- Submissions

Full Text
Environmental Analysis & Ecology Studies
Influence of Neem and Moringa Leaf Extract on Quality and Shelf Life of Tomato
Muhammad Anas Mehboob Malik1, Umar Khan Swati4*, Akhi Badrunnesa4, Raja Ahmad Ali1, Zia Ur Rehman1, Alexander Yesaya3, Zhenyu Yao4, Ravelomanana Julio Stanislas5, Sohail Aslam2, Muhammad Abbas Khan2, Imtiaz Ahmed2, Amina Qamar6, Muhammad Affan Khan1 and Malik Faizan Shaukat1
1Department of Horticulture, The University of Haripur, Pakistan
2National Tea and High Value Crops Research Institute, Shinkiari, Pakistan
3Department of Crop & Soil Sciences, Lilongwe University Of Agriculture & Natural Resource Malawi, Malawi
4Zhengzhou Institute of fruit trees, Chinese Academy of Agriculture Sciences Zhengzhou 450009, China
5Anyang Institute of Cotton Research, Crop Genetics and Breeding, CAAS, China
6Jiangxi Provincial Key Laboratory for Bamboo Germplasm Resources and Utilization, Forestry College, Jiangxi Agricultural University, Nanchang 330045, P. R. China
*Corresponding author:Umar Khan Swati, Zhengzhou Institute of fruit trees, Chinese Academy of Agriculture Sciences Zhengzhou 450009, China
Submission: March 19, 2024; Published: April 12, 2024

ISSN 2578-0336 Volume12 Issue1
Abstract
Edible film coatings are frequently employed as a protective barrier to lower transpiration and respiration, which slows down the ripening process and improves the quality of fruits and vegetables. The present investigation “Influence of neem and moringa leaf extract on quality and shelf life of tomato” was conducted in Horticulture at the University of Haripur, Khyber Pakhtunkhwa, Pakistan during 2022, with the objective of finding the best coating material to increase the storage duration of tomato. Four treatments i-e T0=Control, T1=10% Moringa Leaf Extract, T2=10% Neem Leaf Extract, T3=10%+10% Moringa leaf extract+Neem leaf extract was used. Fruits were stored for maximum of 12 days. Various parameters like, Fruit weight (g), Fruit firmness (Nm), Fruit Decay (%), Fruit pH, Total phenolic contents, Total antioxidant activity (% DPPH Inhibition), Total Soluble Solids (%), Catalase Determination, Peroxidase Determination, Superoxide dismutase and phytochemical screening of tomato fruit were observed. Tomato fruit coated with 10%+10% MLE+NLE showed promising results regarding increasing the shelf life and maintaining the quality of the fruits. From this study it is concluded that coating of 10% MLE+10% NLE helps in enhancing the shelf life of tomato fruits and also found very effective in maintaining the quality of tomato fruit under different storage durations. Post-harvest application of these organic extracts can be helpful in increasing the shelf life of tomato.
Keywords: Tomato; Shelf life; Moringa; Neem
Introduction
Tomato (Lycopersicon lycopersicum), remains as one of the most frequently produced and consumed vegetable across the world. Depending on their growth tendencies, tomato cultivars are classified as determinate or indeterminate; the former type is good for the production of uniformly ripened fruits, whilst the latter type is suitable for the continuous production of fruit throughout the year [1]. Tomato is botanically classified as a fruit. The producers have been enticed to produce the crop all year long, especially in areas with warmer weather, due to the low cost, ease of tomato production, short duration, and significant economic returns [2]. Prior to being accepted as safe for consumption, tomatoes were only cultivated as aesthetic garden plants since they were formerly believed to be toxic. One of the most significant commercial and nutritional vegetable crops today is the tomato [3].
Due to high perishability, tomatoes cannot be kept for an extended period of time. Farmers are losing a significant amount of product each year due to perishability. According to Zewdie et al. [4] post-harvest tomato loss might reach 50%. Since tomatoes are climacteric fruits, they gradually deteriorate following harvest. As a result, they have a limited postharvest life since many processes that lead to quality degradation begin after the crop is collected [5]. After harvesting, there may be several changes that impact quality factors including color, texture, and flavor as tomato fruits transition from the ripe green to the fully mature stage. Tomato fruit preservation is hampered by transpiration, senescence, and fungus infection [6]. Fresh-market tomatoes are a widely consumed and adaptable fruit. Moreover, it is a vegetable that significantly improves human nutrition by providing a diversity of food nutrients [7]. The chemical and nutrient makeup of tomatoes is directly impacted by the ripening procedures and storage temperature [8]. Additional elements that impact fruit and vegetable quality include harvesting techniques, biological maturity and postharvest handling [9].
Neem (Azadirachta indica A. Juss) is a large, evergreen, hardy tree, native to the Indian sub-continent [10]. Neem compound show many biological activities different extracts of neem are medicinal the most closely related neem species are Melia azedarach Linn A. indica and A.juss, due to many medicinal uses neem is used in wide range of homeopathic, unani and ayuryeda medicines. About 135 different compounds are extracted from neem different parts having different structural and chemical diversity the extracted compounds are divided in to two groups triterpenoids and diterpenoids which contain azadirone, protomeliacins, limonoids, gedunin and its derivatives [11]. C-secomeliacins like salanin, azadirchtin nonisopreniods and nibin and also a compound vilasinin which act as carbohydrate, proteins and amino acid, polyphenolics like flavonoids and its glycosides, coumarin tannins and dihydrochalcone, and also aliphatic compounds [12].
Depending on the growth circumstances, ripening stage, and genotypic diversity, antioxidants serve different functions in tomatoes [13]. The chemical and nutrient makeup of tomatoes is directly impacted by the ripening procedures and storage temperature [8]. Additional elements that impact fruit and vegetable quality include harvesting techniques, biological maturity, the environment after harvest, handling, and storage conditions [14]. Because of its numerous uses, Moringa Leaf Extract (MLE), which is derived from the moringa plant, is one of the most popular plant bio stimulants [15]. Amino acids, cytokinins such as zeatin, flavonoids, antioxidants such ascorbic acid, carotenoids, phenolics, vitamin A, and macro- and micronutrients are all present in MLE [16,17]. The family Moringaceae, to which Moringa oleifera belongs, is said to have the most widely distributed tropical plants [18]. The Moringa Leaf Extract (MLE), which contains proteins, vitamins E, phenolics, ascorbic acid, essential amino acids, and a number of mineral components, is thought to be a natural plant growth regulator. This makes it a possible natural growth stimulant. Additionally, Moringa leaf extract significantly enhanced the average plant height, number of leaves, number of branches, and yield of tomato plants, according to [19]. According to Mehmood et al. [20], MLE foliar spray acquired the greatest values of black cumin plant growth and yield metrics. Considering these facts, the current study was conducted to evaluate different concentrations of moringa leaf extract and neem leaf extract for improving the storage of tomato fruits. The study was conducted with the following objectives.
Objectives
A. What are the effects of different concentrations of Moringa leaf
extract and Neem leaf extract on tomato during storage life?
B. Does storage interval have any influence on physical and
biochemical characteristics of tomato?
C. Do Moringa leaf extracts and Neem leaf extracts differs in their
action in preserving the quality and postharvest quality of
tomato?
Materials and Methods
Current study “Influence of Neem and Moringa leaf extract on quality and shelf life of tomato” was conducted at Department of Horticulture, The University of Haripur, Khyber Pakhtunkhwa Pakistan during the year 2022.
Collection of fruits
Fresh and uniform sized tomatoes will be collected from Haripur.
Preparation of moringa and neem leaf extracts
Tomatoes were treated with following concentration of MLE
and NLE. Each treatment was prepared by methods described by
AOAC 2001.Fruits were dipped for 15 minutes in each treatment.
T0= Control
T1= 10% Moringa Leaf Extract
T2= 10% Neem Leaf Extract
T3= 10%+10% MLE+NLE
Storage duration
After MLE and NLE application Tomato fruits were stored for 12 days at ambient temperature in horticulture laboratory. The storage duration was comprised of following durations. Storage Duration 1 (SD)=0-day, Storage Duration 2 (SD)=3 day, Storage Duration 3 (SD)=6 day, Storage Duration 4 (SD)=9 day, Storage Duration 5 (SD)=12 day
Parameters
Data regarding enzymatic activity, physical and biochemical attributes, phytochemical analysis along with phytochemical screening were carried out during the study period.
Physical analysis
Fruit weight loss (%): Fruit weights were measured using a digital balance (MJ-W176P, Panasonic Japan) both before and after storage. Using the formula below, the fruits’ percentage weight loss was calculated [21].
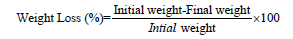
Fruit firmness (Nm): Ripe, uniform tomatoes were chosen,
and damaged specimens were excluded. Fruit firmness was
measured in Newton Meters (Nm) with a calibrated penetrometer.
The measurements were repeated for accuracy, and the data was
examined by computing average hardness values and assessing
statistical significance [22].
Titratable acidity: In a 100mL conical flask, ten mL of juice
was extracted and mixed with purified water. After being titrated
against 0.1N NaOH, the solution was used to indicate 2-3 drops of
phenolphthalein before pink was observed. Using the thousand
equivalent factor for malic acid, the primary acid in the tomato at
maturity was 0.067 [23]. The proportion was then calculated using
the following formula: TA (%)=0.1 NaOH used multiplied by 0.067
per 100mL of juice consumed.
Fruit decay (%): Genanew et al. [24] described the method
of fruit decay. Fruit decay from each treatment was investigated at
each storage interval as the part of the fruit where the peel became
softer. Fruit decay percentage was recorded for this purpose. After
observing the physical look of the fruit, the fruit decay percentage
was calculated every storage day. Total number of decayed fruits
from each treatment was counted.
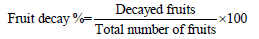
Total phenolic contents (mg GAE/100g): Vallverdú-Queralt et al. [26] used the Folin Ciocalteu reagent procedure to calculate Total Phenolic Contents (TPC). In 100ml of distilled water, 10ml of FC-reactive was dissolved. Each sample (100ml) received a generous quantity of FC-reagent (200L) and a vortex. The 700mM Na2Co3 (800L) is introduced and incubated for 2 hours in each sample. Each sample (200L) was then transferred to a transparent 96-pit plate and estimated at 765nm. TPC concentration was determined using a Gallic acid reference curve. The findings were equivalent to gallic acid.
sTotal antioxidant activity (mg 100mL-1): Absolute plantbased antioxidant activities were measured in samples using the spectrophotometrically performed 2, 2-diphenyl-1-picrylhydrazyl stable radicles reported by García-Alonso et al. [27]. 5mL 0.004 percent methanol is added to aliquots (50L) of peeling and pulp extraction at various concentrations (50-150g mL-1). After a 30-minute incubation period at room temperature, the absorption was measured at a blank distance of 517nm.
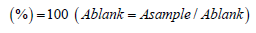
The absorbance of the control reaction (containing DPPH but no sample) is a blank. A sample is the absorbance of DPPH after the sample has been added.
Bio-chemical analysis
pH: pH of 1% and 10% aqueous solutions of fruit juice was
determined according to the AOAC (1990) Method No: 981.12. The
pH metre was adjusted, 60ml of sample was added to the 100ml
clean beaker, and the key was pressed to determine the pH of the
tomato juice. As the metre turned “Ready,” the pH metre’s digital
screen was read, measured, and computed at 18 oC±2 OC (HI
98107, Hanna, Mauritius) [28].
Total soluble solid (Brixo): Using a hand-held refractometer
(KROSS HRN-16), the fruit juice from the selected fruit was
collected, and the gross soluble solid was calculated. First, distilled
water was used to adjust the refractometer to a zero reading. One
drop of each sample was put onto the refractometer’s prism plate.
On the prism plate, the readings were recorded at a single decimal
place. After each examination, the prism plate was cleaned with
distilled water and a soft tissue. The average and recorded data was
expressed in Brixo degrees [29].
Enzymes activities: a) Catalase Determination (U mg-1
protein): With few modifications, the Ye et al. [30] method has been
used to calculate the catalase activity in tomato peel. To stimulate
enzyme reactions, a freshly prepared 5.9mM 100μL H2O enzyme
extract (100μL) has been mixed. Utilising an ELX800 Microplate
Reader (Bio-Tek Instruments, Inc., Winooski, VT, USA), catalastic
activity at 240nm was generated and expressed as U mg-1 Protein.
Catalysis was defined as absorbance shifts of 0.01 units per minute.
b) Peroxide Determination (U mg-1 protein): The Liu et al. [31]
method has been used to investigate peroxidase activity with a
specific modification (2009). A novel reaction mechanism was
created by adding guaiacol (20mM 100μL) and phosphate buffer
(pH 5) of 50 mM 800μL in 40mM 100μL H2O2. After adding 100μL
of enzyme extract absorption to 100μL of reaction mixtures,
the microplate reader (ELX800) from Winooski, Vermont, USA,
detected the results as U mg-1 protein at 470nm. It was said that one
unit of peroxide activity corresponded to the absorbant shift of 0.01
units per minute.
C) Superoxide dismutase Determination (U mg-1 protein): The
method Rahman et al. [32] described for determining the 50%
photochemical reduction in Nitro Blue Tetrazolium (NBT) was used
to the superoxide dismutase study. Each test tube included the
following: 200μL (22μm) of methione (12μM), 500μL phosphate
buffer (50mM, pH 5), and a combination of 100μL filtered water and
100μL enzyme extract. Phosphate buffer (50mM, pH 5) containing
500μL was placed in each test tube. A 15-minute container held
test tubes equipped with fluorescent bulbs. The ELX800 Microplate
Reader (Bio-Tek Instruments, Inc., Winooski, VT, U.S.) was used to
report the absorbance at the level of 560nm.
Statistical analysis
Data was analyzed by application of standard error and Analysis of Variance (ANOVA) using two factorial designs under Complete Randomized Design (CRD) using Least Significant Difference (LSD) at p<0.01 by using latest version of Statistix 8.1 [33].
Results and Discussion
Fruit weight loss (%)
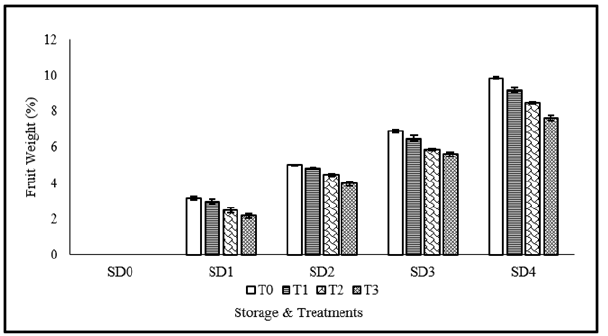
Figure 1 shows fruit weight loss as affected Moringa Leaf Extract (MLE) and Neem Leaf Extract (NLE) coatings. Analysis of variance showed that fruit weight loss was significantly influenced by coating materials and storage durations as well as their interaction. Weight loss in tomato fruits gradually increases with an increase in storage durations. Fruits stored for 12 days showed maximum fruit weight loss. Treatments applications also helped to reduce the fruit weight loss. Maximum fruit weight loss was observed in fruits stored for 12 days without application of MLE and NLE. Due to an increase in transpiration and respiration, this weight loss is associated with an increase in water loss [34]. This might be explained by the tomato high surface to volume ratio and low skin diffusion resistance [35]. Similar findings with different edible coatings were reported in earlier investigations, which came to the conclusion that edible films acted as a physical barrier against moisture loss and decreased transpiration rates [36].
Figure 1:Fruits weight loss of tomato as affected from storage durations and treatments application. LSD for treatments=0.17 LSD for storage durations=0.2 LSD for treatments×storage durations=0.54
Fruit firmness (Nm)
Figure 2:Fruits firmness of tomato as affected from storage durations and treatments application. LSD for treatments=0.17 LSD for storage durations=0.2 LSD for treatments×storage durations=0.55
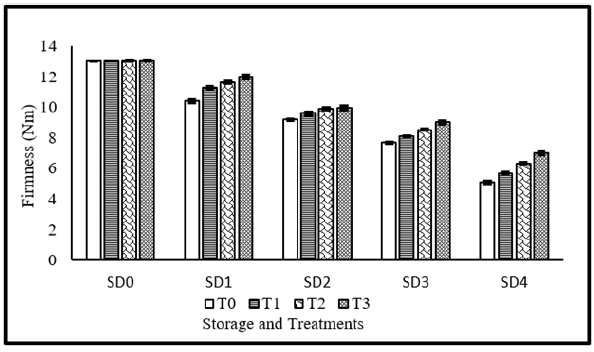
The degree of firmness, which has a significant impact on customer acceptance, is primarily correlated with the food’s water content and metabolic processes. Loss of firmness may have an impact on financial gains. Figure 2 presents the effect of storage durations and application of treatment’s extract coating on fruit firmness of tomatoes. Fruit firmness was significantly affected from storage durations, treatments application as well as their interaction during the study. Maximum fruit firmness was observed with application of combined treatment of 10%+10% MLE+NLE, whereas, minimum fruit firmness was observed in control. Regarding storage durations, maximum fruit firmness was observed in fresh fruits (without storage), whereas, minimum fruit firmness was observed in fruits stored for maximum duration of time. Concerning the interaction, maximum fruit firmness was observed in zero day stored fruits, regardless of the treatment’s application.
Whereas, storage gradually decreases fruit firmness and minimum value was observed in fruits stored for 12 days without any coating. With a longer length of storage, the firmness loss grew over time. This may be caused by the coordinated activity of cell wall-modifying enzymes and proteins, which results in the destruction of cell wall components [37]. Similar observations were reported by Znidarcic et al. [38], who came to the same conclusion that hardness reduces with storage. Neem leaf extract’s and MLE ability to suppress the growth of bacteria that cause rotting and increase metabolic rate may also contribute to the lesser firmness losses of tomatoes treated with the extract [39]. Previous studies showed that the cell wall-degrading enzymes’ inhibitory activities were directly associated to softening and helped to maintain firmness by slowing down the pace of metabolic process during ripening [40]. LSD for treatments×storage durations=0.55
pH
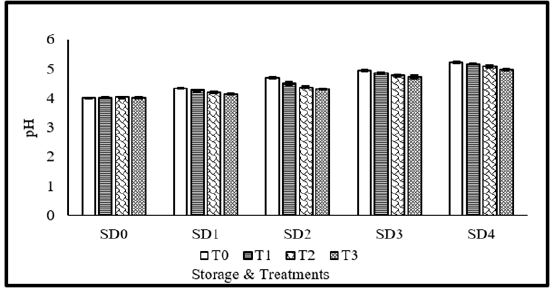
Application of coating materials, days of storage as well as their interaction significantly affected pH of tomato fruit juice. Maximum pH (4.63) was recorded without coating materials, followed by 10% MLE, whereas, minimum pH (4.42) was observed with application of MLE 10%+NLE 10%. Regarding storage durations, maximum pH was recorded in 12 days stored fruits, followed by 9 days, whereas, fresh fruits showed minimum pH. Concerning the interaction, maximum pH was recorded in 12 days stored fruits without application of coatings. pH gradually increases with increase in storage durations (Figure 3). The gradual pH increases in tomatoes treated with neem leaf extract may be due to the extract’s ability to prevent microbial deterioration during storage as a result of its antimicrobial activity. This increase in pH over the time of storage suggests that organic acids in tomatoes degrade during storage. The stability of the pH value of tomatoes during storage is greatly influenced by the slowing in physiological activity and the reduced breakdown of organic acid by neem leaf extract. Odriozola-Serrano et al. [41] & Workneh et al. [42] both came to similar conclusions.
Figure 3:pH of tomato as affected from storage durations and treatments application. LSD for treatments=0.03 LSD for storage durations=0.04 LSD for treatments×storage durations=0.11
Fruit decay (%)
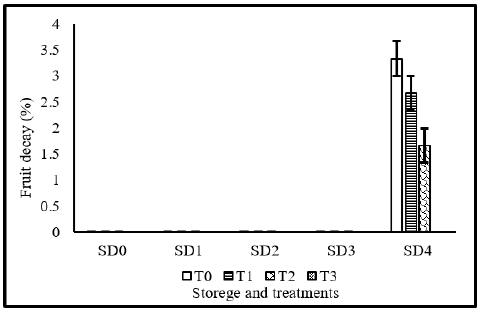
Figure 4 depicting tomato fruit decay as affected from different coatings. Analysis of variance indicated that there was significant effect of coating materials, storage days as well as their interaction on fruit decay. There was no decay in the tomato for 9 days. Decay started after 9th day. Decay was observed in controlled fruits stored for 12 days. Fruits coated with 10% MLE only and fruits coated with NLE 10% also showed decay on 12th day. The lower decay or rotting rate in MLE+NLE treated tomatoes may also be attributable to the incorporation of bioactive compounds from neem leaf extract in fruit that enhanced the fruit’s resistance to pathogen-induced decay. Similarly, the lower decay or rotting rate in neem leaf extracttreated tomatoes may also be attributable to the incorporation of bioactive compounds from neem leaf extract in fruit that enhanced the fruit’s resistance to pathogen-induced decay Our findings are in agreement with the conclusion reached by Genanew et al. [24] & Sanchez-Gonzalez et al. [43].
Figure 4:Fruit decay of tomato as affected from storage durations and treatments application. LSD for treatments=0.22 LSD for storage durations=0.26 LSD for treatments×storage durations=0.7
Total soluble solids (Brix⁰)
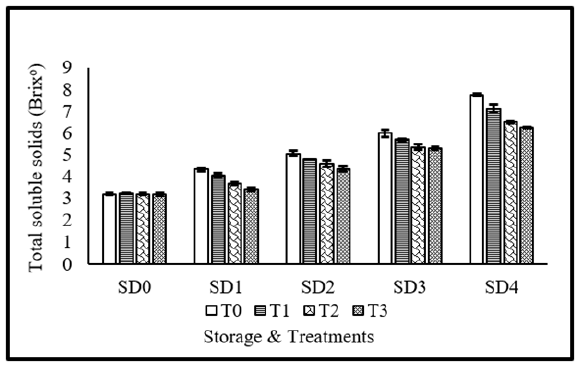
Total soluble solid contents are regarded as the fundamental criterion for evaluating fruit ripening. Coating materials, storage durations as well as their interaction significantly affected total soluble solids of tomato fruits. Maximum TSS was recorded without application of coating materials, whereas application of MLE 10%+NLE 10% resulted in minimum TSS. Regarding storage durations, maximum TSS was observed in 12 days stored fruits, whereas, lowest TSS value was observed in fresh fruits. Concerning the interaction, maximum TSS was recorded in 12 days stored fruits without coatings, whereas, fresh fruits showed minimum TSS. Its sluggish ripening process and respiration may be the cause of the little rise in sugar levels in NLE+MLE treated tomatoes compared to control and other treatments [44]. Due to its natural yield intrinsic activity, 10% NLE combined with 10% MLE decreased the rate of respiration, transpiration, and other metabolic processes [45]. The highest concentration of sugars in the untreated control might be the consequence of starch converting quickly to sugars due to moisture loss and a drop in acidity brought on by physiological changes during storage. Our findings concur with those of Melkamu et al. [46]. By employing tomato varieties Krammes et al. [47] & Opiyo and Ying [48] reported similar outcomes (Figure 5).
Figure 5:Total soluble solids of tomato as affected from storage durations and treatments application. LSD for treatments=0.15 LSD for storage durations=0.18 LSD for treatments×storage durations=0.49
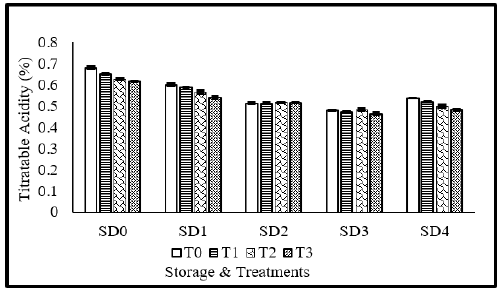
Titratable acidity (%)
Generally speaking, as fruit ages, its titratable acidity tends to decline while its total soluble solid content rises. Figure 6 illustrating titratable acidity of tomato fruits as affected by MLE and NLE coatings. Analysis of variance suggested that titratable acidity was significantly affected from coating materials, storage durations as well as their interaction during the study. Maximum titratable acidity was observed in fresh fruits, as time goes on, titratable acidity of tomato fruits tends to decline and minimum value was recorded for the fruits stored for 12 days. The anti-microbial capabilities of neem leaf extract, which slow down the microbial decomposition of organic acids, may be the cause of the tomatoes treated with neem’s sluggish drop in titratable acidity [23]. It is further reinforced by Tigist et al. [49], who found that coated fruits’ rate of titratable acidity decrease is slower than uncoated fruits’ because of oxygen availability restrictions, which slow down respiration rate.
Figure 6:Titratable acidity of tomato as affected from storage durations and treatments application. LSD for treatments=0.007 LSD for storage durations=0.009 LSD for treatments×storage durations=0.02
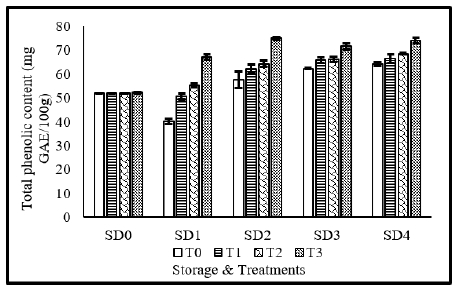
Total phenolic content (mg GAE/100g)
The effect of MLE and NLE coating on total phenolic content of tomato is presented in Figure 7. Total phenolic content of tomato was significantly affected from coating materials, storage durations as well as their interaction. Maximum TSS was recorded without application of coating materials, whereas application of MLE 10%+NLE 10% resulted in minimum TSS. Regarding storage durations, statistically similar results of total phenolic content was observed in 12 days stored fruits and fruits stored for 9 days, whereas, lowest TSS value was observed in fresh fruits. Concerning the interaction, maximum TPC was recorded with application of T3 in fruits stored for 9 and 12 days respectively. Tzortzakis et al. [50] also reported significant differences in phenolic content of tomato fruits treated with different organic coatings.
Figure 7:Total phenolic content of tomato as affected from storage durations and treatments application. LSD for treatments=2.18 LSD for storage durations=2.6 LSD for treatments×storage durations=6.91
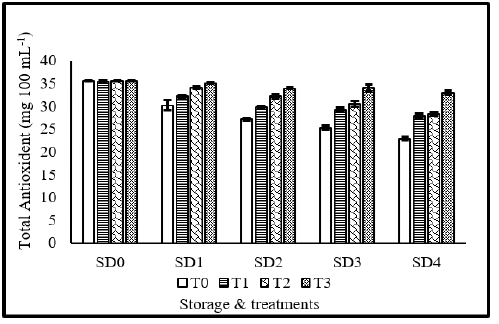
Total antioxidants activity (mg 100 mL-1)
Total antioxidant activity of tomato fruits was significantly affected from storage durations, treatments application as well as their interaction during the study (Figure 8). Maximum total antioxidant activity was observed in fruits treated with 10% MLE+10% NLE, whereas, lowest total antioxidant activity was observed in control treatments. Regarding storage durations, minimum total antioxidant activity was observed in 12 days stored fruits. Total antioxidant activity gradually decreases with increase in number of storage days. Maximum total antioxidant activity was recorded in fresh fruits. Concerning the interaction, maximum antioxidant activity was observed in fresh fruits regardless of the treatment application. The alteration of the internal environment brought on by edible coatings, which initially causes the buildup of phenolic chemicals, may have contributed to the rise in overall antioxidant activity [51]. It was discovered that coating coated Ficus hirta fruit and grapefruits with MLE+NLE increased their overall antioxidant activity [52]. Within the first six days, strawberry fruit coated with chitosan showed a rise in the AOA, according to Vieira et al. [53]. The Maillard process linked to the synthesis of brown melanoidins with high AOA may also be able to explain this phenomenon [54].
Figure 8:Total antioxidants activity of tomato as affected from storage durations and treatments application. LSD for treatments=0.76 LSD for storage durations=0.91 LSD for treatments×storage durations=2.43
Catalase determination (U mg-1protein)
Table 1 depicting catalase determination (U mg-1 protein) of tomato fruits. Analysis of variance showed that Catalase (U mg-1 protein) activity of tomato was significantly affected from coating treatments as well as storage durations, whereas, their interaction remained non-significant during the study. Maximum CAT was observed with application of MLE 10%+NLE 10%, whereas, control treatment showed lowest CAT activity. Regarding storage durations, maximum CAT was observed in fresh fruits, increasing number of days of storage gradually resulted in decrease in CAT activity of tomato fruits. Lowest catalase activity was recorded in 12 days stored fruits. The use of organic coatings on citrus fruits increased the activity of plant defense-related enzymes like CAT, as was previously noted [55]. The findings support the conclusion reached by Masood et al. [2].
Table 1:Catalase Determination (U mg−1 protein) of tomato as affected from storage durations and treatments application.
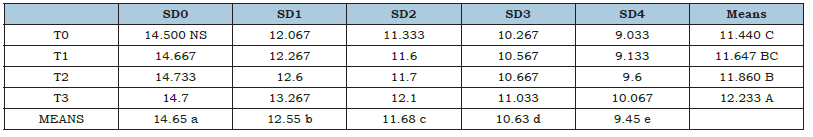
LSD for treatments=0.23 LSD for storage durations=0.28
LSD for treatments×storage durations=NS.
Peroxide determination (U mg-1 protein)
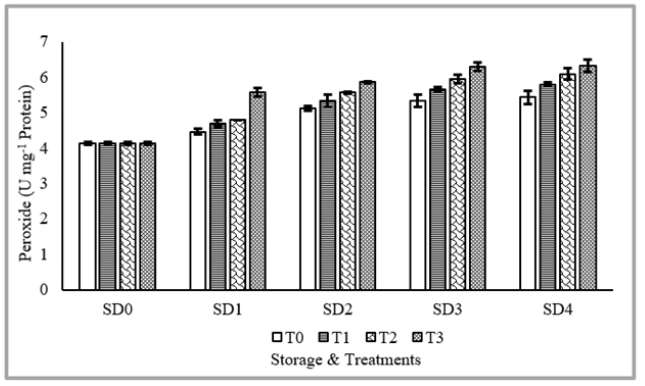
Figure 9 shows Peroxide Determination (U mg-1 protein) of tomato as affected from different coatings and various storage durations. Peroxide Determination (U mg-1 protein) was significantly affected from coating application, storage durations as well as their interaction. Maximum POD (5.64U mg-1 protein) was recorded with application of 10% MLE+10% NLE, whereas, minimum was observed in control. Regarding storage durations, maximum POD was recorded in fruits stored for 12 days, statistically similar observations were recorded in fruits stored for 9 days. Fresh fruits showed minimum POD. Concerning the interaction, maximum POD was recorded in fruits stored for 12 days with application of MLE 10%+NLE 10%. In general, it is understood that fruits’ POD rises when being stored [4]. Analysis showed that as storage time came to a close, POD of the fruits increased in response to ripening and treatments. This research closely resembles that of Kator et al. [1]. Particularly, MLE therapies were more successful than controls at maintaining POD. The same findings were recorded by Hosea et al. [56].
Figure 9:Peroxide Determination (U mg-1 protein) of tomato as affected from storage durations and treatments application Peroxide.
Superoxide dismutase (U mg-1 protein)
All live cells contain the enzyme Superoxide Dismutase (SOD). An enzyme is a substance that quickens some bodily chemical processes. In cells, superoxide dismutase aids in the degradation of potentially damaging oxygen molecules [32]. Table 2 shows Superoxide dismutase value of tomato fruits as affected from different coatings and different storage durations. Analysis of Variance (ANOVA) showed that Superoxide dismutase showed nonsignificant results for treatments applications, storage durations as well as their interaction during the study.
Table 2:Superoxide dismutase (U mg−1 protein) of tomato as affected from storage durations and treatments application.
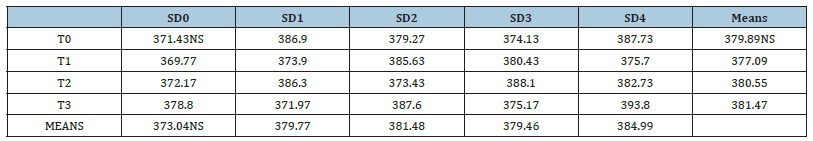
LSD for treatments=NS LSD for storage durations=NS
LSD for treatments×storage durations=NS
Phytochemical screening
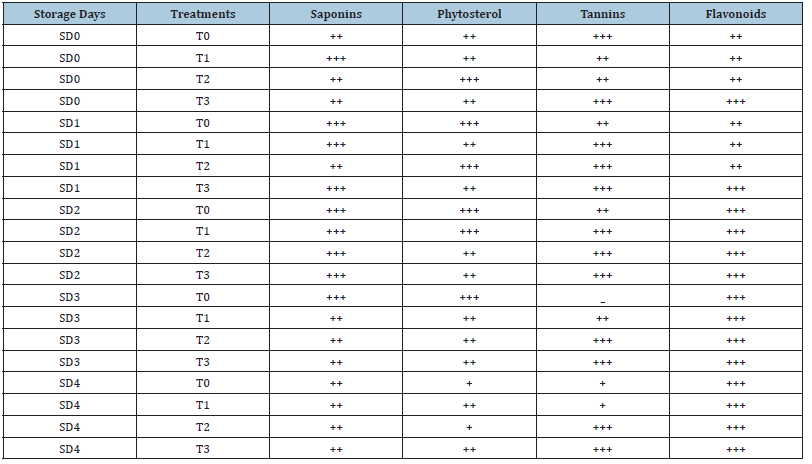
Quantitative phytochemical analysis of tomato fruits is presented in Table 2. Saponins shows their high presence in fruits treated with 10% MLE+10% NLE stored for 3 and 6 days. Phytosterol showed high presence in fruits treated with 10% MLE+10% NLE. Regarding Tannins, it showed the resence regardless of the storage duration and chemical application. Flavonoids are moderately present in all treatments stored for 3 and 6 days’ fruits. After increasing the storage duration of the fruit, flavonoids presence gradually increases and showed its highest presence when stored for more than 12 days (Table 3).
Table 3:Qualitative tests of phytochemical in tomato. +++=Highly present, ++=Moderate presence, -=Absent.
In this investigation, secondary metabolites that differ from one another in polarity and structure were extracted using water (aqueous) as a polar solvent, ethanol as a solvent of intermediate polarity, and chloroform as a solvent of excellent polarity. Each solvent had unique biological characteristics. Solvents often penetrate into plant materials during the extraction process, and substances with comparable polarities solubilize [57]. The polarity of the solvent has an impact on the content and quality of secondary metabolites in an extract. Traditional healers prepare plant extracts with water, but organic solvents like methanol provide more constant antibacterial action and high-quality secondary metabolites than aqueous solutions [58]. For antibacterial research, aqueous, methanolic, chloroform, hexane, and ethanol were most often utilised as solvents [59]. Although tomato lacks extensive chemical analysis, in recent years many saponins, sapogenins, furostane and spirostane glycosides have been isolated [60]. Nevertheless, there are studies of qualitative phytochemical analyses in Safed Musli. It comprises a large variety of phytochemicals, including saponins, alkaloids, flavonoids and phenolic acids [61].
Conclusion
Our extensive research reveals that the best strategy to extend the shelf life and maintain the quality of tomato fruits during storage is to apply a coating containing 10% Moringa leaf extract and 10% Neem leaf extract. Our findings provide persuasive evidence of these organic extracts’ exceptional effects on several crucial variables required for post-harvest fruit management. Throughout the study, the MLE and NLE coatings consistently demonstrated superior performance in terms of reducing fruit weight loss, maintaining fruit firmness, preventing decay, and influencing key biochemical characteristics such as pH, total soluble solids, titratable acidity, total phenolic content, and total antioxidant activity. Furthermore, the aforementioned coatings displayed effective modulation of enzyme activities such as catalase and peroxide determination, which are critical for minimizing oxidative stress and preserving fruit quality. The synergistic action of MLE and NLE, which offer a strong physical barrier against moisture loss, limit microbial development, and regulate metabolic processes during storage, is responsible for the reported improvements in fruit quality and shelf life. Furthermore, the fact that coated fruits include bioactive substances such tannins, flavonoids, phytosterols, and saponins highlights the possible contributions of these substances to the advantages that have been noted.
References
- Kator L, Oche OD, Hosea ZY, Agatsa TD (2019) Effect of aqueous extract of moringa leaves on postharvest shelf life and quality of tomato fruits inoculated with fungal pathogens in Makurdi. Asian Journal of Agricultural and Horticultural Research 3(1): 1-13.
- Masood S, Randhawa MA, Ahmad W, Butt MS, Asghar M, et al. (2018) Quality of tomatoes as influenced by bio-chemicals and controlled atmosphere during storage: Tomato quality in response to biochemicals. Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences 55(4): 39-48.
- Bauer S, Schulte E, Thier HP (2004) Composition of the surface wax from tomatoes. European Food Research and Technology 219(3): 223-228.
- Zewdie B, Shonte TT, Woldetsadik K (2022) Shelf life and quality of tomato (Lycopersicon esculentum) fruits as affected by neem leaf extract dipping and beeswax coating. International Journal of Food Properties 25(1): 570-592.
- El-Ramady HR, Domokos-Szabolcsy É, Abdalla NA, Taha HS, Miklos Fari (2015) Postharvest management of fruits and vegetables storage. Sustainable Agriculture Reviews, Volume 15, Springer Cham, Switzerland, pp. 65-152.
- Zapata PJ, Guillen F, Martınez-Romero D, Castillo S, Valero D, et al. (2008) Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. Journal of the Science of Food and Agriculture 88: 1287–1293.
- Toor RK, Savage GP (2006) Changes in major antioxidant components of tomatoes during post-harvest storage. Food Chemistry 99(4): 724-727.
- Sahlin E, Savage GP, Lister CE (2004) Investigation of the antioxidant properties of tomatoes after processing. Journal of Food composition and Analysis 17(5): 635-647.
- Kader AA (2008) Flavor quality of fruits and vegetables. Journal of the Science of Food and Agriculture 88(11): 1863-1868.
- Gajalakshmi S (2002) Development of methods for the treatment and reuse of municipal and agricultural solid wastes appropriate for rural/urban households, PhD Thesis, Pondicherry University, Pondicherry, India.
- Kharwar RN, Sharma VK, Mishra A, Kumar J, Singh DK, et al. (2020) Harnessing the phytotherapeutic treasure troves of the ancient medicinal plant Azadirachta indica (Neem) and associated endophytic microorganisms. Planta Medica 86(13-14): 906-940.
- Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U (2002) Biological activities and medicinal properties of neem (Azadirachta indica). Current Science 82(11): 1336-1345.
- Leonardi C, Ambrosino P, Esposito F, Fogliano V (2000) Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes. Journal of Agricultural and Food Chemistry 48(10): 4723-4727.
- Tesfay SZ, Magwaza LS (2017) Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana) fruit. Food Packaging and Shelf Life 11: 40-48.
- Phiri C, Mbewe DN (2010) Influence of Moringa oleifera leaf extracts on germination and seedling survival of three common legumes. International Journal of Agriculture and Biology 12(2): 315-317.
- Abdalla MM (2013) The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int J Plant Physiol Biochem 5(3): 42-49.
- Safi-naz S, Rady MM (2015) Moringa oleifera leaf extract improves growth, physio-chemical attributes, antioxidant defence system and yields of salt-stressed Phaseolus vulgaris L. plants. Int J ChemTech Res 8(11): 120-134.
- Shahzad U, Khan MA, Jaskani MJ, Khan IA, Korban SS (2013) Genetic diversity and population structure of Moringa oleifera. Conservation Genetics 14(6): 1161-1172.
- Bashir KA, Bawa JA, Mohammed I (2014) Efficacy of leaf extract of drumstick tree (Moringa oleifera Lam.) on the growth of local tomato (Lycopersicon esculentum). Journal of Pharmacy and Biological Sciences 9(4): 74-79.
- Mehmood A, Naveed K, Ayub Q, Alamri S, Siddiqui MH, et al. (2021) Exploring the potential of moringa leaf extract as bio stimulant for improving yield and quality of black cumin oil. Scientific Reports 11(1): 24217.
- Kapsiya J, Gungula DT, Tame VT, Bukar N (2015) Tomato (Solanum lycopersicum ) Fruits. Journal of Bioscience 1(3): 41-46.
- Rowe P (2015) Optical techniques for fruit firmness assessment, Doctoral dissertation, University of Waikato, New Zealand.
- Nielsen SS (2017) Standard solutions and titratable acidity. Food Analysis Laboratory Manual, (3rd edn), Berlin, Germany, pp. 179-184.
- Genanew T (2013) Effect of post harvest treatments on storage behavior and quality of tomato fruits. World Journal of Agricultural Sciences 9(1): 29-37.
- Bhandari SR, Chae Young CY, Lee Jun Gu LJ (2016) Assessment of phytochemicals, quality attributes, and antioxidant activities in commercial tomato cultivars. Horticultural Science and Technology 34(5): 677-691.
- Vallverdú-Queralt A, Arranz S, Medina-Remón A, Casals-Ribes I, Lamuela-Raventos RM (2011) Changes in phenolic content of tomato products during storage. Journal of Agricultural and Food Chemistry 59(17): 9358-9365.
- García-Alonso FJ, Bravo S, Casas J, Perez-Conesa D, Jacob K, et al. (2009) Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. Journal of Agricultural and Food Chemistry 57(15): 6815-6822.
- Anthon GE, LeStrange M, Barrett DM (2011) Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. Journal of the Science of Food and Agriculture 91(7): 1175-1181.
- Paul V, Singh A, Pandey R (2010) Estimation of total soluble solids (TSS), Laboratory Manual on Post-Harvest Physiology of Fruits and Flowers, Division of Plant Pathology, Indian Agricultural Research Institute, New Delhi, India, pp.41-43.
- Ye RJ, Wu VC (2011) Use of a simple catalase assay for assessment of aerobic microbial contamination on vegetables. Annals of Microbiology 61: 231-236.
- Liu L, Wang J, Qu L, Li S, Wu R, et al. (2012) Effect of nitric oxide treatment on storage quality of Glorious oranges. Procedia Engineering 37: 150-154.
- Rahman SL, Mackay WA, Nawata E, Sakuratani T, Uddin AM, et al. (2004) Superoxide dismutase and stress tolerance of four tomato cultivars. Hort Science 39(5): 983-986.
- Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, (2nd edn), Wiley India Pvt Ltd, New Delhi, India.
- Wissam Z, Ali A, Rama H (2016) Optimization of extraction conditions for the recovery of phenolic compounds and antioxidants from Syrian olive leaves. Journal of Pharmacognosy and Phytochemistry 5(5): 390.
- Wani AA, Singh P, Gul K, Wani MH, Langowski HC (2014) Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packaging and Shelf Life 1(1): 86-99.
- Shamloo M, Sharifani M, Garmakhany AD, Seifi E (2013) Alternation of flavonoid compounds in Valencia Orange fruit (Citrus sinensis) peel as a function of storage period and edible covers. Minerva Biotecnologica 25(3): 191-7.45
- Lai T, Wang Y, Li B, Qin G, Tian S (2011) Defense responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biology and Technology 62(2): 127-132.
- Žnidarčič D, Ban D, Oplanić M, Karić L, Požrl T (2010) Influence of postharvest temperatures on physicochemical quality of tomatoes (Lycopersicon esculentum ). Journal of Food, Agriculture and Environment 8(1): 21-25.
- Khater HF (2012) Prospects of botanical biopesticides in insect pest management. Pharmacologia 3(12): 641-656.
- Zhou R, Mo Y, Li Y, Zhao Y, Zhang G, et al. (2008) Quality and internal characteristics of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biology and Technology 49(1): 171-179.
- Odriozola‐Serrano I, Soliva‐Fortuny R, Martín‐Belloso O (2008) Antioxidant properties and shelf‐life extension of fresh‐cut tomatoes stored at different temperatures. Journal of the Science of Food and Agriculture 88(15): 2606-2614.
- Workneh TS, Osthoff G, Steyn MS (2009) Integrated agrotechnology with preharvest ComCat® treatment, modified atmosphere packaging and forced ventilation evaporative cooling of tomatoes. African Journal of Biotechnology 8(5).
- Sánchez-González L, Pastor C, Vargas M, Chiralt A, González-Martínez C, et al. (2011) Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biology and Technology 60(1): 57-63.
- Kumah P, Olympio NS, Tayviah CS (2011) Sensitivity of three tomato (Lycopersicon esculentum) cultivars-Akoma, Pectomech and power-to chilling injury. Agriculture and Biology Journal of North America 2(5): 799-805.
- Cha DS, Chinnan, MS (2004) Biopolymer-based antimicrobial packaging: a review. Critical Reviews in Food Science and Nutrition 44(4): 223-237.
- Melkamu M, Seyoum T, Woldetsadik K (2008) Effects of pre-and post-harvest treatments on changes in sugar content of tomato. African Journal of Biotechnology 7(8).
- Krammes JG, Megguer CA, Argenta LC, Amarante CVTD, Grossi D (2003) Use of 1-methylcyclopropene to delay tomato ripening. Brazilian Horticulture 21: 611-614.
- Opiyo AM, Ying TJ (2005) The effects of 1‐methylcyclopropene treatment on the shelf life and quality of cherry tomato (Lycopersicon esculentum cerasiforme) fruit. International Journal of Food Science & Technology 40(6): 665-673.
- Tigist M, Workneh TS, Woldetsadik K (2013) Effects of variety on the quality of tomato stored under ambient conditions. Journal of Food Science and Technology 50(3): 477-486.
- Tzortzakis N, Xylia P, Chrysargyris A (2019) Sage essential oil improves the effectiveness of Aloe vera gel on postharvest quality of tomato fruit. Agronomy 9(10): 635.
- Frusciante L, Carli P, Ercolano MR, Pernice R, Di Matteo A, et al. (2007) Antioxidant nutritional quality of tomato. Molecular Nutrition & Food Research 51(5): 609-617.
- Wang SY, Gao H (2013) Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa ). LWT-Food Science and Technology 52(2): 71-79.
- Vieira JM, Flores-López ML, de Rodríguez DJ, Sousa MC, Vicente AA, et al. (2016) Effect of chitosan-Aloe vera coating on postharvest quality of 46 blueberry (Vaccinium corymbosum) Postharvest Biology and Technology 116: 88-97.
- Aslanova D, Bakkalbasi E, Artik N (2010) Effect of storage on 5 hydroxymethylfurfural (HMF) formation and color change in jams. International Journal of Food Properties 13(4): 904-912.
- Chen C, Cai N, Chen J, Wan C (2019) Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 9(5): 197.
- Hosea ZY, Kator L, Owoicho AL, Agatsa TD (2019) Effect of Neem (Azadirachta indica) Leaf Coating on Shelf Life and quality Retention of Tomato 41(Solanum lycopersicum L.) Fruits during Storage. Current Trends in Food Science 1: 118-131.
- Yusoff SF, Haron FF, Tengku Muda Mohamed M, Asib N, et al. (2020) Antifungal activity and phytochemical screening of Vernonia amygdalina extract against Botrytis cinerea causing gray mold disease on tomato fruits. Biology 9(9): 286.
- Sopyan I, Gozali D, Tiassetiana S (2018) Formulation of tomato extracts (Solanum lycopersicum L.) as a sunscreen lotion. National Journal of Physiology, Pharmacy and Pharmacology 8(3): 453-458.
- Mohammed N, Abdullahi I, Bala SK (2022) Preliminary phytochemical screening of healthy and leaf curl virus infected tomato (Solanum lycopersicum) Journal of Applied Sciences and Environmental Management 26(5): 871-876.
- Awere CA, Githae EW, Gichumbi JM (2021) Phytochemical analysis and antifungal activity of Tithonia diversifolia and Kigelia africana extracts against Fusarium oxysporum in tomato. African Journal of Agricultural Research 17(5): 726-732.
- Emiri UN, Enaregha EB (2020) Phytochemical screening and antifungal potency of Vernonia amygdalina (bitter leaf) extract against post harvest mycodeterioration of tomato (Lycopersicum esculentum). Brazilian Journal of Biological Sciences 7(17): 327-337.
© 2024 © Umar Khan Swati. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






