum of intermediates of metanil yellow.

Conclusion
It was concluded that the steady state condition of the reactor can be obtained by balancing both, OLR as well as feed COD. Decolourization of SB46 wastewater did not correlate with COD concentration, %COD, OLR or methane production. It seems that the colour removal of SB46 wastewater may be attributed to the fermentative bacteria. Inlet pH of feed was 4-5 which was adjusted ~6.5 using NaOH, which increased to 8.5-8.6 due to degradation of acetate in the wastewater in the outlet throughout all the phases, indicating outlet pH to be independent of OLR for SB46 wastewater. Therefore, results of this study suggest that anaerobic treatment can be employed advantageously for SB46 wastewater, resulting in excellent COD removal without any energy input and producing useful methane’s.
References
- Mustafa K, Sukru D, Mehmet EA (2010) The Decolorization of azo dye reactive black 5 in a sequential anaerobic-aerobic system. Ekoloji 19(74): 15-23.
- Subramanian N, Hari C, Bajaj, Rajesh JT (2017) Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J Environ Sci 65: 201-222.
- Nigam P, Armour G, Banat IM, Singh D, Marchant R (2000) Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresource Technology 72(3): 219- 226.
- Avila HE, Castillo DI, Petriciolet A (2016) Relevance of anionic dye properties on water decolorization performance using bone char: Adsorption kinetics, isotherms and breakthrough curves. Journal of Molecular Liquids 219: 425-434.
- Mohammad FA, Mumtaz AZ, Abeer MA (2012) Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iranian Journal of Environmental Health Science & Engineering 9: 17-20.
- Abdelkader A, Younes A, Salah S, Mohammed S, Hicham R (2009) Colour and COD removal of disperse dye solution by a novel coagulant: Application of statistical design for the optimization and regression analysis. J Hazard Mater 166(3): 1302-1306.
- Imen K, Marrot B, Philippe M, Raja BA (2011) Decolorization of the reconstituted textile effluent by different process treatments: Enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination 268(3): 27-37.
- Rui Z, Dong XY, Bao ML (2014) Kinetics and products of ozonation of C.I. Reactive Red 195 in a semi-batch reactor. Chinese Chemical Letters 26(1): 93-99.
- Aouni A, Fersi C, Sik AM, Dhahbi M (2009) Treatment of textile wastewater by a hybrid electrocoagulation/nanofiltration process. Journal of Hazardous Materials 168(3): 868-874.
- Popli S, Upendra DP (2015) Destruction of azo dyes by anaerobicaerobic sequential biological treatment: a review. International Journal of Environmental Science and Technology 12(1): 405-420.
- Ayoob T, Eqbali A, Hashemian SJ (2011) The effect of organic loading rate on the performance of UASB reactor treating slaughterhouse effluent. Resources, Conservation and Recycling 40(1): 1-11.
- Gao M, She Z, Jin C (2007) Performance evaluation of a mesophilic (37 °C) upflow anaerobic sludge blanket reactor in treating distiller’s grains wastewater. Journal of Hazardous Materials 141(3): 808-813.
- Hampannavar US, Shivayogimath CB (2010) Anaerobic treatment of sugar industry wastewater by Up flow anaerobic sludge blanket reactor at ambient temperature. International Journal of Environmental Sciences 1(4): 631-639.
- Jitender KS, Anju S, Rajesh KL (2016) Rice mill wastewater treatment by UASB. International journal of plant animal and environmental sciences 6.
- Kaviyarasan K (2014) Application of UASB Reactor in industrial wastewater treatment a review. International Journal of Scientific & Engineering Research 11(5): 409-418.
- Neha K (2014) Impact of dye industry on environment.
- Nishad NF, Rathinam A, Jonnalagadda RR, Balachandran UN (2008) Dye house wastewater treatment through advanced oxidation process using Cu-exchanged Y zeolite: A heterogeneous catalytic approach. Chemosphere 70(6): 1146-1151.
- Bello MR, Castillo RMF (1998) Start-up of an anaerobic hybrid (UASB⁄Filter) Reactor treating wastewater from a coffee processing plant. Anaerobe 4(5): 219-225.
- Shreeshivadasan C, Thomas W, Sallis PJ (2006) Performance of an upflow anaerobic stage reactor (UASR) in the treatment of pharmaceutical wastewater containing macrolide antibiotics. Water Res 40(3): 507- 516.
- www.fibre2fashion.com/industry-article/5118/the-future-of-indiandyes- dye-intermediates#, May 2010
© 2019 Prablin Kaur ME. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






