- Submissions

Full Text
Environmental Analysis & Ecology Studies
The Mechanism of PGPR on the Photosynthesis System, Plant Resistance and Biofertilizers
Abdullah Msaad Al-Falih*
Department of Botany and Microbiology, College of Science, King Saud University, Saudi Arabia
*Corresponding author:Abdullah Msaad Al-Falih, Department of Botany and Microbiology, College of Science, King Saud University, Saudi Arabia
Submission: October 12, 2023; Published: October 30, 2023

ISSN 2578-0336 Volume11 Issue4
Abstract
In view of urgent need to improve plant growth and increase productivity, the factors affecting plant growth are studied. Since the nutrients around the root are abundant, they support the growth of the organisms around them. Among these organisms, especially the bacterial community, there is a group known as the root bacteria that occupies the rhizosphere, causing a direct stimulation of plant growth or control biology of plant pathogens, it has a common name “Plant Growth-Promoting Rhizobacteria (PGPR). These bacteria contribute to many plant activities, due to the capabilities known to them related to plant activities. These bacteria are characterized by nitrogen fixation and can dissolve phosphorus, iron and others, in addition to improving plant tolerance of some heavy elements. Some strains of plant growth stimulating root bacteria to build some plant growth regulators such as auxin, gibberellin, cytokinin, and ethylene, or produce volatile compounds, so these may contribute to stimulating growth. PGPR affect the photosynthesis system by increasing some plant pigments. Some strains of these bacteria have been known to form antagonistic compounds to certain pathogens such as phenazine, thus protecting plants or increasing plant resistance to Systemic Acquired Resistance (SAR). Some strains have been used as biofertilizers as inoculants or co-inoculants. There is no general agreement on the mechanism of action, the most important is the improvement of selection of inoculants and their quality for a specific need as the effectiveness of selecting PGPR is crucial for this biotechnology, especially increasing the yield of plants.
Keywords:Bacteria; Roots stimulating plant growth; Dissolving phosphorous; Growth regulators; Nitrogen fixation; Biological fertilizers
Introduction
Plant growth in agricultural soil is affected by many factors physical and biological. The human tried and tried throughout the ages improving plant productivity with traditional methods such as fertilization and pest control and plant breeding, but as human knowledge advanced, the attempt continued in that and in modern ways, including genetic engineering or control and change the physical factors surrounding the plant, such as light and relative humidity and the temperature and concentration of carbon dioxide and others in homes protected. For example, the technology of aquaculture is effectively exploited useful, and agriculturally competitive, too, especially for some crop plants like tomato, cucumber, etc. The Green Revolution of the 20th century enabled unprecedented gains in global food production. The Green Revolution was roughly comprised of two main advances; chemical inputs (pesticides, herbicides, and chemical fertilizers) and improved crop plants (through targeted breeding and advanced genetic manipulations). The technique indicates the availability of these crops not in a specific season but for a long time year and at a lower cost, and therefore this technology increased productivity despite not climate and soil suitability in some regions of the world. Likewise, biological factors and growth conditions are now being looked at the plant in nature and surrounding the main plant organ (total radical) of objects and variables, studying them and then trying to control in an effort to increase the productivity of plants growing in nature, in ways the predominant agricultural use in agricultural lands after improving them. A plant growing under field conditions is not an individual community or gathering; it is a complex community [1] with subtle and relatively constant partner relationships. A well-structured and regulated community of microorganisms is always associated with the plant [2-6] This community is the Phyto microbiome [7]; the Phyto microbiome plus the plant is the holobiont [8-9]. Microbiome relationships exist with all multicellular organisms, and probably all eukaryotes. In fact, these probably predate the colonization of the land by plants [10]. This microbial community has been associated with terrestrial plants since their earliest evolution, to assist early land plants faced with challenges such as access to nutrients, novel and often-stressful conditions and pathogens [11].
Among the microorganisms present in the rhizosphere is a group that includes both true phytopathogenic bacteria and nonpathogenic bacteria. Of course, some of these non-pathogenic bacteria can influence plant growth by increasing or delaying it. I knew the importance of this bacterial group of microorganisms and the role of some of them in keeping plant roots healthy and the role of some also in their absorption of carbon and nutrients and in bearing some harsh environmental stresses. The Plant-Growth Promoting Rhizobacteria (PGPR) includes all genera of bacteria that stimulate plant growth. So, this term PGPR includes large number of bacterial genera, including: Acetobacter, Achromobacter, Anabaena, Arthrobacter, Azoarcos, Azospirillum, Azotobacter, Bacillus, Burkholderia, Clostridium, Enterohacter, Flavobacterium Frankia, Hydrogenophaga, Kluyvera, Microcoleus, Phyllobacterium, Pseudomonas, Serratia, Staphylococcus, Streptomyces, Vibrio bacteria, and Rhizobium. In the soil, there is a gradient of intimacy between plant roots and microbes extending away from the plant root: the degree of plant influence over the microbial community increases nearer the root surface in rhizosphere (Figure 1). Diversity and number of microbes is variable between soils, distance from plant roots, crop species, and plant tissue [12].
Figure 1:The degree of intimacy and influence of the plant-microbe interactions. Microbes are represented by small different colored [12].
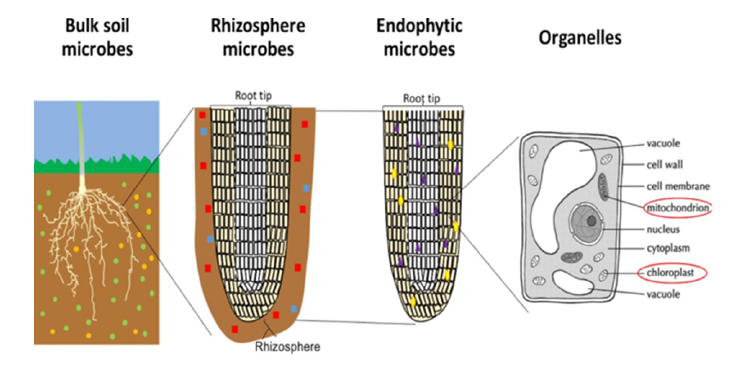
In this review I will mention something in detail about the ability root bacteria stimulator for plant growth, improve growth rate and increase its productivity by reducing the number of harmful organisms in the rhizosphere.
Rhizosphere
One study [13] estimated using the distance over which fungi respond to substances secreted from roots that the average rhizosphere width of about 1mm, however it may reach 12mm for one type of fungus (Gaeumannomyces gramminis). Plant rhizospher is a fertile ground for the growth of some living organisms due to the availability of materials nutrient, where the plant loses about 40% of the material formed in a process photosynthesis by roots [14]. But in another review, the net carbon excreted by roots was estimated to be 5-10% of the net carbon fixed by plant [15]. Plus carbon, roots are excreted from various major compounds normally present in the cell, most notably mucous substances, organic acids and phenols (among the iron holders) and other compounds [16]. These organisms in the Rhizospher contain microorganisms [including bacteria (of which there may be about 1010cells/g) and fungi] and microfauna (including protozoa, nematodes, insects, and others under this heading). In fact, abundance of this grouping of organisms depends on many variables such as soil quality [17], plant type and timing [18], the type of their roots, the age of those roots and their regions [19], the extent of infection with pathogens [20] and the materials excreted from those roots. On the other hand, these organisms affect the composition and quality of various substances secreted from the roots through their influence on cell filtration, metabolism and plant nutrition (see also [21]). Among the microbiota groups is a group that includes real, pathogenic and non-pathogenic bacteria in plants. Of course, some of these non-pathogenic organisms affecting plant growth by increasing or hindering it. It is known the importance of this group of organisms, the role of some of them in keeping plant roots healthy, and the role of some also in absorbing them for nutrients and in bearing some environmental stresses [22-23].
Rhizobacteria
Scholars generally use two directions of definition in an
attempt to learn about the bacterial community in the rhizospher
and others are:
A. Phenotypic methods: The traditional method is to grow
bacteria in a food medium or use logs physiological characteristics
at the Community Level Physiological Profiles (CLPP) with various
usage detection techniques such as Fatty Acid Methyl Ester (FAME)
[24].
B. Genotypic methods: Employing ulture-independent
culture molecular techniques of DNA extraction gene sequence
analysis of ribosomal DNA. (16S-rRNA gene sequence analysis
[25]. Added to this is the revised DNA density method namely, the
sounding isotope fixed by RNA ″ RNA Stable Isotope Probing, as cited
by one of the papers, [26] and increase its efficiency [27]. In short,
treating plants with marked carbon dioxide in their natural location
13CO2 and then extracted the ribosomal DNA and fractionated
it into 12C-RNAs and 13C-RNAs. These results are collected in a
library after back translation followed by PCR amplification. These
are examples of ways to contribute to identifying the structure
of the bacterial community in the root environment and others
so that it can be dealt with. With the advancement of laboratory
technology, the location of bacteria on the roots can be monitored
using Molecular markers such as proteins or antibody tags, and by
confocal laser scanning microscopy [28].
Scientists recognized some of the groups and gave them names
generally based on interest in what the group might play. Some
scholars in this field [29] are using a general term for all types
of bacteria that stimulate plant growth, which is Plant Growth-
Promoting Bacteria (PGPB) either nitrogen-fixing or non-nitrogenfixing
bacteria and suggested dividing them into two groups:
a) A group that stimulates plant growth directly by
influencing its metabolism and
b) another group that activates controlled plant growth
promoter bacteria are called biocontrol-PGPB.
These include endophytic bacteria and diazotrophic bacteria) and rhizobacteria and symbiotic bacteria in root nodes [29]. Of course, this comprehensiveness includes a large number of genera. A survey study indicates a number of bacteria (393 bacteria) associated with different root regions of the peanut plant Arachis hypogaea L indicates that the active isolation of the rhizospher Bacillus firmis GRS 123 and two isolates from the phylloplane leaf plane region namely Bacillus megaterium GPS 55 and Pseudomonas aeruginosa GPS 21, all stimulate plant growth in field, and it was possible to isolate it again after pollination in the field and after a while it may last for 100 days [30]. However, among the bacterial groups that scientists knew was a group that included nitrogenfixing bacteria along with other bacteria that were not known to fix nitrogen and were commonly called all together with a group plant growth root stimulant bacteria group Plant Growth-Promoting Rhizobacteria (PGPR). As the name suggests, it stimulates plant growth and grows in an environment competitiveness [31].
Plant Growth-Promoting Rhizobacteria (PGPR)
This group is represented by bacteria as it was mentioned above
[32] once defined it as the bacteria that occupies the roots cause
stimulation of plant growth (control) biology for plant pathogens
[31]. This definition distinguish three characteristics:
A. The bacterium must settle on a surface root,
B. It must survive and reproduce in its microenvironment,
which is the root surface, and finally
C. It should stimulate plant growth [23].
Research has been active in various laboratories around the world in various fields of the role of these bacteria, that is, “activation” and “control” in an attempt to find out the mechanism of activation by following the capabilities that have been published that stimulate plant growth roots bacteria are specific to them, such as nitrogen fixation growth regulators and dissolving elements and their absorption and reducing effects stress and others, as will be mentioned below, in preparation for this exploitation the group is applied in agriculture. The molecular basis of plant-bacteria interaction mechanisms responsible for the physiological changes are beginning to be discerned, mainly due to the emerging “omics” approaches. Plant-microbe co-evolution has led to some of the bacteria becoming facultative intracellular endophytes [5]. Among these free-living bacteria are PGPR that exert beneficial effects on plants through direct and indirect mechanisms. Beneficial rhizobacteria have been utilized to improve water and different nutrients uptake, abiotic and biotic stress tolerance. Even though numerous soil bacteria have been reported to promote plant growth and development, the mode(s) of action by which the bacteria exhibit beneficial activities are often not well understood.
Activation
In the area of activation, a number of research and reviews
refer [33], for example) until a group of these bacteria contribute
to many processes that affect plant metabolism by providing
substances that the plant needs or affect it. From detailed studies
those that use nitrogen-fixing bacterial systems (especially the
Azospirillum bacterial system, which has been more and more
widely studied [34]. Moreover, the majority of species of this genus
contain strains known to stimulate plant growth. Characteristics in
general reviews such as [35-40]. Subspecies of this genus influence
the appearance of roots and increase the surface area of roots
[33], but the productivity depends on the fertility system used to
produce the crops [41]. This genus is exploited in environmental
studies that have no scope for detail, and you can refer to one of the
reviews about the environmental applications of this species [42]
The review covered three topics mentioned here for importance,
namely:
A. Wastewater bioremediation
B. Mangrove reforestation
C. Desert reforestation
Rhizobacteria affects plant growth in many fields of plant physiology treated by them, and the result is stimulating plant growth as follow:
Mineral Nutrition
This group of bacteria contributes with autotrophic bacteria endophyte where both are completely not symbiotic with the plant in nitrogen fixation as part of Biological Nitrogen Fixation (BNF), where most research indicates that this group of bacteria provides some of the nitrogen needs of the plant [43-44]. Since other essential elements are just as important as nitrogen, including phosphorous and iron, are abundant in soil particles. However, in unavailable form, it has attracted the scientist to study the contribution of these microorganisms of several plants to dissolve these elements and make them easily absorbable. In general, it is a bacterium the stimulating roots of plant growth contribute to dissolving phosphorus and iron and making it available for absorption [45] and follow some of those studies will be mentioned in some plants. Laboratory, and among the 32 isolates from under a group of Plant Growth-Promoting Rhizobacteria (PGPR), which is known as the group of bacteria solvent for inorganic phosphates from hydroxyapatite precipitates Serratia marcescens GPS-5 turned out to appreciate it. Associated with the control of late leaf spot disease of soybean plants. By using the mutagenic compound Ethyl Methane Sulfonate (EMS) and obtain (1700 mutant), it was found that the largest dissolving ability is EMS XVIII Sm-35 from that isolation [46]. It is learned from a study of another genus polyps plant growth stimulant Pseudomonas chlororaphis PCL1391 which is the antibiotic Phenazine-1-Carboxamide (PCN) that this isolation of the roots can dissolve the crystallized iron and manganese oxides, as it is suggested that the compound (PCN) works as a “shuttle” to transport the electron, not as a chelate compound, which is what researchers expect it to occur in nature. It is this compound (PCN) and other antibiotics may be antagonists to oxidize and reduce, which in turn activates the elemental reductase by microbiology [47].
According to some previous studies, inoculation with the bacterium Azospirillum brasilense stimulates growth, but only to a degree varying the absorption of elements such as phosphorous and potassium of [48] and iron in sorghum bicolor [49]. In another study on stimulation growth of inoculated wheat and soybeans with more strains than bacteria A. brasilense results indicate that the bacterium is able to alter distribution and quantity of the studied elements in these two plants included eleven elements [50] these are, (K+, P, Ca2+, Mg2+, S, Na, Mn2+, Fe2+, B, Cu2+, Zn2+ ). Because of the relationship between the absorption of the elements and the electrochemical potential difference of the cell, it was found that the cell membrane activity in the roots of the soybean plant and the proton flow through it were affected by the inoculation with A. brasilense [51]. One study [52] indicates that four types of root bacteria stimulate plant growth are: Bacillus chitinolyticus, B. subtilis var.2 and B. pumillus var.2 and Citrobacter sp., isolated from the root level of Pachycereus pringlei [S. Wats] that grows on volcanic rocks) See [53] had been tested on the seedlings of the same aloe vera, and it was found that untreated plants grew poorly and some died while the treated plant stimulates its growth and increases the amount of elements that benefited from the treated plants (phosphorous, potassium, iron and magnesium) from the significant rocks after being rated at the end of the experiment.
The field of genetic engineering appears attractive for research in studies of the contribution of stimulating rhizobacteria to plant growth and the proposed mechanism of how activation by effect. An example is the contribution of bacteria to dissolve inorganic phosphates, as it was mentioned a general review of published research on this, [54] and the isolated genes and their description called dissolved genes Mineral Phosphate-Solubilizing (mps) genes (Table 1). The field does not accommodate all attempts for each feature of the root bacteria stimulating plant growth, and it needs another review if there are no reviews. While it is theoretically possible to know the gene for a specific characteristic of the root stimulant bacteria for plant growth and use such genes to obtain plants genetically engineered for this property, but the problem is that this property in bacteria does not work alone. Rather, bacteria use several interconnected mechanisms to stimulate plant growth, then the field of genetic engineering at the present time may not have the desired effect.
Table 1:The genes responsible for dissolving inorganic phosphorus explaining the organism, the gene or plasmid and the characteristics. As for the references, they are found in the general reference [54].
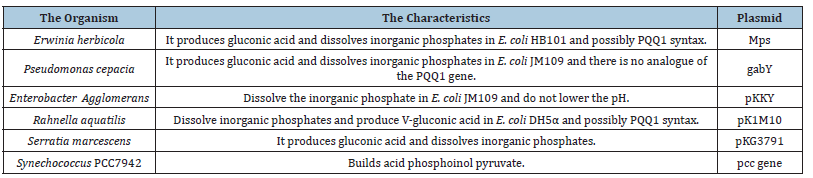
PQQ: Pyrroloquinoline Quinone.
Growth Regulators
Some strains of Plant Growth-Promoting Rhizobacteria (PGPR) are building some plant growth regulators such as auxin, gibberellin, cytokinin and ethylene (for more see [55]. Of the detailed studies showing a bacterial system that fixes nitrogen-fixing, the Pseudomonas putida strain GR12-2, where the mechanism of root elongation activation was studied and its relationship to the ability of this bacterium to produce the growth regulator of Indole Acetic Acid (IAA) but not fix nitrogen, [56,57]. The results of this study indicate the importance of the quantity available for a specific growth regulator such as IAA, as this leads to the formation of another growth regulator, which is ethylene, so that the interaction between the regulators is what determines the extent to which the plant benefits from the presence of the bacterium as shown in the published schematic diagram as a suggested model for the pathway (Figure 2). In summary, this model demonstrates ammonia building pathways and lowering ethylene concentration, ammonia beneficial to the plant and ethylene inhibits root elongation. Building a growth regulator of Indole Acetic Acid (IAA) increases its concentration in the root zone and this leads to activation of building the initiating compound to build ethylene 1-aminocyclopropane-1-carboxylate (ACC) by ACC synthase, but ACC an enzyme from the bacterium, ACC deaminase, converts it to ammonia [56]. Learned from a later study [58] on the effect of some strains of Pseudomonas putida including the GR12-2 strain, its ability to stimulate growth in both plants Canola Brassica napus. L and Lentil culinaris. Medik and growth regulators such as auxin. It was also mentioned in the study that one of the studied strains, P. putida strain 6-8, produces Cytokinins: Isopentenyl Adenosine (IPA), Zeatin Riboside (ZR) and Dihydroxyzeatin Riboside (DHZR) in the pure culture. In addition, this strain is able to form iron carriers and dissolve inorganic phosphates and used the (ACC) compound a precursor of ethylene as a nitrogen source.
Figure 2:Schematic diagram of the IAA growth regulator interaction pathway model and ethylene. (Source: adapted from [56-57]).
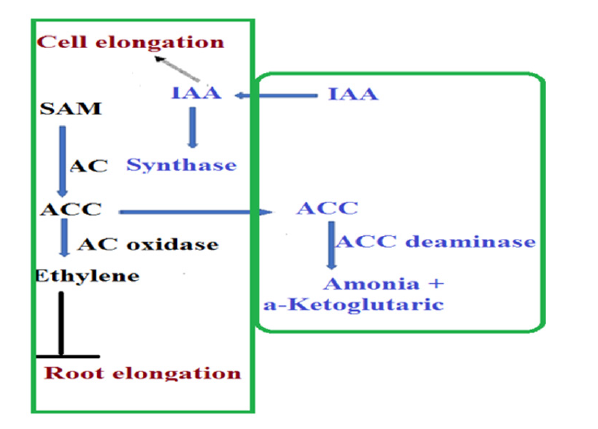
Another study that may support the role of growth regulators is a field study on Malus domestica Borkh apple trees where the use of strains of plant growth promoting rhizobacteria (Bacillus OSU-142, Bacillus M-3, Burkholderia OSU-7 and Pseudomonas BA- 8), producing IAA growth regulator and Cytokinins increase fruit production, but it depends to a large extent on the grafting of rootstocks and agricultural variety and the method of treatment. The study also adds that grown newly apple trees which are pollinated with these organisms, shows an increases in the average shoot length, diameter, and fruit yield. Some researchers think that the effect may be directly to growth regulators [59], while others such as [60] mentioned that the effect is dependent for root bacteria stimulating plant growth on the roots of the graft and that in two species of the genus Citrus namely Citrus limonia and Citrus reshni. In addition, results from a study of two varieties of wheat are added (Pasban-90) and (Inqlab-91) after inoculating with root bacteria that stimulate plant growth, selected from 30 isolates for their ability in vitro to form auxin growth regulator. Laboratory and field results indicate that the strain that was laboratory characterized by the greatest value of auxin formation in the sterilized soil caused the highest growth and productivity for the two varieties. The authors suggest the possibility of exploiting auxin building by root bacteria as a means of identifying the effectiveness of root bacterium strains that stimulate plant growth [61]. But another study on the activation of the growth of corn plants, and the stimulation of root nodules formation in a plant from the cowpea varieties Vigna radiata L by the root bacteria that stimulate plant growth indicates that characteristic of ACC deaminase it may be more efficient for screening strains of plant growth stimulating root bacteria [62].
Improve Plant Tolerance to Stress
Some areas are characterized by a short period of growth and a hindrance to formation root nodes on some low-degree crop plants root zone temperature, In order to avoid this obstacle, the effect of some strains of root stimulant bacteria on plant growth on plants was studied. Deduced from the study on soybeans Glycine max (L.) Merr. cultivar OAC Bayfield after co-injection of a Bacillus thuringiensis strains NEB17 and the nitrogen-fixing bacteria in this plant Bradyrhizobium japonicum strains 532C. An activation of plant growth and root nodule formation occurred where the increase in growth was greater and constant in all experiments when using this strain (NEB17), that is, it improved the tolerance of this plant to the coldness of the root zone, and this could be by stimulating the formation of root nodes, [63]. This is in relation to identifying the appropriate strain, but determining the mode of co-inoculation is also important in order to know the type of interaction between the two types of bacteria (nitrogen fixing and growth stimulant) as mentioned in another study [64] on the plant itself, using strains of three types of root stimulating bacteria, Psedomonas fluorescens, Chryseobacterium balustinum, Serratia fonticola, and the root nodule-forming bacterium Sinorhizobium fredii.
In this regard, a study on bacteria (some are from the genera Bacillus, Actinomadura, and Citrobacter) associated with some desert plants (such as cacti and figs) growing on rock fragments, indicated that they release a significant amount of the elements (phosphorous, potassium, magnesium, manganese, copper and zinc) from rocks, and these bacteria are characterized by being tolerant to heat and salt and drought [53]. Benefiting from the results of a study on Retama sphaerocarpa and interaction root stimulant bacterium Bacillus thuringiensis with the fungus Glomus intraradices is symbiont with the Arctum plant that the bacterium root growth doubled, but the highest value was by co-inoculation with the bacterium and fungi, but the bacterium showed an increase in water absorption in the presence and absence of the fungus, which the researchers consider a positive effect for plants growing in dry environments [65]. Some research related to improving tolerance to water stress, such as Sunflower research on Helianthus annuus L. isolated from its root environment a strain of exopolysaccharide secreting bacterium Rhizobium sp,/. Strain YAS34. The results of seed and soil inoculation with this strain indicated an increase in the adhesion of soil particles and an increase in the size of the interstitial spaces between them, at the same time increased growth in the two vegetative groups and rootstocks of seedlings under normal conditions or water stress (lack of water), and from that this strain improved soil properties and plant tolerance of water stress [66].
It is worth noting that there is a relationship between root bacteria stimulating plant growth and the plant’s tolerance to the presence of heavy elements in the soil. This may be indicated by what was stated in research that a lineage belonging to the genus Brevibacillus isolated from lead contaminated soil stimulate the growth of Trifolium pratense L by increasing the acid concentration Indole Acetic Acid (IAA), nitrogen and phosphorous accumulation and formation root nodes and fungal roots, above which reduce absorption lead [67]. In a later study, [68] on the interaction of Brevibacillus brevis root bacteria and a fungus Glomus mosseae isolated from contaminated soil with cadmium, the results indicated that besides stimulating plant growth, fungal roots formation (Arbuscular mycorrhizae AM) and their properties are activated. The researchers attributed the increased plant resistance to cadmium to an increase in the availability of elements such as phosphorous and potassium and a decrease in the concentration of many other elements including cadmium, chromium, manganese, copper, molybdenum, iron and nickel in plant tissues. When examining strains of PSB7, PSB1 and PSB10 of bacteria Bacillus isolated from alluvial soil laboratory demonstrated its ability to reducing chromium, dissolving phosphorous, zinc and iron, and stimulating growth the plant made researchers anticipate its contribution to stimulating growth plant in vine-contaminated soil, meaning it may improve plant resistance toxicity to the element [69]. The effect of inoculation was also evaluated basalts of Cr6+ Hexa resist bacteria after isolation from the root zone in soil contaminated with vines namely Pseudomonas sp. PsA4 and Bacillus sp. Ba32 to the Indian cabbage Brassica juncea. The results indicate that these strains stimulate plant growth, the growth different concentrations of chromium in the soil had the highest activation when inoculation with Pseudomonas sp. PsA4 but the two strains did not change the amount of chromium accumulated in the root total. The researchers concluded that these bacteria protect plants from the inhibitory effect of chromium [70]. One of the beneficial contributions to soil composition is that plant root cells secrete mucus materials to facilitate their passage in the soil and mucous materials contain six, five sugars and uronic acid, likewise the microorganism cells at the root level secrete additional mucus material and all of them form a gel that binds soil microorganisms and root microorganisms with root, this combination is sometimes known as “rhizosheath”. This composition is important, as the amount of water absorbed by the plant decreases continuously the water begins to contract the gel material, maintaining the stability of the hydraulic conductivity and at the same time the bonding of the root and microorganisms to it. This ensures that the soil composition is not exposed to erosion and is likely to prevent pathogens and other neighborhoods from reaching the soil root cells [71].
Volatile Compounds
Volatile Organic Compounds (VOCs), characterized by low molecular weight and high vapor pressure, are produced by all organisms as part of normal metabolism, and play important roles in communication within and between organisms. Similar to the Azospirillum system, other systems using NPs have been studied, but to a lesser extent, such as Bacillus subtilis GB03 and B. amyloliquefaciens IN937a, where the results of one study on the promising herb Arabidopsis indicate that these two strains may stimulate plant growth by forming a mixture of volatile substances especially the 2,3-butanediol precursor complex and acetoin initiator, these in turn affect plant metabolism [72]. In a study examined the effects of VOCs released by three species of plant growth-promoting rhizobacteria (Pseudomonas fluorescens, Bacillus subtilis, Azospirillum brasilense) on growth parameters and composition of Essential Oils (EO) in the aromatic plant Mentha piperita [73]. The bacteria and plants were grown on the same Petri dish, but were separated by a physical barrier such that the plants were exposed only to VOCs but not to solutes from the bacteria. Growth parameters of plants exposed to VOCs of P. fluorescens or B. subtilis were significantly higher than those of controls or A. brasilense-treated plants. Production of EOs (monoterpenes) was increased 2-fold in P. fluorescens-treated plants. Two major EOs, (+)pulegone and (−)menthone, showed increased biosynthesis in P. fluorescens-treated plants. Menthol in A. brasilense-treated plants was the only major EO that showed a significant decrease. These findings suggest that VOCs of rhizobacteria, besides inducing biosynthesis of secondary metabolites, affect pathway flux or specific steps of monoterpene metabolism. Bacterial VOCs are a rich source for new natural compounds that may increase crop productivity and EO yield of this economically important plant species [74].
Phytopigments
Plants inoculated with root stimulating bacteria appear fresh, vibrant, and greener than non-inoculated plants, indicating that the photosynthesis system is affected. Some research indicates for example, the inoculation of cabbage Brassica oleracea var. botrytis with biological fertilizers leads to an increase in the total chlorophyll content [75]. Likewise, wheat plants inoculated with Azospirillum and Bacillus increases the total chlorophyll content in the field [76]. The rice plant inoculated with Azospirillum brasilense NO40 isolated from Egyptian soil and the plant grown in China increases its chlorophyll content [77]. In the most comprehensive study of chlorophyll and other phytopigments [78] on wheat seedlings inoculated with A. brasilense Cd. The results showed that there was a significant increase in chlorophyll a, b and the pigments including, β-carotene, violaxanthin, zeaxanthin, antheroxanthin, lutein, and neoxanthin, in the first week after inoculation, either with liquid inoculation or in the form of alginate microbeads.
Control of Pathogens
As for “control”, a general review sums up [79] that under another group of root bacteria stimulating plant growth, the plant is activated in an indirect way, preventing the harmful effect of microorganisms and pathogens on the plant. Other review [29] indicated it is the bacteria in this group that stimulate plant growth indirectly through the formation of compounds harmful to other organisms (bacteria, fungi and viruses) which are not harmful to plants, or limit the availability of some essential elements for these harmful organisms, or plant inductions to increase plant resistance to disease..
Traditionally, methyl bromide is used to sterilize soil to eliminate plant pathogens when planting it, as a result of the devastating effect of this compound on the ozone layer after its volatilization, many people and environmental preservation agencies were interested in that. They demanded to stop its use, and some governments responded and enacted laws limiting its use so that it gradually stopped using, so in the United States of America, the use of this compound was stopped since 2001 [80]. Further claim by reducing the use of chemicals and fertilizers in traditional agricultural practices. The trend was towards finding alternatives to the use of this compound that were not harmful to the environment. So, the best candidate to control this was the group of “root bacteria stimulating plant growth”, a candidate to play the role of sterilizing and reducing material use chemical and fertilizers, from which this group is still a wide field of scientific research. Hence, research in the field of control was so prevalent in the eighties of the twentieth century AD, a tremendous momentum of research and reviews emerged about the ability of “root bacteria stimulant for plant growth” to reduce the number of harmful microorganisms in the rhizospher and thus produce stimulation of plant growth [31] This research in this field resulted in the introduction of a new term, which is “harmful root bacteria”, meaning “Deleterious Rhizobacteria” (DRB), to assume that there are bacteria in the root zone causing damage to the plant (inhibiting its normal growth). Then accordingly, inoculating the plant with “root bacteria stimulating plant growth” will inhibits these bacteria and thus stimulates plant growth.
Such an assumption is not correct until now, as it means that there are strains of “harmful root bacteria” that can be isolated and proven harmful according to the rules of infection the pathogen that is based on the definition of the pathogen as defined by the scientist Koch’s definition. A description of the group has not yet been published symptoms of damage associated with the use of the so-called “harmful root bacteria”, moreover, most of this research was not under competitive environmental conditions or that the inoculation used contains huge numbers of organisms (about the logarithm of 8.7 bacteria to 11.8 bacteria in inoculation medium), this of course does not represent natural soil competition [31] Scientists are beginning to realize the basic mistakes in research on “harmful root bacteria” and this is still the field that is researched, but to a lesser extent, as the number of studies gradually decreased in the 1990s from the twentieth century AD.
Of the microorganisms present in the soil and which propagate it producer of antibiotics [81] Pseudomonas, Streptomyces, Sorangium, Arthrobacter, Nocardia, Burkholderia, Brevibacterium and others. It is the most well-known microorganism characterized by the formation of antagonists the pathogenic of the genus Pseudomonas.sp, which is also considered as organisms associated with plant roots, and can refer to the previous source, which covers primary studies and lists of organisms that have been observed to be antibiotics [81]. One of the first studies that demonstrates direct antagonism and induction of plant resistance to the pathogen and the formation of an antibiotic was shown by a study on root bacteria stimulating plant growth Pseudomonas fluorescens with an antibiotic composition is phenazine, which is against the pathogenic fungus Gaeumannomyces graminis var. tritici, which causes Take-all stem disease of wheat plants [82]. Then research continued to clear the root rhizobacteria in search of bacteria that stimulates growth and has an anti-organism effect the pathogen of the plant. Modern techniques such as the use of microscopy immunofluorescence and reporter genes have contributed in improving field studies of inoculation with the bacterium of the genus Pseudomonas and broadening knowledge about its behavior in this environment [83]. It is noted that surveying a large number of members of the bacterial community around the roots of a plant may lead to obtaining isolates with high capacities that contribute to the benefit of the plant in all stages of its growth and exposure to disease. This is reported by a survey in the survey vegetable chili peppers and tomato susceptible to peptic degeneration disease before and after treatment with a fungus of the genus Pythium sp. and Phytophthora sp. Strains of the bacterium were selected Pseudomonas sp, which is FQP PB-3, FQA PB-3, and GRP (3). It was reported that these strains stimulated growth and reduced the incidence rate to a large extent for both plants [84]. Added to this is a study on lettuce that is exposed to infection by the fungus Fusarium?. sp. 12 isolates of ?Pseudomonas sp. were found has anti-fungal activity, but only three of them activated growth in the plant [83].
Some previous studies reported that cyanide formation is a major and direct agent in antagonism against the Pseudomonas fluorescens, Rhizotrophin strains CHAO, that was also associated with the pathogen of the tobacco plant Thielaviopsis basicola black root rot occurs without the plant being affected [84] and induces the formation of cyanide by increasing the concentration triple iron [85]. A Systemic Acquired Resistance (SAR) develops in the plant after initial infection with a pathogen, i.e., uninfected areas of the plant body those who have been infected will gain greater resistance to the pathogen again and salicylic acid contributes to its pathway [86]. On the other hand, some research has reported that strains of root growth stimulating bacteria induces systemic resistance in the shoot system when the roots are infected with a pathogen, as this has been observed in many types of plants such as beans, cloves, cucumbers and others [87]. Characterized this type of resistance as the rhizobacteria-mediated Induced Systemic Resistance (ISR). A review indicates that this mode of resistance, particularly in the herbivore Arabidopsis thaliana has both jasmonic acid and ethylene play an effective role in the pathway of the excitation signal from the roots to the shoots [86].
Plant Growth Stimulation Mechanism
After mentioning some researches and their results after
inoculation, there are many opinions and proposals to explain the
phenomenon of activation as discussed in some reviews [88,89],
they include:
A. Increase in nitrogen fixation.
B. Production of growth regulators; auxin, gibberellin,
cytokinin and ethylene.
C. Dissolving phosphorus and sulfur oxidation.
D. Increase the availability of nitrates.
E. Production of extracellular antibiotics.
F. Production of hydrolyzed enzymes.
G. Hydrocyanide acid production.
H. Increase root permeability.
I. Tight competition for available nutrients at a site root.
J. Induction of systemic plant resistance.
Another possibility adds another mechanism: change the composition of the bacterial community around the root as a result of pollinator addition. This research reported [90] that when two soil types were used for European alder and inoculation with Bacillus licheniformis the response was different, as in one type of soil the leaf area increased significantly, while the other growth parameters (root growth, for example), the response did not differ, in addition to the disappearance of the inoculation bacterium in the first soil after 6 weeks, while on the other soil after only two weeks. From here it was concluded that the revitalization of growth was a change the bacterial community in or around the root, however, before doing so it is important to consider the composition of the soil and the availability of the necessary elements for the plant. Which may support the role of changing the bacterial community around the roots, other research reported on Quercus ilex ssp. ballota oak seedlings and co-inoculation with rhizobacteria strains plant growth stimulant Bacillus licheniformis CECT 5106 and the fungus Pisolithus tinctorius, which is usually symbiotic with these trees, that although growth was stimulated, the growth of the fungus was inhibited and changed slightly both the total bacterial community around the roots and the potential cultivated bacterial individuals [91].
Despite the abundance of research on this topic on the various physiological and molecular phenomena of these strains root bacteria stimulating plant growth all proposals and opinions to explain this phenomenon, but the general picture is its ability to fix nitrogen when cultivated and when it is associated with the plant, [31]. Before that, some scientists [92] believed that the mechanism of reducing the severity of the disease by root bacteria stimulated the growth of the plant as an effective biological control agent may be the result of a combination of several mechanisms but not standardized with a specific name. After that, I directly put forward the hypothesis that there are several mechanisms that affect each one of them are few, and these mechanisms cooperate with some in influencing addition, and it can be called the “synergy” hypothesis or the ”Additive theory”. In the Encyclopedia of Soils in the Environment [29] several mechanisms were mentioned (about 16 mechanisms) each one could be a mechanism that explains the observation of one to affect after inoculation with one or a mixture of bacteria of the roots or the rhizobacteria or internal bacteria such as nitrogen fixation or the production of growth regulators and others. At the moment there is no general agreement on a specific mechanism, although the production of the growth regulator of Indole Acetic Acid (IAA) may explain what has been observed in the increase in root length, the number of root hairs, root branches and the surface area of the roots of the treated seedlings, but other growth regulators such as gibberellin and nitrogen fixation may also increase productivity and contribute to the availability and uptake of some nutrients by the treated plant [31].
Several autotrophic bacteria have been described and studied nitrogenous and non-symbiotic and tested for use as a bio-fertilizer such as BioGro as mentioned in a previous review [43], for more on this, see also [44]. Likewise, the results and indications of these researches about the groups of bacteria associated with the plant and stimulate its growth were exploited, so that a number of commercial inoculations were available on the market to stimulate the growth of a specific plant (corn, wheat and rice) or a group of crop plants and tree nurseries, Table 2 represents such inoculations, for example, are not limited to. Although there are studies on the use of strains of the genus Pseudomonas in understanding general aspects of the influence mechanism, its use limits its lifetime (within several weeks) [93]. Some field studies indicate after inoculation with the bacterium Azospirillum suggests that 30 to 40% of inoculation operations are unsuccessful and that the increase in yield ranges from 5 to 30% only [29], although this genus is not specific to a specific plant but is rather broad and stimulates the growth of many plants as mentioned in this review. Also, a commercial product has been marketed in Sweden, Cedomon BioAgri AB, Uppsala (Sweden) to provide protection from pathogens carried by barley and oats seeds by treating the seeds with strain inoculation Pseudomonas chlororaphis, which produces the antibiotic phenazine [94].
Table 2:Some examples of commercially available inoculations and effective bacteria [93].

In general, field applications of plant growth stimulating bacteria produce satisfactory benefits only under controlled conditions, but not under conditions and agricultural practices. Hence, it is important to optimize the selection and quality of the inoculation when a specific application is required, because the effectiveness of selection of nitrogen-fixing and stimulating rhizobacteria is crucial to the development of this technique [23]. An example of this is reported by a study on late leaf spot disease of the peanut plant Arachis hypogaea, which uses the fungicide chlorothalonil for its resistance in the field. This study reported that the combination of inoculation with a vaccine containing a strain of the root bacterium Pseudomonas aeruginosa GSE18 and tolerance to the fungicide and a quarter of the fungicide concentration (2000 micrograms/liter) and in a field treatment that doubled the productivity [95].
PGPR Applications
Bacteria with multiple benefits can be advantageous in commercial agriculture and are relevant to the bio-economy. Many cultivated plants of economic significance are grown in monoculture and require amendments for optimal growth and highly yield, as well as protection against disease organisms [96,97].
Increasing yield and decreasing fertilizer inputs
Utilization of bacterial consortia has inconsistent effects on crop yield [98]. It is known that the mixing of a bacterium (B. amyloliquefaciens) with a fungus (Trichoderma virens) improves yields of corn and tomato, among other crops [99,100] and is available in the market place. The company Excalibre-SA (ABM) combines Trichoderma with Bradyrhizobium for improved growth of soybean while BioGrow Endo (Mycorrhizal Applications) combines arbuscular mycorrhizal fungi and Trichoderma for improved growth and treatment of pathogens microorganisms involved in the soil; both of which are commercially available. A consortium of bacteria (Bacillus cereus PX35, Bacillus subtilis SM21, and Serratia sp XY2) reduced the incidence of root knot nematode (Meloidogyne incognita) in tomato, increased fruit yield (31.5 to 39%) and quality (soluble sugars, vitamin C, and titratable acids) [101]. Inoculation with N-fixing bacteria (Azospirillum and Azobacter) allowed halfrate N-fertilizer application and increased sesame seed yield and oil quality [102]. Similar effects were shown for the bacterium Azospirillum vinelandii inoculated Brassica carinata cv. Peela raya [103,104]. On the other hand, biofuels are derived from non-food biomass [105], often lignocellulosic material, to minimize any competition with food production; the long-term goal is provision of renewable fuels, along with high value bio-products, to reduce the atmospheric CO2 emissions associated with fossil fuels [106]. With the use of PGPR that contain natural potential to cope with soil contaminants, the biofuel crops could be used efficiently for phytoremediation and also to reduce high levels of agrochemicals residues in agriculture lands [107]. Conversion of lignocellulosic material to fuel needs to become easier and less expensive to make this biologically fuel economically competitive [108]; in addition to that, there needs to be improved biomass availability from purposegrown biomass crops (e.g., Miscanthus, switchgrass, and Sorghum bicolour) [109,110]. The growth and productivity of selected grown biofuel crops can be improved through inoculation with PGPR [11] as has been demonstrated for switchgrass [111-114]. Marginal and contaminated lands can be used to grow biofuel crops in order to avoid conflicts around the food versus energy crops.
Plant disease control and rationalize agrochemicals consumption
One of the tangible benefit of PGPR is the Improving Disease Control and Reducing the Use of Agrochemicals. Biologicals are an alternative method for combating plant pathogens [115], and there are commercially available examples [116]. Beneficial rhizobacteria may secrete antibiotics and other compounds antagonistic to plant pathogens. Production of antibiotics is one of the more common biocontrol mechanisms [117,118]. There are commercially available examples of biocontrol agents [116]. However, microbes pathogens often develop resistance to the antibiotics and other mechanisms of biocontrol, so that they cannot be fully controlled in the long-term. A holistic approach with multiple controlling methods is probably better than excessive dependency on a single solution when confronting pathogens. Over the long term, pathogen-antagonistic bacteria will also evolve their mode of action to counteract the pathogens. PGPR also produce antibiotics such as lipopeptides, polyketides and antifungal metabolites that suppress pathogens [119].
References
- Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, et al. (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488(7409): 86-90.
- Turner TR, James EK, Poole PS (2013) The plant microbiome. Genome Biol 14(6): 209.
- Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. ISME J 8(4): 790-803.
- Lebeis SL (2014) The potential for give and take in plant-microbiome relationships. Front Plant Sci 5: 287.
- Bulgarelli D, Garrido-Oter R, Munch PC, Weiman A, Droge J, et al. (2015) Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17(3): 392-403.
- Smith DL, Subramanian S, Lamont JR, Bywater-Ekegard M (2015) Signaling in the phytomicrobiome: Breadth and potential. Front Plant Sci 6: 709.
- Smith DL, Gravel V, Yergeau E (2017) Editorial: Signaling in the phytomicrobiome. Front Plant Sci 8: 611.
- Berg G, Rybakova D, Grube M, Koberl M (2016) The plant microbiome explored: Implications for experimental botany. J Exp Bot 67(4): 995-1002.
- Theis KR, Dheilly NM, Klassen JL, Brucker RM, Baines JF, et al. (2016) Getting the hologenome concept right: An eco-evolutionary framework for hosts and their microbiomes. mSystems 1(2): e00028-e00016.
- Berg G, Grube M, Schloter M, Smalla K (2014) Unraveling the plant microbiome: Looking back and future perspectives. Front Microbiol 5: 148.
- Smith DL, Praslickova D, Ilangumaran G (2015) Inter-organismal signaling and management of the phytomicrobiome. Front. Plant Sci 6: 722.
- Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, et al. (2018) Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9: 1473.
- Huisman OC (1982) Interrelations of root growth dynamics to epidemiology of root-invading fungi. Annual Review of Phytopathology 20: 303-327.
- Lynch JM, Whipps JM (1991) Substrate flow in the rhizosphere. In: Keister DL, Cregan B (Eds.), The Rhizosphere and Plant Growth(Beltsville Symposia in Agricultural Research, BSAR Volume 14), Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 15-24.
- Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizosphere. Ecology 84: 827-837.
- Morgan JAW, Bending GD, White PJ (2005) Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exper. Botany 56(417): 1729-1739.
- Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biology and Biochemistry 33(11): 1437-1445.
- Smalla K, Wieland G, Buchner A, Zock A, Parzy J, et al. (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Applied and Environmental Microbiology 67(10): 4742-4751.
- Yang CH, Crowley DE (2000) Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Applied and Environmental Microbiology 66(1): 345-351.
- Yang CH, Crowley DE, Menge JA (2001) 16S rDNA fingerprinting of rhizosphere bacterial communities associated with healthy and Phytophthora infected avocado roots. FEMS Microbiology Ecology 35(2): 129-136.
- Passioura JB (2006) Rhizosphere biology and crop productivity. Australian Journal of Soil Research 44(4): 299-317.
- Cook RJ (2002) Advances in plant health management in the twentieth century. Ann Rev Phytopathol 38: 95-116.
- Barea JM, Pozo MJ, Azcon R, Anguilar CA (2005) Microbial co-operation in the rhizosphere. J Exp Botany 56(417): 1761-1778.
- Germida JJ, Siciliano SD, de Freitas JR, Seib AM (1998) Diversity of root-associated bacteria associated with field-grown canola (Brassica napus ) and wheat (Triticum aestivum). FEMS Microbiol Ecol 26(1): 43-50.
- Rondon MR, Goodman RM, Handelsman J (1999) The earth’s bounty: Assessing and accessing soil microbial diversity. Trends Biotechnol 17(10): 403-409.
- Manefield M, Whiteley AS, Griffiths RI, Bailey MJ (2002) RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Applied and Environmental Microbioloy 68(11): 5367-5373.
- Lueders T, Manefield M, Friedrich MW (2004) Enhanced sensitivity of DNA-and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environmental Microbiology 6(1): 73-78.
- Bloemberg GV, Lugtenberg, BJJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4(4): 343-350.
- Bashan Y, de-Bashan LE (2005) Bacteria/plant growth promotion. Encyclopedia of Soils in the Environment, Elsevier, Oxford, UK, pp. 103-115.
- Kishore GK, Pande S, Podile AR (2005) Phylloplane bacteria increase seedling emergence, growth and yield of field-grown groundnut (Arachis hypogaea L). Letters in Applied Microbiology 40(4): 260-268.
- Kloepper JW (2003) A Review of mechanisms for plant growth promotion by PGPR. 6th International PGPR Workshop, Calicut, India, pp. 81-92.
- Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Angers, France, pp. 879-882.
- Bashan Y, Holguin G (1997) Azospirillum-plant relationships environmental and physiological advances (1990-1996). Can. J Microbiol 43(2): 103-121.
- Vande Broek A, Dobbelaere S, Vanderleyden J, Van Dommelen A (2000) Azospirillum-plant root interactions signaling and metabolic interactions. In: Triplett EW (Ed.), Prokaryotic Nitrogen Fixation: A Model System for the Analysis of a Biological Process. Horizon Scientific Press, Wymondham, UK, pp.761-777.
- Holguin G, Patten CL, Glick BR (1999) Genetics and molecular biology of Azospirillum. Biol Fertil Soils 29: 10-23.
- James EK (2000) Nitrogen fixation in endophytic and associative symbiosis. Field Crops Res 65(2-3): 197-209.
- Dobbelaere S, Croonenborghs A, Thys A, Ptacek A Vanderleyden J, et al. (2001) Responses of agronomically important crops to inoculation with Azospirillum. Aust J Plant Physiol 28(9): 871-879.
- Kennedy IR, Islam N (2001) The current and potential contribution of asymbiotic nitrogen fixation to nitrogen requirements on farms: A review. Aust J Exp Agric 41(3): 447-457.
- Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum plant relationships: physiological, molecular, agricultural, and environmental advances (1997-2003). Canadian Journal of Microbiology 50(8): 521-577.
- Botelho GR, Mendonça-Hagler LC (2006) Fluorescent Pseudomonads associated with the rhizosphere of crops -An Overview. Brazilian Journal of Microbiology 37: 401-416.
- Fallik E, Sarig S, Okon Y (1994) Morphology and physiology of plant roots associated with Azospirillum. In: Okon Y (Ed.), Azospirillum/Plant Associations, CRC Prees, Boca Raton, Florida, USA, pp. 77-85.
- Bashan Y (2000) Environmental application for Azospirillum Proceedings of the 5th International plant growth promoting rhizobacteria conference.
- Al-Whaibi MH (2005) Plants and diazotrophic bacteria. King Saud University Journal (Agriculture Section) 5(2): 60-73.
- Kennedy IR, Choudhury ATM, Kecskes ML (2004) Non-symbiotic bacterial diazotrophs in crop-farming systems: Can their potential for plant growth promotion be better exploited? Soil Biology & Biochemistry 36(8): 1229-1244.
- Seshadri S, Muthukumarasamy R, Lakshminarasimhan C, Ignacimuthu S (2000) Solubilization of inorganic phosphates by Azospirillum halopraeferans. Curr Sci 79(5): 565-567.
- Tripura C, Sashidhar B, Podile AR (2007) Ethyl methanesulfonate mutagenesis-enhanced mineral phosphate solubilization by groundnut-associated Serratia marcescens GPS-5. Curr Microbiol 54(2): 84-79.
- Hernandez ME, Kappler A, Newman DK (2004) Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl Environ Microbiol 70(2): 921-928.
- Lin W, Okon Y, Ralph W, Hardy F (1983) Enhanced mineral uptake by Zea mays and Sorghum bicolor roots Inoculated with Azospirillum brasilense. Applied and Environmental Microbiology 45(6): 1775-1779.
- Barton LL, GV Johnson, SO Miller (1986) The effect of Azospirillum brasilense on iron absorption and translocation by sorghum. J Plant Nutr 9(3-7): 557-565.
- Bashan Y, Harrison SK, Whitmoyer RE (1990) Enhanced growth of wheat and soybean plants inoculated with Azospirillum brasilense is not necessarily due to general enhancement of mineral uptake. Applied and Environmental Microbiology 56(3): 769-775.
- Bashan Y, Levanony H (1991) Alterations in membrane potential and in proton efflux in plant roots induced by Azospirillum brasilense. Plant and Soil 137: 99-103.
- Puente ME, Li CY, Bashan Y (2004) Microbial populations and activities in the rhizoplane of rock-weathering desert plants, II. Growth promotion of cactus seedlings. Plant Biology 6(5): 643-650.
- Puente ME, Bashan Y, Li CY, Lebsky VK (2004) Microbial populations and activities in the rhizoplane of rock weathering desert plants, I. Root colonization and weathering of igneous rocks. Plant Biology 6: 629-642.
- Rodriguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant and Soil 287: 15-21.
- Arshad M, Frankenberger WT (1998) Plant growth promoting substances in the rhizosphere: Microbial production and function. Advances in Agronomy 62: 45-151.
- Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentration by plant growth promoting bacteria. J Theor Biol 190(1): 63-68.
- Hall JA, Peirson D, Ghosh S, Glick BR (1996) Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Israel Journal of Plant Sciences 49(1): 37-42.
- Pallai R (2005) Effect of plant growth-promoting-rhizobacteria on canola (Brassica napus. L) and lentel (Lens culinaris. Medik) plants. Master of Science, Department of Applied, Microbiology and Food Science, University of Saskatchewan, Saskatoon, Saskatchewan.
- Aslantas R ,Cakmakci R, Sahin F (2007) Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Scientia Horticulturae 111(4): 371-377.
- Freitas SS, Aguilar Vildoso CI (2004) Rhizobacteria and growth promotion of citrus plants. Rev Bras Ciênc Solo 28(6): 987-994.
- Khalid A, Arshad M, Zahir, ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. Journal of Applied Microbiology 96(3): 473-480.
- Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Letters in Applied. Microbiology 42(2): 155-159.
- Bai Y, Zhou X, Smith DL (2003) Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci 43(5): 1774-1781.
- Lucas Garcia JA, Probanza A, Ramos B, Barriuso J, Gutierrez Mañero FJ (2004) Effects of inoculation with Plant Growth Promoting Rhizobacteria (PGPRs) and Sinorhizobium fredii on biological nitrogen fixation, nodulation and growth of Glycine max Osumi. Plant and Soil 267: 143-153.
- Marulanda A, Barea J, Azcon R (2006) An indigenous drought-tolerant strain of glomus intraradices associated with a native bacterium improves water transport and root development in retama sphaerocarpa. Microbial Ecology 52(4): 670-678.
- Alami Y, Achouak W, Marol C, Heulin T (2000) Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl Environ Microbiol 66(8): 3393-3398.
- Vivas A, Azcon R, Biro B, Barea JM, Ruiz-Lozano JM (2003) Influence of bacterial strains isolated from lead-polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pratense under lead toxicity. Can. J Microbiol 49(10): 577-588.
- Vivas A, Barea JM, Azcon R (2005) Interactive effect of Brevibacillus brevis and Glomus mosseae, both isolated from Cd contaminated soil, on plant growth, physiological mycorrhizal fungal characteristics and soil enzymatic activities in Cd polluted soil. Environmental Pollution 134(2): 257-266.
- Wani PA, Khan MS, Zaidi A (2007) Chromium reduction plant growth-promoting potentials, and metal solubilizatrion by Bacillus sp. isolated from alluvial soil. Current Microbiology 54(3): 237-243.
- Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62(5): 741-748.
- Czarnes S, Hallett PD, Bengough AG, Young IM (2000) Root- and microbial-derived mucilages affect soil structure and water transport. European Journal of Soil Science 51(3): 435-443.
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei H-X, et al. (2003) Bacterial volatiles promote growth in Arabidopsis. PNAS 100(8): 4927-4932.
- Santoro M, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiology and Biochemistry 49(10): 1177-1182.
- Cappellari LR, Chiappero J, Palermo TB, Giordano W, Banchio E (2020) Volatile organic compounds from rhizobacteria increase the biosynthesis of secondary metabolites and improve the antioxidant status in Mentha piperita grown under salt stress. Agronomy 10(8): 1094.
- Bambal AS, Verma RM, Panchbhai DM, Mahorkar VK, Khankhane RN (1998) Effect of biofertilizers and nitrogen levels on growth and yield of cauliflower (Brassica oleracea var. botrytis). Orissa J Hort 26(2): 14-17.
- Panwar JDS, Singh O (2000) Response of Azospirillum and Bacillus on growth and yield of wheat under field conditions. Indian J Plant Physiol 5: 108-110.
- Omar MNA, Fang P, Jia XM (2000) Effect of inoculation with Azospirillum brasilense NO40 isolated from Egyptian soils on rice growth in China. Egypt J Agric Res 78(3): 1005-1014.
- Bashan Y, Bustillos JJ, Leyva LA, Hernandez JP, Bacilio M (2006) Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol Fertil Soils 42: 279-285.
- Glick BR, Bashan Y (1997) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnology Advances 15(2): 353-378.
- Al-Whaibi MH (2008) Plant growth promoting rhizobacteria. Saudi Journal of Biological Sciences 15(3): 3-16.
- Madigan MT, Martinko JM, Parker J (2000) Brock biology of microorganisms. (8th edn), Prentice-Hall Inc, Englewood Cliffs, New Jersey, USA.
- Thomashow LS, Weller DM (1998) Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. Journal of Bacteriology 170(8): 3499-3508.
- Sottero AN, Freitas S, Melo AMT, Trani PE (2006) Rhizobacteria and lettuce: Root colonization, plant growth, promotion and biological control. Rev Bras Ciênc Solo 30(2): 225-234.
- Sharma A, Wray V, Johri BN (2007) Rhizosphere Pseudomonas sp strains reduce occurrence of pre- and post-emergence damping-off in Chile and tomato in central Himalayan region. Archives of Microbiology 187(4): 321-335.
- Weisbeek PJ, Gerrits H (1999) Iron and biocontrol. In: Stacey G, Keen NT (Eds.), Plant-Microbe Interactions, APS Press, St. Paul, Minnesota, USA, pp. 217-250.
- Pieterse CMJ, Van Wees SCM, Ton J, Van Pelt JA, Van Loon LC (2002) Signalling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biol 4: 535-544.
- Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology 36: 453-483.
- Chanway CP (1997) Inoculation of tree roots with plant growth promoting soil bacteria: An emerging technology for reforestation. For Sci 43(1): 99-112.
- Kloepper JW(1993) Plant growth-promoting rhizobacteria as biological control agents. In: Metting B (Ed.), Soil Microbial Ecology: Applications in Agricultural and Environmental Management, Marcel Dekker, New York, USA, pp. 255-274.
- Ramos B, García JAL, Probanza A, Barrientos ML, Gutiérrez Mañero FJ (2003) Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis. Environmental and Experimental Botany 49(1): 61-68.
- Domenech J, Ramos-Solano B, Probanza A, Lucas-Garcia JA, Colon JJ, et al. (2004) Bacillus and Pisolithus tinctorius effects on Quercus ilex ssp. ballota a study on tree growth, rhizosphere community structure and mycorrhizal infection. Forest Ecology and Management 194(1-3): 293-303.
- Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. Journal of Experimental Botany 52(Suppl 1): 487-511.
- Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology 3(4): 307-319.
- Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ (2003) Phenazines and their role in biocontrol by Pseudomonas New Phytologist 157(3): 503-523.
- Kishore GK, Pande S, Podile AR (2005) Management of late leaf spot of groundnut (Arachis hypogaea) with chlorothalonil-tolerant isolates of Pseudomonas aeruginosa. Plant Pathology 54(3): 401-408.
- Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21(5): 573.
- Andreote FD, Pereira ESMC (2001) Microbial communities associated with plants: Learning from nature to apply it in agriculture. Curr Opin Microbiol 37: 29-34.
- Backer RGM, Saeed W, Seguin P, Smith DL (2017) Root traits and nitrogen fertilizer recovery efficiency of corn grown in biochar-amended soil under greenhouse conditions. Plant Soil 415: 465-477.
- Akladious SA, Abbas SM (2012) Application of Trichoderma harziunum T22 as a biofertilizer supporting maize growth. Afr J Biotechnol 11(35): 8672-8683.
- Molla AH, Haque MM, Haque MA, Ilias G (2012) Trichoderma-enriched biofertilizer enhances production and nutritional quality of tomato (Lycopersicon esculentum mill.) and minimizes NPK fertilizer use. Agric Res 1: 265-272.
- Niu DD, Zheng Y, Zheng L, Jiang CH, Zhou DM, et al. (2016) Application of PSX biocontrol preparation confers root-knot nematode management and increased fruit quality in tomato under field conditions. Biocontrol Sci. Technol 26(2): 174-180.
- Shakeri E, Modarres-Sanavy SAM, Amini Dehaghi M, Tabatabaei SA, Moradi-Ghahderijani M (2016) Improvement of yield, yield components and oil quality in sesame (Sesamum indicum) by N-fixing bacteria fertilizers and urea. Arch Agron Soil Sci 62(4): 547-560.
- Nosheen A, Bano A, Ullah F (2016) Bioinoculants: A sustainable approach to maximize the yield of Ethiopian mustard (Brassica carinata) under low input of chemical fertilizers. Toxicol Ind Health 32(2): 270-277.
- Nosheen A, Bano A, Yasmin H, Keyani R, Habib R, et al. (2016) Protein quantity and quality of safflower seed improved by NP fertilizer and Rhizobacteria (Azospirillum and Azotobacter). Front Plant Sci 7: 104.
- Ajjawi I, Verruto J, Aqui M, Soriaga LB, Coppersmith J, et al. (2017) Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat Biotechnol 35(7): 647-652.
- Rokem JS, Greenblatt CL (2015) Making biofuels competitive: The limitations of biology for fuel production. JSM Microbiol 3(2): 1023.
- Evangelou MW, Deram A (2014) Phytomanagement: A realistic approach to soil remediating phytotechnologies with new challenges for plant science. Int J Plant Biol Res 2: 1023.
- Kuhad RC, Gupta R, Singh A (2011)Microbial cellulases and their industrial applications. Enzyme Res 2011: 280696.
- Margaritopoulou T, Roka L, Alexopoulou E, Christou M, Rigas S, et al. (2016) Biotechnology towards energy crops. Mol Biotechnol 58(3): 149-158.
- McCalmont JP, Hastings A, Mcnamara NP, Richter GM, Robson P, et al. (2017) Environmental costs and benefits of growing Miscanthus for bioenergy in the UK. Glob Chang Biol Bioenergy 9(3): 489-507.
- Ker K, Seguin P, Driscoll BT, Fyles JW, Smith DL (2012) Switchgrass establishment and seeding year production can be improved by inoculation with rhizosphere endophytes. Biomass Bioenergy 47: 295-301.
- Ker K, Seguin P, Driscoll BT, Fyles JW, Smith DL (2014) Evidence for enhanced N availability during switchgrass establishment and seeding year production following inoculation with rhizosphere endophytes. Arch Agron Soil Sci 60(11): 1553-1563.
- Shanta N, Schwinghamer T, Backer R, Allaire SE, Teshler I, Vanasse A, et al. (2016) Biochar and plant growth promoting rhizobacteria effects on switchgrass (Panicum virgatum Cave-in-rock) for biomass production in southern Québec depend on soil type and location. Biomass Bioenergy 95: 167-173.
- Arunachalam S, Schwinghamer T, Dutilleul P, Smith DL (2017) Multi-year effects of biochar, lipo-chitooligosaccharide, thuricin 17, and experimental bio-fertilizer for switchgrass. Agron J 110(1): 77-84.
- Harman GE (2000) Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis 84: 377-393.
- Velivelli SL, De Vos P, Kromann P, Declerck S, Prestwich BD (2014) Biological control agents: From field to market, problems, and challenges. Trends Biotechnol 32(10): 493-496.
- Doumbou CL, Salove MKH, Crawford DL, Beaulieu C (2001) Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection 82(3): 85-102.
- Company S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9): 4951-4959.
- Prashar P, Kapoor N, Sachdeva S (2013) Biocontrol of plant pathogens using plant growth promoting bacteria. In: Lichtfouse E (Ed.), Sustainable Agriculture Reviews, Springer, Dordrecht, Netherlands, pp. 319-360.
© 2023 © Abdullah Msaad Al-Falih. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






