- Submissions
Full Text
Examines in Marine Biology and Oceanography: Open Access
Oxygen Consumption of Nauplii of Marine Planktonic Copepods
Gustav-Adolf Paffenhofer1* and Marion Koster2
1Skidaway Institute of Oceanography, USA
2Ernst-Moritz-Arndt Universitat Greifswald, Germany
*Corresponding author: Gustav Adolf Paffenhofer, Skidaway Institute of Oceanography, 10 Ocean Science Circle Savannah, Georgia 31411, USA
Submission: February 13, 2018; Published: February 20, 2018

ISSN: 2578-031X Volume1 Issue3
Abstract
The objective of our study was to determine the oxygen consumption rates of nauplii of two small calanoid species, Paracalanus quasimodo and Temora turbinata, and compare their rates with those of three other planktonic species. The comparisons reveal that motion results in higher oxygen consumption i.e. higher energy expenditure than little or no motion. In addition, the type of motion results in different oxygen consumption rates, and therefore different energy expenditures. Our findings also show that nauplii consume more energy per unit body weight than their copepodids. This implies that small calanoid nauplii, having few energy reserves, are at a disadvantage in relation to copepodid stages as they require continuous food supply and continuous feeding which here is accompanied by continuous motion. That leaves them vulnerable to predators. Thus, not only predation but also discontinuous/intermittent feeding activity could result in increased mortality of calanoid nauplii.
Keywords: Copepod nauplii; Oxygen consumption; Metabolism
Introduction
Marine planktonic copepods are the most abundant metazoa on our planet [1]. Within this taxon the early juveniles, the nauplii, are the most abundant. So far, the vast majority of research on planktonic copepods has been on late juveniles and adult stages i.e. nauplii were rarely included [2]. In his Biology of Calanoid Copepods of 710 pages devoted about 1% (less than 10 pages) to nauplii. More than four decades ago the significance of copepod nauplii was addressed within process studies on calanoid copepods: [37] since nauplii of planktonic copepods usually do not accumulate energy reserves as copepodid stages and adults do [8] they most likely will not survive food shortages over longer periods of time. Yet our knowledge on their food requirements and energy needs is limited [9,10]. Studies in temperate waters revealed that in situ mortality of nauplii and older stages is related to invertebrate and vertebrate predators [11,12]. We know from laboratory studies that survival of nauplii and older stages of calanoid is also a function of food abundance [5]. So far, our knowledge of nauplius mortality in relation to food abundance in warmer waters is scarce. We know that energy consumption per unit body weight decreases from younger to older stages of copepods [10,13]. The goal of this study was to quantify the oxygen consumption rate of two species of small calanoid copepods, Paracalanusquasimodo and Temoraturbinata which often occur abundantly on the U.S. southeastern continental shelf [14] and compare their rates to those of other planktonic copepod species, and with older stages. We calculated their daily energy requirements by transforming oxygen consumption into energy units and compared this with their food intake assuming 93% assimilation efficiency [9].
Material and Methods
Rearing of nauplii
Late copepodid stages and adult females were collected from the U.S. southeastern continental shelf during summer of 2007 and late winter 2008 (Temora turbinata), and January/February 2015 and March 2016 (Paracalanus quasimodo). The copepodid stages moulted to adults followed by egg production. Eggs were also obtained from the collected females (age unknown) which had a short reproductive period (age unknown). Rearing occurred at 19.5 to 20.1 °C during 12h dim light/12h darkness in glass vessels of 960 to 1,900ml volume on a plankton wheel rotating at 0.5rpm. The nauplii were offered simultaneously the flagellates Isochrysis galbanaand Rhodomonas sp. and the diatom Thalassiosira weiss flogii at total average food concentrations of near 60|ig C L-1. This approach provided food levels close to frequently 60|ig C L-1 occurring phytoplankton abundances on the middle to outer shelf. The jars were inspected daily and the concentrations of each of the three food species were determined by using a Beckman-Coulter Multisizer IV. At the beginning of each experiment additional food particles were added to reach food levels of near 20-30% higher than the average food concentration (Table 1).
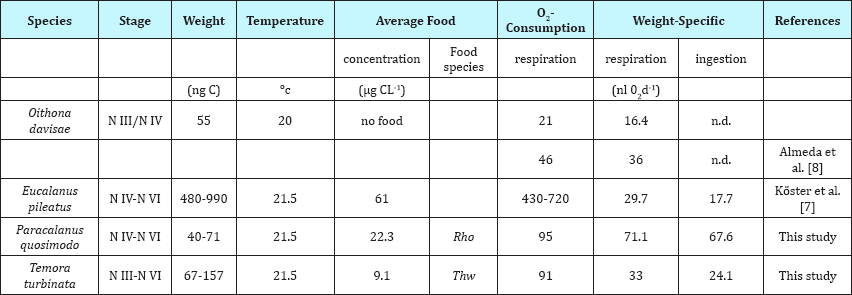
Table 1: O2 Consumption rates, weight-specific respiration and ingestion of different planktonic copepods. Rho Rhodomonas sp., Thw Thalassiosira weissflogii.
The oxygen consumption of nauplii of the calanoid copepods P. quasimodo and T. turbinata was quantified using optical fluorescence-based oxygen respirometry [15]. A 24-channel Sensor Dish Reader (SDR, Presens, Regensburg, Germany) was used to quantify oxygen consumption in 8ml and 10.5ml respiration chambers containing nauplii and food. Changes in oxygen concentrations were recorded continuously at 5-minute intervals over 4 to 6h i.e. in time-series. All oxygen measurements were performed in darkness. For further information [9]. All experiments were run at 21.5 to 21.8 °C.
Nauplii experiments
The oxygen consumption experiments with nauplii of T. turbinata were run in September 2007 and March 2008, and those with nauplii of P. quasimodo in March 2015 and September 2016, all at a temperature of 21.5 °C. The T. turbinate studies occurred in standing vials of 10.5ml volume with the number of nauplii ranging from 39 to 65 per vial; stages ranged from N III to N VI, weighing 67 to 157ng C. We decided to offer food at environmental abundances because the absence of food would result in an underestimation of metabolic rates of copepods [10]. The food species was T weissflogii (1,000 to 1,200|im3 cell volume). The more recent studies with nauplii of P. quasimodo were conducted in 8.0ml vessels being on a plankton wheel rotating at 0.5rpm. The number of nauplii per vial ranged from 39 to 64 weighing on average 40 to 71ng C Nauplius-1 (N IV to N VI). Here the food was Rhodomonas sp. (300 to 350|im3 cell volume); and later lsochrysis galbana (40|im3 cell volume). Four vials, serving as controls, were always run simultaneously. The control vessels had the same phytoplankton species and concentration as were offered to the nauplii. The oxygen consumption by the nauplii in each vessel was quantified from the linear part of the time-series oxygen concentration data by linear regression. The nauplius' individual respiration rate was quantified by subtracting the average rates in the control vials from the individual respiration rates in order to eliminate the net oxygen consumption or production by bacteria and phytoplankton. The daily metabolic expenditures per Nauplius were calculated as 1nl oxygen respired = 0.43ng C respired [4].
After the oxygen time-series experiments the nauplii's morphology and motion behavior were inspected under a dissecting microscope. The contents of each vial were placed in a settling chamber, preserved with 2% Lugol's solution to count the remaining phytoplankton cells. Initial and final phytoplankton concentrations served to calculate clearance and ingestion rates of all nauplius experiments [16]. Each nauplius' body length was measured to obtain an average weight (ng C) from a previously obtained regression of Nauplius length vs. weight for each of the two copepod species: P. quasimodo log y = 2.088 log x - 2.921; T. turbinata log y = 3.195 log x - 5.446.
Statistics
We calculated linear regressions of Nauplius weight (ng C Nauplius-1) vs. oxygen consumption (nl oxygen respired Nauplius-1 h-1), and then compared slopes and elevations [17].
Oxygen consumption
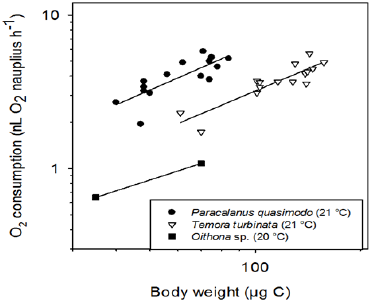
Nauplii of T. turbinate which create a feeding current and move slowly at 0.5mms-1 [18] consumed 2.15|il h-1 while those of P. quasimodo of the same weight (70ng C), moving continuously at 1.34mms-1 consumed 4.5nl h-1 i.e. over 100% more than T. turbinatanauplii. Nauplii of Oithonadavisae of the same weight but without food consumed only 1.1nl O2-1h-1 at 20 °C [10]. It appears that oxygen consumption increased with increased motion as shown earlier for adult female copepods [19]. The fast-moving females of Clausocalanus furcatus consumed about 65% more oxygen than the slowly-moving and feeding current-producing Paracalanus aculeatus females [10]. Also determined oxygen consumption of O. davisae nauplii at the extraordinarily high food concentration of 2.75mg C L-1 resulting in an O2 consumption increase by factor 2.3 i.e. 2.5nl O2h-1 of a 70ng C Nauplius. Such an increase in oxygen consumption is thought to be associated with Specific Dynamic Action (SDA) which has been related to the expense associated with biosynthesis of new tissue from recent ingestion [20]. They showed that respiration of the copepod Acartia tonsa increased with markedly increasing ingestion. We intentionally offered food near environmental levels in our experiments because of our generally environmentally-oriented research. This implies that no experiments were conducted without food. We are also comparing our findings with those of [21] on non-feeding copepods because the taxa we used in our experiments were part in their metabolic rate quantifications (Temora turbinata and the genus Paracalanus). Their equation of oxygen consumption vs. body carbon at 21.5 °C gives a value of 3.0nl O2h-1 for a copepod of 70ngC, ranging between our values of T.turbinata and P. quasimodo. Would there be no food shortage effects of oxygen consumption by starving copepods? At 20 °C O.davisae nauplii without food are lowering their oxygen consumption rate 10h after starting the experiment, implying that immediate effects of food absence were not observed (Figure 1). It appears that oxygen consumption rates of copepods measured for several hours just after they were removed from their food are close to those or even identical as when they were feeding.
Figure 1: Oxygen consumption in relation to body weight of nauplii of three species of planktonic copepods. (O.davisae data from Almeda et al. [8]). Regression equations:
-
Oithona davisae log y = 0.766 log x -1.376
Temora turbinata log y = 1.028 log x -1.554
Paracalanus quasimodo log y = 1.013 log x -1.175
Significance of metabolic expenditures and behaviors for copepod nauplii and older stages
When comparing metabolic expenditures of the various juvenile stages of planktonic copepods it became apparent that expenditures per unit body weight decrease with increasing stage [10]. Nauplii of many calanoid species are at an evolutionary disadvantage because they not only have a higher metabolic demand than their older brethren but also have to find food by moving continuously, thus leaving them exposed to predators. In addition, their mechanosensory performance is inferior to that of the copepodid stages as shown by results from [22] late nauplii of the genera Paracalanus and Acartia were more heavily preyed upon than early copepodids of the same species by the females of the carnivorous calanoid Labidocera trispinosa. While the nauplii of T. turbinata and P. quasimodo are moving all their appendages and setae continuously their copepodid stages possess the nonmoving first antennae with 3-D oriented setae at their tips which perceive oncoming predators, and thus avoid heavy predation. While we observe a major change in morphology from Nauplius VI (N VI) to copepodid stage I (C I), particularly for the first antennae (A1), there can be no change in motion behavior, e.g. the genus Temora producing continuously feeding currents, which should be accompanied by hardly any change in oxygen consumption from N VI to C I (as their weights hardly differ). Or, a major change is observed as for the genus Paracalanus where the nauplii move hop- wise continuously fast whereas the copepodid stages produce a feeding current and moving slowly [18] which should result in less energy expenditure.
The opposite is observed for the cyclopoid Mesocyclops brasilianus: Here the nauplii hardly move; right after moulting the C I moves continuously consuming 250% more oxygen than the N VI of about the same weight [18]. Are there any in situ indications that nauplii may suffer higher mortality than their older stages in continental shelf waters? Time-series observations during summer of 1978 were made possible by following a drogue in up welled water over 10 days sampling each vertical layer (warm upper mixed layer=UML, thermocline=TH, and intruding cold up welled water mass= IN) with 100 and 30 micron mesh. This allowed quantitative sampling of nauplii and older stages of abundant copepods [24]. The average concentrations of nauplii of T. turbinata of 7 stations was 6067, 3903 and 3244 m-3 in the UML, TH and IN, respectively. The average concentrations of copepodids and adults of the same species were only 641, 1042, and 1873 m-3, respectively. At each depth range of each of the 7 stations the concentrations of the nauplii surpassed those of the older stages clearly. From those data the probability of substantial mortality of nauplii cannot be excluded, as compared to the post naupliar stages. The question then would remain whether food and/or predators were the cause. During autumn the omnivorous calanoid Centropages velificatus intermittently dominates on the southeastern middle shelf while the often dominant mainly herbivorous P. quasimodo hardly occurs (unpubl. results). It is assumed that the strongly carnivorous omnivore [25] affects the P. quasimodo assemblage by consuming its nauplii.
The relatively high metabolic rates of the nauplii (near 33% of body C for T. turbinata, and 69% for P. quasimodo) require near continuous food supply because these nauplii have hardly any energy reserves which older stages have [8]. Will there be a continuously sufficient food supply on the SE shelf during much of the year? Since the range of perception and ingestion is limited for those small nauplii, mainly in the range of 4 to 11 microns (pers. obs.), and competition is provided by heterotrophic dinoflagellates and mixotrophs, sufficient food concentrations may not always be found [26]. Analyzed the effects of starvation on nauplii of Calanus pacificus: when initial feeding was delayed for about 10h after moulting to N III survivorship was reduced. For well-feeding stages of N III to N VI survival was reduced when starving for more than 6h. However, complete starvation may not often occur, as abundances of phytoplankton will never be below 0.04|ig chlorophyll L-1 [19] i.e. about 2|ig C L-1. However, major reductions of small phytoplankton will occur on the SE shelf as e.g. abundances of more than 5,000 P. quasimodo copepodids/adult females were intermittently encountered which would substantially reduce cells in the range of 5 to 12|im ESD (Equivalent Spherical Diameter and Paffenhofer, unpubl. results).
At which food concentrations will nauplii of T. turbinate and P. quasimodo meet their metabolic expenditure? In our oxygen consumption experiments the weight-specific ingestion rate of T. turbinata nauplii was 24.1% at an average food concentration of 9.1|ig C L-1 of T weissflogii while the respiration expenditure was 33% of its body carbon. In jars of 480 and 960 ml capacity T turbinate nauplii ingested daily 36% of their body carbon at 10|ig C L-1 of T weissflogii (unpubl. results). It appears that the small oxygen consumption vessels of 10.5ml capacity, and the not-moving, could have affected the nauplii's ingestion rates. Nauplii of P. quasimodo, being smaller, were studied in vessels of 8.0ml capacity, starting at higher food concentrations (flagellate Rhodomonas sp.) than for T turbinata, being mounted on a slowly-moving plankton wheel. At an average food concentration of 22.3|ig C L-1 those nauplii ingested Rhodomonasat a rate of 71% of their body carbon d-1 while respiring 69% daily at 21.6 °C. When feeding on T weissflogiiat 20 °C at about 8|ig C L-1a N VI of Paracalanus sp. ingested daily near 70% and at 24|ig C L-1 near 105% of its body carbon. That occurred in vessels of 960ml [28] where hardly any wall effects should be encountered. Post naupliar stages spend far less energy per unit body weight than nauplii despite being able to compete with the nauplii for part of the same food: Adult females of Paracalaus aculeatus expend only 15% of their body carbon per day at 20 °C [19]. At the same time copepodid stages can accumulate energy reserves; they also are able to vertically explore food reserves more readily than nauplii.
Outlook
For future studies we plan to record the motion, and feeding of various stages of calanoid copepod species in 100ml respiration chambers using a sensitive infrared digital camera, and monitor simultaneously the oxygen consumption at environmental food levels (including food limitation) in extended time-series experiments (more than 12h).
Conclusion
Survival of its nauplii is essential for the persistence of a species. How could we determine survival and associated variables, and find out whether Nauplius losses are caused by predation and/ or food shortage? [29] obtained in Australian river estuaries in situ evidence that the calanoid Gladioferens imparipesis replaced by the omnivorous calanoid Sulcanus conflictus because the latter preys heavily on the nauplii of the former. It appears that on a continental shelf a closely spaced time-series, building on earlier data, would be needed to track a defined body of water in vertical layers over time. Nauplii, copepodid stages and potential predators and associated needed variables would be collected quantitatively [30]. The respective prey species would require to be staged [31-35]. This would be readily possible for each of the two Temora species on the SE shelf [36-40].
Financial Support
This research was supported by NSF Grant OCE 1031263.
Acknowledgement
Captain and crew of the R/V Savannah always supported us with competence and can-do attitude. We gratefully acknowledge their support.
References
- Fryer G (1986) Structure, function and behavior, and the elucidation of evolution in copepods and other crustaceans. Syllogeus 58: 150-157.
- Mauchline J (1998) The biology of calanoid copepods. In: Blaxter JHS, Southward AJ, Tyler PA (Ed.), Advances in Marine Biology. Academic Press, San Diego, Boston, New York, London Vol. 33, p. 710.
- Marshall SM, Orr AP (1956) On the biology of Calanus finmarchicus. IX. Feeding and digestion in the young stages. Journal of the Marine Biological Association of the United Kingdom 35(3): 587-603.
- Mullin MM, Brooks ER (1970) Growth and metabolism of two planktonic, marine copepods as influenced by temperature and type of food. In: Steele JH (Ed.), Marine Food Chains, Oliver and Boyd, Edinburgh, UK, pp. 74-95.
- Paffenhöfer GA (1970) Cultivation of Calanus helgolandicus under controlled conditions. Helgolander wissenschaftliche Meeresuntersuchungen 20: 346-359.
- Harris RP, Paffenhofer GA (1976) The effect of food concentration on cumulative ingestion and growth efficiency of two small marine planktonic copepods. Journal of the Marine Biological Association of the United Kingdom 56(4): 875-888.
- Fernandez F (1979) Nutrition studies in the nauplius larva of Calanus pacificus (Copepoda: Calanoida). Marine Biology 53(2): 131-147.
- Lee RF, Nevenzeland JC, Paffenhofer GA (1972) The presence of wax esters in marine planktonic copepods. Naturwissenschaften 59(9): 406411.
- Köster M, Krause C, Paffenhofer GA (2008) Time-series measurements of oxygen consumption of copepod nauplii. Marine Ecology Progress Series 353:157-164.
- Almeda R, Alcaraz M, Calbet A, Saiz E (2011) Metabolic rates and carbon budget of early developmental stages of the marine cyclopoid copepod Oithona davisae. Limnology Oceanography 56(1): 403-414.
- Eiane K, Aksnes DL, Ohman MD, Wood M, Marinussen MB (2002) Stage- specific mortality of Calanus spp. under different predation regimes. Limnology and Oceanography 47(3): 636-645.
- Eiane K, Ohman MD (2004) Stage-specific mortality of Calanus finmarchicus, Pseudocalanus elongatus and Oithona similis on Fladen Ground, North Sea, during a spring bloom. Marine Ecology Pogress Series 268:183-193.
- Epp RW, Lewis WM (1980) The nature and ecological significance of metabolic changes during the life history of copepods. Ecology 61(2): 259-264.
- Bowman TE (1971) The distribution of calanoid copepods off the Southeastern United States between Cape Hatteras and Southern Florida. Smithsonian Contribution to Zoology 9: 1-64.
- Kautsky H (1939) Quenching of luminescence by oxygen. Transaction of the Faraday Society 35: 216-219.
- Frost BW (1972) Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnology and Oceanography 17(6): 805-815.
- Zar JH (1974) Biostatistical analysis. In: Zar JH (Ed.), Biostatistical analysis. Prentice-hall, Englewood Cliffs, US, pp. 620.
- Paffenhöfer GA, Strickler JR, Lewis KD, Richman S (1996) Motion behavior of nauplii and copepodid stages of marine planktonic copepods. Journal Plankton Research 18: 1699-1715.
- Paffenhöfer GA (2006) Oxygen consumption in relation to motion of marine planktonic copepods. Marine Ecology Progress Series 317: 187192.
- Kiθrboe T, Mθhlenberg F, Hamburger K (1985) Bioenergetics of the planktonic copepod Acartiatonsa: relation between feeding, egg production and respiration, and composition of specific dynamic action. Marine Ecology Progress Series 26: 85-97.
- Ikeda T, Kanno Y, Ozaki K, Shinada A (2001) Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Marine Biology 139(3): 587-596.
- Landry MR (1978) Predatory feeding behavior of a marine copepod, Labidocera trispinosa. Limnology and Oceanography 23(6): 1103-1113.
- Epp RW, Lewis ML (1979) Metabolic responses to temperature change in a tropical freshwater copepod (Mesocyclops brasilianus) and their adaptive significance. Oecologia 42(2): 123-138.
- Paffenhöfer GA (1983) Vertical zooplankton distribution on the northeastern Florida shelf and its relation to temperature and food abundance. Journal Plankton Research 5(1): 15-33.
- Paffenhöfer GA, Knowles SC (1980) Omnivorousness in marine planktonic copepods. Journal Plankton Research 2(4): 355-365.
- Lopez MDG (1996) Effect of starvation on development and survivorship of naupliar Calanus pacificus (Brodsky). Journal Experimental Marine Biology and Ecology 203(2): 133-146.
- Paffenhöfer GA Koster M (2005) Digestion of diatoms by planktonic copepods and doliolids. Marine Ecology Progress Series 297: 303-310.
- Paffenhöfer GA (1984) Food ingestion by the marine planktonic copepod Paracalanus in relation to abundance and size distribution of food. Marine Biology 80(3): 323-333.
- Rippingale, R.J. and Hodgkin, E.P. (1974) Predation effects on the distribution of a copepod. Australian Journal of Marine and Freshwater Research 25: 81-91.
- Beers JR, Stewart GL (1971) Micro-zooplankters in the plankton communities of the upper waters of the eastern tropical Pacific. Deep Sea Research and Oceanographic Abstracts 18(9): 861-883.
- Ueda H (1983) Small-scale ontogenetic and diel vertical distributions of neritic copepods in Maizuru Bay, Japan. Marine Ecology Progress Series 35: 65-73.
- Damkaer DM (2002) The copepodologist's cabinet. In: Damkaer DM (Eds.), The copepodologist's cabinet. American Philosophical Society Philadelphia, US, pp. 300.
- Gerritsen J, Strickler JR (1977) Encounter probabilities and community structure in zooplankton: a mathematical model. Journal of the Fisheries Research Board of Canada 34(1): 73-82.
- Ikeda T (1985) Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Marine Biology 85(1): 1-11.
- Jahnke RA Craven DB (1994) The influence of organic matter diagenesis on CaCO3 dissolution at the deep-sea floor. Geochimica et Cosmochimica Acta 58(13): 2799-2809.
- Klekowski RZ, Kukina IV, Tumanseva NI (1977) Respiration in the micro zooplankton of the equatorial upwelling's in the eastern Pacific Ocean. Polish Archives Hydrobiology 24(Suppl): 467-489.
- Mullin MM, Brooks ER (1967) Laboratory culture, growth rate, and feeding behavior of a planktonic marine copepod. Limnology Oceanography 12(4): 657-666.
- Paffenhöfer GA Lewis KD (1989) Feeding behavior of nauplii of the genus Eucalanus (Copepoda, Calanoida). Marine Ecology Progress Series 57: 129-136.
- Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs. Zoological Studies 43(2): 255266.
- Uchima M, Hirano R (1986) Food of Oithona davisae (Copepoda: Cyclopoida) and the effect of food concentration at first feeding on the larval growth. In: Uchima M, Hirano R (Eds.), Food of Oithona davisae (Copepoda: Cyclopoida) and the effect of food concentration at first feeding on the larval growth. Bulletin of the Plankton Society of Japan, Japan 33: 21-28.
© 2018 Gustav-Adolf Paffenhofer, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






