- Submissions

Full Text
Environmental Analysis & Ecology Studies
Companion Plants May Increase Beneficial Populations in Horticultural Crops: An Evaluation of Arthropod Fauna of Three Companion Aromatic Plants
Clovel Pancarte1*, Dominique Carval2 and Philippe Ryckewaert3
1CIRAD, Persyst, UPR 103 HORTSYS, CAEC Petit Morne, BP214 - 97285 Le LAMENTIN cédex 2, France
1CIRAD, Persyst, UPR 26 GECO, Station de Bassin Plat - BP 180 97455 Saint-Pierre Cedex Réunion, France
1CIRAD, Persyst, UPR HORTSYS, Antenne de Mayotte - 69 rue Moussa Oili - Tsararano 97660 Dembéni Cedex Mayotte, France
*Corresponding author:Clovel Pancarte, CAEC CIRAD Petit Morne, BP 214, 97285 Le Lamentin Cedex 2, Martinique, France
Submission: February 02, 2024; Published: March 27, 2024

ISSN 2578-0336 Volume122 Issue1
Abstract
Introduction
Under tropical climates, vegetable crops are highly susceptible to pressure from numerous arthropod pests, which cause considerable damage [1,2]. However, as Ratnadass et al. [3] highlighted in their review, pesticide use can be reduced with an agro-ecological approach, which includes multispecies cropping systems [4]. Scherber et al. [5] showed that plant diversity regulates both the abundance and species richness of organisms. Associating companion plants within a main crop may be a useful agro-ecological practice, as these species can be chosen to attract or repel the pests that damage the main crop, and can increase the diversity of beneficials (parasitoids or predators) by increasing the diversity of food resources and refuges [6]. In this context, traditional tropical “home gardens” are of great interest, because of their multispecies structure and the principles of their functioning could be used as foundations for the design of improved agroforestry practices [7], the home garden as an agroforestry system [8]. The many advantages of such agro-ecosystems are exploiting the regulatory effect of the natural enemies of crop pests, through the diversification of spontaneous or cultivated biotopes [3,9]. In home gardens, aromatic species are omnipresent, intercropped or mixed with vegetable or tuber crops, or in agroforestry plots [7,10] and such traditional aromatic plants are an integral part of home gardens of the Caribbean islands, called “Creole gardens” [11,12]. These plants are used for their medicinal properties [10] but also because farmers observed their positive effects in reducing crop pests and diseases. Indeed, the attractive or repellent chemicals produced by the plants may promote plant defenses and reduce pest damage [13].
Aromatic plants are thus good candidates to improve agroecosystems but little attention have been paid in agricultural research [10], and their potential beneficial effects on crop pest and diseases need to be assessed. Pharmaceutical studies have focused on the repellent effects of these plants and identified natural chemicals, such as essential oils, which are used for the production of insecticides or repellents [14,15]. However, very few field studies have been conducted to characterize the performance of repellent biotic species associated in situ with crops for the purpose of pest management. Some of these investigations focused on induced, rather than constitutive, plant defenses [16] and a few authors, showed that intercropping with aromatic plants, compared with intercropping with natural herb vegetation, was able to reduce both the abundance and species richness of herbivorous arthropods in the specific conditions of a pear orchard [17,18]. A companion plant might have repelling and/or intercepting effects on pests and pathogens and attract natural enemies, or provide food for natural enemies [19]. Indeed, the companion plants can control insect pests directly by deterring pest establishment or indirectly by attracting natural enemies [20]. In addition, increasing plant diversity at the local scale is recognized to support populations of natural enemies while reducing the abundance of insect pests and their damages to crops [21].
We hypothesized that field observations of insect fauna of aromatic plants could help to guide the choice of companion plants in vegetable crops. In Martinique (French West Indies), we inventoried populations of phytophagous and beneficial arthropods on three traditional aromatic plants cultivated in separated plots, known for their repellent essential oils [22,23]: lemongrass (Cymbopogon citratus), basil (Ocimum basilicum) variety with small leaves and chives (Allium fistulosum). Allium is known for its insect repellent properties [24,25]. The repellent properties of the allelochemicals (sulfur compounds) of Allium in associated crops were revealed by Yu [24] in a test associating Allium and tomato and by Auger et al. [26] for its anti-palatable effects on insects. We made the same inventory on tomato (Lycopersicon esculentum) plots, as beneficiary plants, tomato being one of the main vegetable crops grown on the island. In Martinique, tomato cultivation is greatly reduced due to Ralstonia solanacearum, the causal agent of bacterial wilt disease [27] and two main insect pests Bemisia tabaci and Helicoverpa zea. Bemisia tabaci, the whitefly vector of Tomato yellow leaf curl virus (TYLCV), seriously reduces tomato production and quality [28]. Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae), is a major pest of tomato. It is present throughout the island all year round and its population increases during the tomato growing season [29]. We assessed (i) the abundance of phytophagous and beneficial arthropods on the three aromatic plants and on tomato, (ii) the correlation between the abundance of beneficials and the abundance of phytophagous arthropods and (iii) the dynamics of these abundances.
Materials and Methods
Study site and experimental design
We conducted our study from early October 2010 to mid- January 2011 in the Caribbean Island of Martinique (French West Indies) at the CIRAD research station located at Lamentin (14 °37’ N, 60 °58’ W, altitude 16ma.s.l). The site has a mean annual rainfall of 2007mm and a mean annual temperature of 26.5 °C. The climate is tropical and humid with a rainy season from June to October, and a dry season from November to May. The soil at the study site is an alluvial continental Ferralsols. The experiment was conducted in field conditions during the tomato growing season. The plants were purchased from a nursery and plotted about 10cm high. Before planting, the plants used were selected to be of a similar size. At the start of the experiment, 20 days after planting, the average plant height was 20cm for basil, 27.4cm for lemongrass, 12.8cm for onion and 12cm for tomato. A randomized complete block experimental design was set up, with three replications, each comprising four 5x2m plots spaced 5m apart [30]. Each plot was planted with one of the four species. The same crop management was used for four plant species and no pesticide or fertilizer treatments were carried out during the experiment. To avoid weeds and stagnating water, the inter-crop spaces were covered with a canvas made of UV resistant woven polypropylene. The ground in the vicinity of the plots was regularly mowed and the ground in the plots was regularly hand weeded.
Observations and sampling
The observations were made during from November 2010 to January 2011. The arthropod populations were monitored from December 1 to January 12 by combining two trapping methods (D-vac suction and pitfall traps) in 1m2 located in the center of each plot to avoid edge effects [31]. During this period, at intervals of 20 days, three samplings of arthropods were made on the same day with each trapping method. The D-vac suction was switched on for 16 seconds to capture arthropods circulating on foliage [32]. Captured arthropods were placed at -20 °C for one hour before identification and counting. Pitfall traps (12cm in diameter) were filled with water and a few drops of detergent (commercial dish cleaner) to weaken water surface tension and placed on the ground for 24 hours at each collection to collect arthropods that are active on the soil [33-36]. The trapped arthropods were placed in 70° alcohol for several hours before identification and counting. Identification was made to the at least to the family level [37-39].
Statistics and data processing
We calculated two diversity indices, the Shannon Weaver Index to account for the diversity of the insect fauna in the treatments and distributions. The Shannon Weaver Index (H’) was calculated as follows Shannon [40] & Jost [41]: H’-- Σsi-1 pi∗ in (pi) ,where i is one species relative to the total number of species, S is the total number of species, p(i) the study area calculated as p(i) is the proportion of species I, where ni is the number of individuals of the species i, and N the total number of individuals of all species. Overdispersion in the data was taken into account using a quasi-generalized linear model (quasi GLM) with a Poisson error to assess the effect of plant type, sampling date, and the abundance of beneficials on the abundance of phytophagous arthropods. To account for heterogeneity in the data, we used a Generalized Least Square model (GLS) Zuur et al. [42] with the date as a variance of error function to assess the effect of the plant type, the sampling date and the abundance of phytophagous taxa on the abundance of beneficials. For GLS, the abundance of beneficials was log transformed to reach normal distribution. We used Likelihood Ratio Tests (LRT), Zuur et al. [42] to assess the significance of explanatory variables in the GLS models. For all statistical analyses, we used a significance level of 0.05.
Results
General features of the arthropods we collected
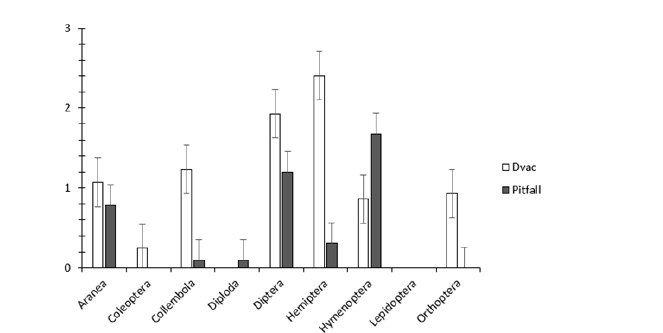
In total we trapped 1,817 arthropods, representing 45 taxa in 9 orders. D-vac suction accounted for 1,534 arthropods from 41 taxa, 8 orders and 33 families were identified (Table 1). Pitfall traps accounted for 283 arthropods from 21 taxa, 8 orders and 15 families were identified (Table 2). Seven of the nine identified orders were common to both trapping methods: Araneae, Collembola, Diptera, Hemiptera, Hymenoptera, Lepidoptera, and Orthoptera. Coleoptera was only trapped with D-vac, and individuals of the order Diplopoda only with pitfall. Regardless the crop and sampling dates, hemipterans represented the highest number of arthropods trapped by D-Vac, with 252.0±80.8, mean±SE (65%) of all arthropods trapped) while hymenopterans (almost exclusively ants) represented the highest number of arthropods trapped by pitfall traps with 47.0±7.9 (66%) of all arthropods) (Figure 1).
Table 1:List of arthropods collected in the 12 plots with the D-vac (4 cropsx3 replications: 12 plots, trapping done in the central (1m2) of each plot.
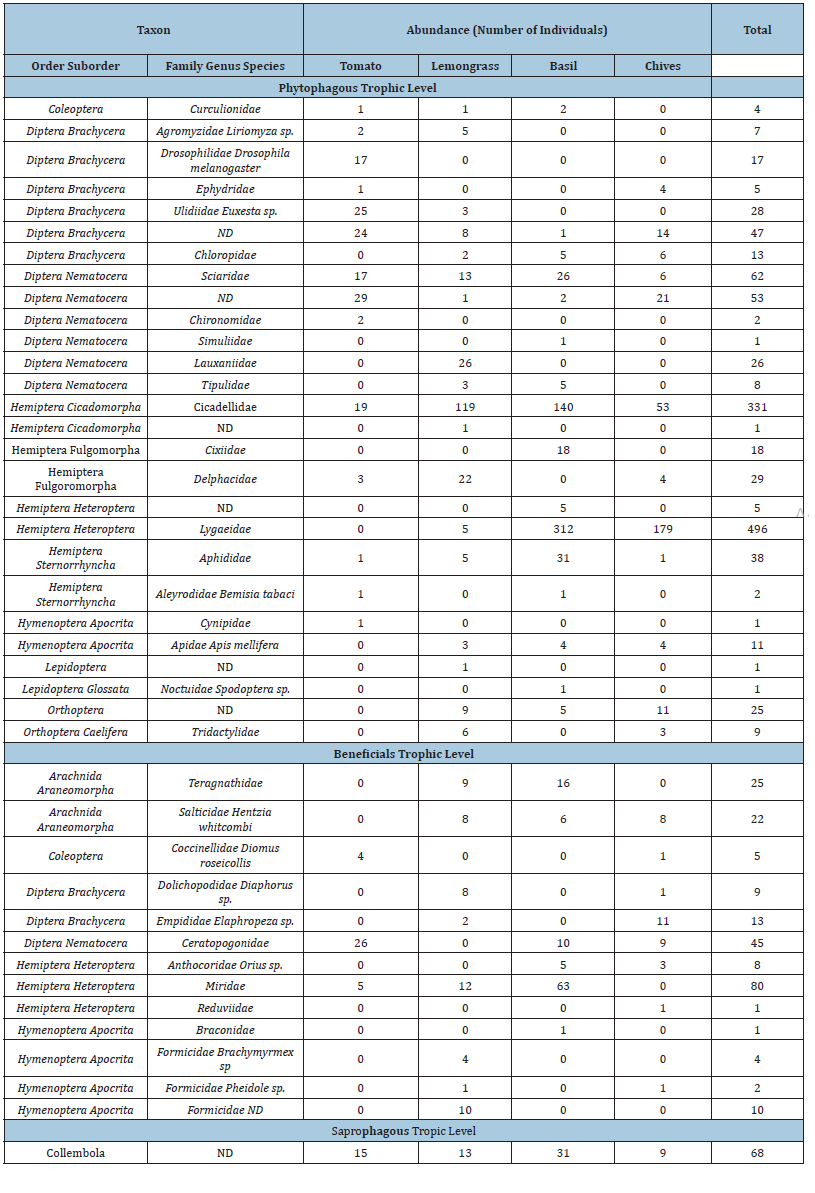
Table 2:Arthropods collected on the 12 plots with Pitfall traps (4 cropsx3 replications: 12 plots, trapping done in the central 1m2 of each plot.
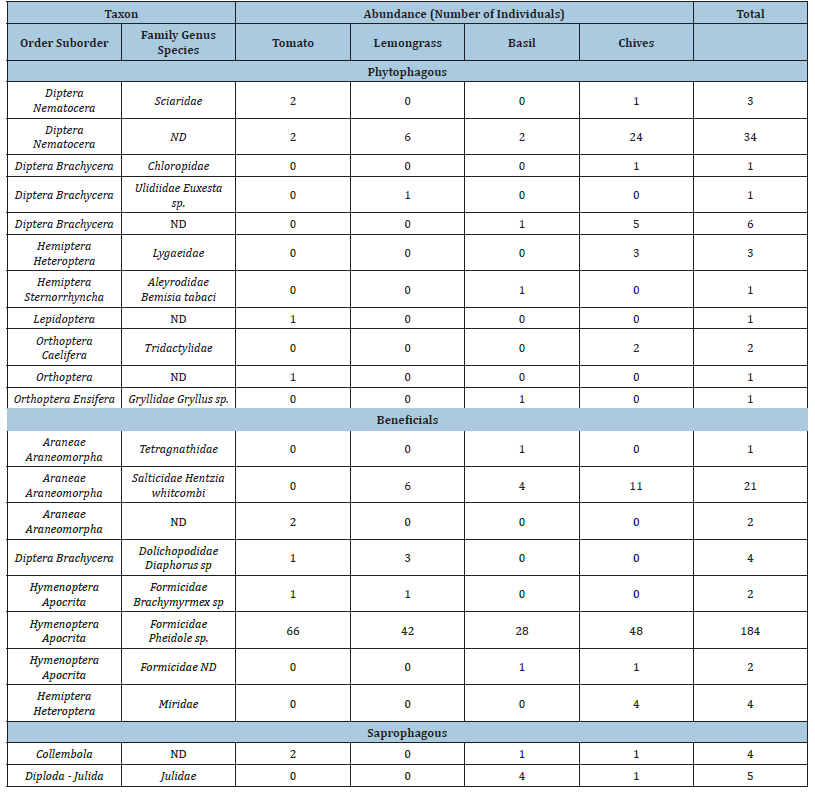
ND: Not determined.
Figure 1:Distribution of arthropod orders collected with D-vac and Pitfall traps. Average numbers of individuals (mean±SE) for the four plants studied (tomato, lemongrass, basil, chives), replicates (3), and collections (3) (Martinique, 2010-2011).
We identified 1241 phytophagous, 225 beneficials and 68 saprophagous individuals. In the D-vac samples, beneficials accounted for 14.7% of the total number of individuals collected distributed across 5 orders and 11 families. Among these, we observed three families of Hemiptera (38% of individuals), five families of Diptera (32% of individuals), two families of Araneae (20% of individuals), five families of Hymenoptera (7.6% of individuals) and only one familie of Coleoptera (2.1% of individuals). The first collection accounted for 17.9% of all the arthropods collected, the second collection for 30.5% and the third collection for 51.6%. This trend coincided with an increase in arthropod populations observed both in the D-vac samples and in the pitfall samples. Thus, the third collection comprised 52% of individuals of samples collected of D-vac and 47% of individuals of samples collected of pitfall samples (Tables 1 & 2)
Arthropod diversity: Shannon
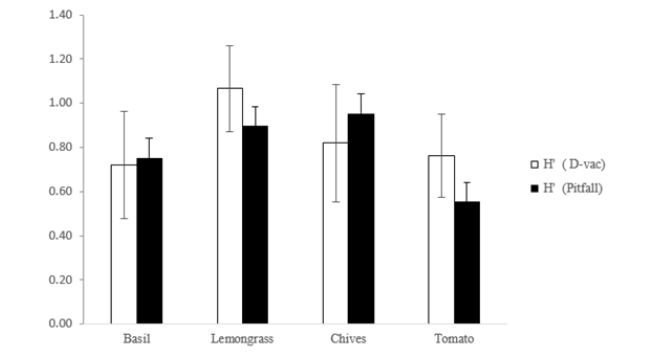
The mean Shannon index (H’) as determined by D-vac sampling was 0.84, with values ranging between 0.7±0.2, mean with SE and 1.1±0.2 mean with SE. The mean diversity index as determined by pitfall trap sampling was 0.79 (H’ ranging between 0.5±0.1 and ±0.3, mean with SE) depending on the plant (Figure 2). The Shannon indices of lemongrass and chives were higher (H’=1.1±0.2, mean±SE and H’=0.8±0.3, respectively). The mean Shannon index of the Pitfall samples was 0.79 (with values ranging between 0.6±0.2, mean±SE and 1.0±0.3, mean±SE). The fluctuation of this index revealed lower diversity (0.6±0.2, mean±SE) on the three aromatic plants than on tomato (Figure 2). The Shannon indices of lemongrass and chives were similar (Figure 2).
Figure 2:Shannon index (H’) showing arthropod diversity, collection with D-vac and Pitfall traps. Average values of indices (means±SE) of the three replications on each of the four plants.
Arthropod abundance
There was a significant effect of plant species and collection date on the abundance of phytophagous arthropods (Table 3). The model with plant and date as explanatory variables of the abundance of phytophagous arthropods was better than the model with only date (ANOVA test: F(2, 31)=3.72, P=0.021), and then the model with only the plant (ANOVA test: F(3, 31)=5.24, P=0.010). The effect of plant was mostly due to the effect of basil, on which the abundance of phytophagous arthropods was 1.32 more than that observed on tomato (t-value=2.73, df=6, P=0.01). The abundance of phytophagous arthropods on chives and lemongrass was not significantly higher than that observed on tomato (t-value=0.83, df =6, P=0.410 and t-value =0.41; df=6, P=0.223. The abundance of phytophagous at different sampling dates was significantly higher at sampling date 3 with 1.2307 more beneficials arthropods than on tomato (t-value=2.88, df=6; P=0.007), but not at sampling date 2 (t-value=1.35, df=6, P=0.185). The abundance of beneficials had no significant effect on the abundance of phytophagous arthropods (P>0.05). The abundance of phytophagous arthropods and the date of sampling had no significant effect on the abundance of beneficials (P>0.05). The effect of the plants was mostly explained by basil for which the log-abundance of beneficials was 1.728 greater than that observed on tomato (t-value=1.97, df=7, P=0.0004). The log-abundance of beneficials on chives and lemongrass was not significantly greater than that observed on tomato (t-value=3.97, df=7, P=0.1 and t-value=1.69, df=7, P=0.114, respectively).
Table 3:Abundance of phytophagous arthropods analyzed with a quasi-generalized linear model with a Poisson error and log of abundance of beneficials analyzed with a generalized least square model with date as a variance function error.
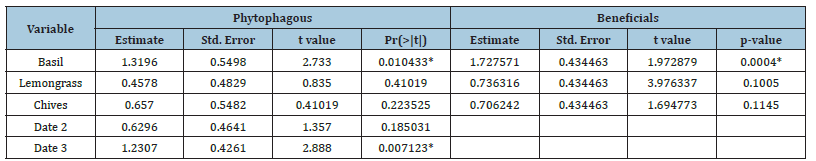
*: Indicates significant effect between aromatic plants and the abundance of phytophagous arthropods and beneficial arthropods.
To assess the influence of aromatic plants on ground-dwelling arthropods, we selected the three most representative arthropod orders based on the number of individuals, and analyzed them using a quasi-generalized linear model with a Poisson error. Aromatic plants had a significant effect on the abundance of arthropods (P>0.05). Plant species and sampling date had a significant effect on cumulated abundance. The model with plant and data as explanatory variables of the abundance of arthropods was bette than the model with only data (ANOVA: F(3, 41) =3.732, P=0.018) than the model with only plant (ANOVA: F(3, 44)=9.248, P<0.001). The abundance of arthropods on tomato was 0.88 higher than on basil (t-value=2.693, df=3, P=0.01). The abundance of arthropods on lemongrass and chives was respectively lower than on basil (t-value=2.77 and 1.335; df=3, P=0.008 and 0.18 respectively (Table 4).
Table 4:Abundance of the three most frequent arthropod orders analyzed with a generalized least square model with the date as the error variance function.
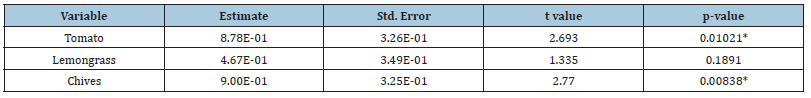
*: Indicates significant effect between aromatic plants and the abundance of arthropod orders.
Discussion
To our knowledge, this is the first inventory of populations of phytophagous and beneficial arthropods on traditional aromatic plants known for their repellent essential oils [23]. Indeed, results show that the three traditional aromatic plants lemongrass, basil, chives could be used with tomato, one of the main crops produced in Martinique. Of the three plants, lemongrass had an effect on both the abundance and diversity of arthropod populations. For sustainable agriculture and integrated pest management, the multispecies system consists in combining plants with attractants and repellents or biocides to control aerial arthropod pests of the target crop. Ample evidence accumulated in the last few years shows that volatiles from vegetative plant parts can directly repel herbivores and also protect plants by attracting herbivore enemies, including beneficials [43]. The attraction or the repulsion exerted on a pest depends on the physiology and biochemistry of the plant, while the emission of a particular volatile compound into the atmosphere depends on both the rate of its biosynthesis and the rate of its release [44]. Similarly, the extra floral nectars developed by some of these plants (e.g. basil) are important dietary components for the attraction of natural enemies such as omnivorous arthropod predators, but which are able to shift between a diet comprised of arthropod prey and one composed of plant-based resources during periods when prey are scarce [45].
Species richness of the arthropod fauna was completely distributed on basil, not on the other aromatic plants or on tomato on which one to two orders of arthropods were absent. In pitfall traps, a similar phenomenon was observed concerning diversity on tomato where ants largely dominated. A more even distribution of populations of arthropods was found on the soil close to lemongrass, whereas near the tomato plants, one species dominated the entire population. Hemipterans accounted for the great majority of arthropods collected on the three aromatic species during the campaign. Individuals of the Cicadellidae family were found on all four crops, with the exception of the two of three plots of chives. The hemipterans of the family Lygaeidae were the most abundant representatives of the Hemiptera order and seem to be attracted to basil, but nevertheless occupied one plot of chives. Populations of dipterans, representing by a single family (Ceratopogonidae), were numerically dominant on tomato plants. According to Werner and Pont [46], the larvae of the subfamily of Ceratopogoninae, which are essentially predators that feed on small animals as well as all adult female Ceratopogoninae except Culicoides, are also predators of small insects such as midges. Nearly half of these arthropods are potential predators and they were the same families that we found on the tomato crop. Thus, on chives, dipterans belonging to the Ceratopogonidae, Dolichopodidae and Empididae families were denoted as predatory. Otherwise, on lemongrass, only one family of predaceous dipterans (Dolichopodidae) was observed. Species of this family feeds mainly on the larvae of other Diptera [46]. Some families, including Dolichopodidae and Ceratopogonidae, which may play a role in predation, were present in large numbers on tomato.
In pitfall traps, potential ants accounted for the majority of arthropod populations collected on tomato plants Among the ants, the genus Pheidole, which is often the most prevailing ant genus in warm climates such as tropics [47], accounted for a very large majority of potential beneficials, Based on individuals counted in the D-vac samples, the aromatic plants were more attractive than tomato to the hemipterans. The high abundance of hemipterans presence at the third sampling date coincided with more abundant flowering of basil than at the two previous sampling dates. Members of the Lygaeidae prefer the reproductive organs of host plants [48,49]. This was also confirmed in an experiment on the natural food needs of Oncopeltus fasciatus, a member of the Lygaeidae family, by Ralph [49] using plant species as host plants, which revealed that the nutrients needed for good reproduction of the species are flowers or seeds rather than stalks or leaves. Holway et al. [50] speculate that the pheromones produced by male member of the Heteroptera order may play a defensive role but also play an important role in the orientation of colonization of new habitats. However, some foods that are good for breeding are poor for growth, and vice versa. Under this hypothesis, these species may play a significant role in the natural reduction of populations of insect pests [51]. Similarly, numerous studies have shown that members of the Miridae family are omnivorous predators. Using the example of Dicyphus hesperus, Gillespie & McGregor [52] showed that without leaves or supplementary water, D. hesperus cannot complete its life cycle or feed on prey as an adult. Thus, species belonging to the Miridae family are among the many taxa of omnivorous predators reported to be improvers of life-history traits when prey food is supplemented by plant food [52-54]. Plant feeding by omnivorous predators should promote top-down control and may increase the likelihood of tropic cascades [55,56].
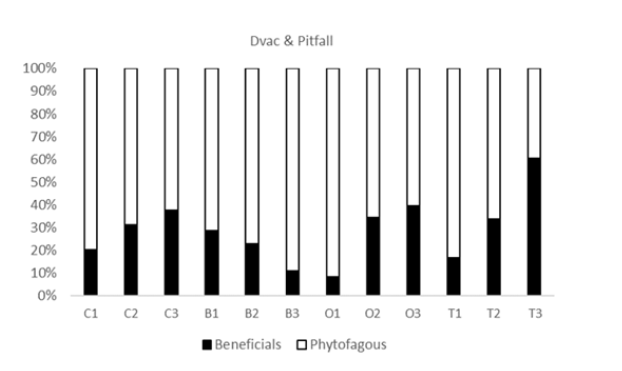
Most collembolans were caught on the leaves of aromatic plants. Thus, although the majority of collembolans inhabit the soil, some can colonize plant litter, the herbaceous layer that covers the soil under shrubs and trees, and even bare soil and rock [57]. Many studies highlighting food preferences (mycophagy) of collembolans, have suggested climate condition are of secondary importance. Similarly, the effects of the wet or the dry season could be reflected in numeric responses in arthropod populations [58]. The different approaches we used allowed us to assess the effects of aromatic plants on beneficial populations, which may have great potential for the control of arthropod pests of vegetable crops. Phytophagous insects were abundant on aromatic plants, plant volatiles are also known to attract enemies of plants [43]. Unlike their essential oils, which are used in the manufacture of insecticides or repellents, aromatic plants did not show the same performance as their essential oils, but seem mostly to increase the presence of phytophagous and beneficials. However, in the case of aromatic plants associated with crops, moving herbivores toward these plants could itself be a beneficial effect because the herbivore-challenged plants also emit volatiles that attract insect predators and bolster resistance to future threats [59]. Song et al. [60] assessed five aromatic plants as intercrops in a pear orchard, and all significantly reduced the pest population compared with that in the plot with natural grasses. Similar results were obtained by Lopez & Shepard [61] in an experiment with a medicinal plant species, feverfew (Tanacetum parthenium. L), an aromatic plant which attracts and maintains populations of arthropod predators. Our survey data to our knowledge provides the first assessment of the beneficial and pest arthropod populations present on aromatic plants that could serve as companion plants, and enhance biological control of tomato crop. The changes in the abundance of beneficial and harmful insects, in Figure 3, are undoubtedly one of the most important results of this study for the choice of companion plants.
Figure 3:Abundance behavior of beneficial and phytofagous arthropods during the test The abundance behavior of beneficial and phytophagous arthropods during the trial was markedly different for basil compared to other crops.
Acknowledgement
We thank Eddy and Thierry Dumbardon-Martial of FREDON Martinique of FREDON Martinique for help with the identification of arthropods. This work was funded by Project “Dynamics pest and communities under ecological intensification conditions” from European Regional Development Fund (FEDER) and the Territorial Community of Martinique (CTM) in the framework of the AGROBIODEV project. Thanks to Alain Ratnadass, Philippe Tixier and Béatrice Rhino (CIRAD research center) for helpful comments.
References
- Carvalho FP (2006) Agriculture, pesticides, food security and food safety. Environmental Science & Policy 9(7-8): 685-692.
- Daam MA, Van Den Brink PJ (2010) Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology 19(1): 24-37.
- Ratnadass A, Fernandes P, Avelino J, Habib R (2012) Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agronomy for Sustainable Development 32: 273-303.
- Malézieux E, Crozat Y, Dupraz C, Laurans M, Makowski D, et al. (2009) Mixing plant species in cropping systems: Concepts, tools and models: A review. Agronomy for Sustainable Development 29: 43-62.
- Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, et al. (2010) Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468: 553-556.
- Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecological Monographs 43(1): 95-124.
- Kumar BM, Nair PR (2004) The enigma of tropical homegardens. Agroforestry Systems 61: 135-152.
- Soemarwoto O (1987) Homegardens: A traditional agroforestry system with a promising future. Agroforestry: A decade of development, Section 3, World Agroforestry Centre, Nairobi, Kenya, pp. 157-170.
- Chaubet B (1992) Ecological diversity, development of agro-ecosystems and promotion of natural enemies of pests: Case of aphidiphages. INRA environmental letter 18: 45-63.
- Rao M, Palada M, Becker B (2004) Medicinal and aromatic plants in agroforestry systems. Agroforestry Systems 61: 107-122.
- Degras L (2005) The creole garden: Cultural, scientific and technical landmarks.
- Wezel A, Bender S (2003) Plant species diversity of homegardens of Cuba and its significance for household food supply. Agroforestry systems 57: 39-49.
- Dugravot S (2004) Secondary sulfur compounds of Allium: Role in leek defense systems and actions on insect biology. François Rabelais-Tours University, Tours, France.
- Negrelle R, Gomes E (2007) Cymbopogon citratus (DC.) Stapf: Chemical composition and biological activities. Rev Bras Pl Med 9: 80-92.
- Tchoumbougnang F, Dongmo PMJ, Sameza M, Mbanjo E, Fotso G, et al. (2009) Larvicidal activity on Anopheles gambiae Giles and chemical composition of essential oils extracted from four plants cultivated in Cameroon. Biotechnol Agron Soc Environ 13(1): 77-84.
- Wittstock U, Gershenzon J (2002) Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology 5(4): 300-307.
- Beizhou S, Jie Z, Jinghui H, Hongying W, Yun K, et al. (2011) Temporal dynamics of the arthropod community in pear orchards intercropped with aromatic plants. Pest Management Science 67(9): 1107-1114.
- Tang GB, Song BZ, Zhao LL, Sang XS, Wan HH, et al. (2012) Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agroforestry systems 87: 273-285.
- Parolin P, Bresch C, Desneux N, Brun R, Bout A, et al. (2012) Secondary plants used in biological control: A review. International Journal of Pest Management 58(2): 91-100.
- Parker JE, Rodriguez-Saona C, Hamilton GC, Snyder WE (2013) Companion planting and insect pest control. Weed and Pest Control-Conventional and New Challenges, Intech Open Access Publisher, London, UK.
- Letourneau DK, Armbrecht I, Rivera BS, Lerma JM, Carmona EJ, et al. (2011) Does plant diversity benefit agroecosystems? A synthetic review. Ecological Applications 21(1): 9-21.
- Amarawardana L, Bandara P, Kumar V, Pettersson J, Ninkovic V, et al. (2007) Olfactory response of Myzus persicae (Homoptera: Aphididae) to volatiles from leek and chive: Potential for intercropping with sweet pepper. Acta Agriculturae Scandinavica Section B-Soil and Plant Science 57(1): 87-91.
- Koul O, Walia S, Dhaliwal G (2008) Essential oils as green pesticides: Potential and constraints. Biopestic Int 4(1): 63-84.
- Yu JQ (1999) Allelopathic suppression of Pseudomonas solanacearum infection of tomato (Lycopersicon esculentum) in a tomato-chinese chive (Allium tuberosum) intercropping system. Journal of Chemical Ecology 25: 2409-2417.
- Deberdt P, Perrin B, Coranson-Beaudu R, Duyck PF, Wicker E (2012) Effect of Allium fistulosum extract on Ralstonia solanacearum populations and tomato bacterial wilt. Plant Disease 96(5): 687-692.
- Auger J, Arnault I, Thibout É (2008) Sulfur-containing substances from Allium and cruciferous plants: Phytosanitary potential and applications to bio fumigation. Biopesticides of Plant Origin, (2nd edn), Tec & Doc-Lavoisier, Paris, France, pp. 101-123.
- Wicker E, Grassart L, Coranson‐Beaudu R, Mian D, Prior P (2009) Epidemiological evidence for the emergence of a new pathogenic variant of Ralstonia solanacearum in Martinique (French West Indies). Plant Pathology 58(5): 853-861.
- Fang Y, Wang J, Luo C, Wang R (2018) Lethal and sublethal effects of clothianidin on the development and reproduction of Bemisia tabaci (Hemiptera: Aleyrodidae) MED and MEAM1. Journal of Insect Science 18(2): 37.
- Rhino B, Verchere A, Thibaut C, Ratnadass A (2016) Field evaluation of sweet corn varieties for their potential as a trap crop for Helicoverpa zea under tropical conditions. International Journal of Pest Management 62: 3-10.
- Mommertz S, Schauer C, Kösters N, Lang A, Filser J (1996) A comparison of D-vac suction, fenced and unfenced pitfall trap sampling of epigeal arthropods in agro-ecosystems. Phoenician Zoological Annales 33(1): 117-124.
- Debras JF, Senoussi R, Rieux R, Buisson E, Dutoit T (2008) Spatial distribution of an arthropod community in a pear orchard (southern France): Identification of a hedge effect. Agriculture Ecosystems & Environment 127: 166-176.
- Elliott N, Tao F, Fuentes-Granados R, Giles K, Elliott D, et al. (2006) D-vac sampling for predatory arthropods in winter wheat. Biological Control 38(3): 325-330.
- Le Berre J, Roth M (1969) Water traps.
- Mckey D, Gaume L, Dalecky A (1999) Symbioses between plants and arboreal ants. The Organic Year 38: 169-194.
- Ward DF, New TR, Yen AL (2001) Effects of pitfall trap spacing on the abundance, richness and composition of invertebrate catches. Journal of Insect Conservation 5: 47-53.
- Work TT, Buddle CM, Korinus LM, Spence JR (2002) Pitfall trap size and capture of three taxa of litter-dwelling arthropods: Implications for biodiversity studies. Environmental Entomology 31(3): 438-448.
- Delvare G, Aberlenc HP (1989) Insects from Africa and tropical America: Keys to recognizing families, Editions Quae.
- Bolton B (1994) Identification guide to the ant genera of the world, Harvard University Press, Cambridge, Massachusetts, USA.
- Bordat D, Arvanitakis L (2004) Arthropods of vegetable crops in West and Central Africa, Mayotte and Reunion, CIRAD, Montpellier, France.
- Shannon CE (1948) A note on the concept of entropy. Bell System Tech J 27: 379-423.
- Jost L (2006) Entropy and diversity. Oikos 113(2): 363-375.
- Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1(1): 3-14.
- Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Current Opinion in Plant Biology 12(4): 479-485.
- Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiology 135(4): 1893-1902.
- Limburg DD, Rosenheim JA (2001) Extrafloral nectar consumption and its influence on survival and development of an omnivorous predator, larval Chrysoperla plorabunda (Neuroptera: Chrysopidae). Environmental Entomology 30(3): 595-604.
- Werner D, Pont A (2003) Dipteran predators of Simuliid blackflies: A worldwide review. Medical and Veterinary Entomology 17(2): 115-132.
- Wilson EO (2003) Pheidole in the new world-a dominant, hyperdiverse ant genus, Cambridge, Massachusetts, USA.
- Bongers J, Eggermann W (1971) The influence of the subsocial behavior of the specialized seed suckers Oncopeltus fasciatus Dall. and Dysdercus fasciatus on their diet. Ecology 6: 293-302.
- Ralph CP (1976) Natural food requirements of the large milkweed bug, Oncopeltus fasciatus (Hemiptera: Lygaeidae), and their relation to gregariousness and host plant morphology. Ecology 26: 157-175.
- Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annual review of Ecology and Systematics 33: 181-233.
- Carayon J (1961) Some remarks on Hemiptera-Heteroptera: Their importance as auxiliary insects and the possibilities of their use in biological control. Entomophage 6: 133-141.
- Gillespie D, Mcgregor R (2000) The functions of plant feeding in the omnivorous predator Dicyphus hesperus: Water places limits on predation. Ecological Entomology 25(4): 380-386.
- Coll M (1996) Feeding and ovipositing on plants by an omnivorous insect predator. Ecology 105(2): 214-220.
- Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annual Review of Entomology 47: 267-297.
- Eubanks MD, Denno RF (2000) Host plants mediate omnivore-herbivore interactions and influence prey suppression. Ecology 81(4): 936-947.
- Eubanks MD, Styrsky JD, Denno RF (2003) The evolution of omnivory in heteropteran insects. Ecology 84(10): 2549-2556.
- Betsch JM (1990) The reproductive behavior of Springtails. Insects 77: 2-5.
- Pinheiro F, Diniz I, Coelho D, Bandeira M (2002) Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecology 27(2): 132-136.
- Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41-66.
- Song BZ, Wu HY, Kong Y, Zhang J, Du YL, et al. (2010) Effects of intercropping with aromatic plants on the diversity and structure of an arthropod community in a pear orchard. BioControl 55: 741-751.
- Lopez R, Shepard BM (2007) Arthropods associated with medicinal plants in coastal South Carolina. Insect Science 14(6): 519-524.
© 2024 © Clovel Pancarte. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






