- Submissions

Full Text
Environmental Analysis & Ecology Studies
Microclimate Variability Under Forest Canopies Along an Altitudinal Gradient in Western Himalaya
Pradeep Singh1* and GCS Negi2
1GB Pant National Institute of Himalayan Environment, India
2Retd Scientist-G, Forest Ecology, GB Pant National Institute of Himalayan Environment, India
*Corresponding author:Pradeep Singh, GB Pant National Institute of Himalayan Environment, India
Submission: August 31, 2023; Published: September 20, 2023

ISSN 2578-0336 Volume11 Issue3
Abstract
It is well known that forest microclimates contrast strongly with the climate outside forests. We studied microclimate (air temperature, air humidity, soil temperature and soil moisture) beneath the forest canopies of four dominant forest types along an altitudinal gradient (300-2200masl) in Central Himalaya. Microclimatic conditions across the under canopy of the four forests varied significantly. In the study area May was the warmest month (mean across the under canopy of four study sites=29.1 °C) and January was the coldest month (mean=11.0 °C), with mean annual temperature of 22.9 °C. The under-canopy temperature was found to be about 4 °C lower than the mean atmospheric temperature (27.0 °C) reported in the study area. With respect to air temperature the south aspects were slightly warmer than the north aspects. The mean annual soil temperature (18.0 °C) was found to be about 5 °C lower than the mean annual air temperature (22.9 °C) across the forest under canopies. The RH was recorded in a narrow range of 72-75% across the forests that decreased with altitude significantly. The N aspects recorded markedly higher RH than the S aspects. The soil moisture was recorded maximum during rainy season (mean=16.4%) and minimum during summer (mean=11.7%). Both air temperature, soil temperature and RH decreased significantly with increasing altitude. ANOVA indicates that both air temperature (p<0.026) and soil temperature (p<0.001) were significantly different across the forests and sampling months. These existed a significant negative relationship between air temperature and altitude (r=0.996; p<0.004) and between relative humidity and altitude (r=-0.993; p<0.007). The soil temperature and altitude were negatively correlated (r=0.975; p<0.025). The dampening of air temperature, higher humidity and soil moisture under the canopy of these forests has several advantages to the plants and the fauna dependent upon them. These forests with distinct microclimate may act as a micro-refugia to provide congenial habitat and conserve rich biodiversity in the face of global warming in this region.
Keywords:Forest microclimate; Altitudinal gradient; Global warming; Biodiversity; Microrefugia; Central himalayan forests
Introduction
Microclimate is the suite of climatic conditions measured in localized areas near the earth’s surface [1]. Microclimatic variables, which include temperature, light, wind speed and moisture, provide meaningful indicators for habitat selection and other activities in forest management. In forests, the tree canopy functions as a thermal insulator and buffers sub-canopy micro-climatic conditions, thereby affecting biological and ecological processes [2]. Forest ecosystems have a distinct below-canopy microclimate, regulated by diverse bio-physical processes, and of eminent importance to the growth and survival of understory vegetation and seedlings. The below canopy microclimate may substantially differ from comparable open areas [3]. The importance of microclimate in influencing ecological processes such as plant regeneration and growth, soil respiration, nutrient cycling, and wildlife habitat selection has now become an essential component of current ecological research [4]. Plants respond to these microclimatic conditions differently that affects their growth and development. The physiological and ecological importance of forest microclimates has long been recognized [3,5]. It is well known that forest microclimates contrast strongly with the climate outside forests. Below forest canopies, direct sunlight and wind speed are strongly reduced, leading to a dampening of temperature and humidity [6]. Temperature extremes are often strongly buffered in forests compared to open habitats, with cooler below-canopy maximum temperatures, warmer minimum temperatures, and lower seasonal and inter-annual variability [7-9]. Microclimate is known to shape (i) species distributions, (ii) species interactions, and (iii) ecosystem functioning in forest ecosystems. Spatial and temporal variation in forest microclimates results from an interplay of forest features, local water balance, topography and landscape composition. Forest organisms living below or within tree canopies experience distinct climatic conditions that deviate considerably from the climate outside forests [3,6,9].
The role and importance of microclimate vary widely among forests over time and under different weather conditions. Microclimatic variables, particularly solar radiation, air temperature at the ground surface, and soil temperature, are highly sensitive to changes in the over storey canopy and exhibit relatively high spatial and temporal variability within a forest [10-11]. Differences in insolation period and solar intensity change with aspect, thereby forming a range of microclimates in forested landscapes [12]. It holds particularly true for the Himalayan mountains where the topography varies dramatically from one place to another within a landscape [13,14]. Pook and Costin [15] reported that opposing slopes vary in their microclimate, light intensity, soil and air temperature, humidity, soil moisture and evaporation, and duration of growing periods, and these differences are closely associated with differences in vegetation composition and structure. Forest microclimates can determine the distribution of individuals, populations, and species. Due to the increasing impacts of current macroclimate warming on biodiversity, studies on forest microclimates are receiving much attention in global change biology [16-17]. It has been emphasized that to fully understand and better predict how forests biodiversity and functions relate to climate and climate change, microclimates need to be integrated into ecological research [16]. Major ecological processes, such as production, mineralization, and the spread of diseases, insects, and natural disturbances (e.g., fire), are controlled directly or have been related empirically to microclimatic conditions [4,18]. Manipulating microclimate by altering the structural environment can thus be a useful tool in both wildlife and ecosystem conservation. The poor understanding of forest microclimate contrasts with its high ecological relevance for many forest ecosystem processes [19] such as survival and growth of young tree seedlings, seed germination of annuals, buffering against frost etc. [20]. Microclimatic information is vital for empirical field studies, theoretical modelling exercises, and management decision-making. In Himalayan forestry research less, attention has been given to variability in microclimate or to the differences among microclimatic patterns across spatial and temporal scales. In this article, we present microclimatic characteristics associated with the dominant forest ecosystems (those differ both structurally and functionally) of Central Himalaya occurring along an altitudinal gradient in Central Himalaya. We hypothesized that the four forest types differing with regard to their physiognomy, growth cycle, topography etc. should have distinct variations with regards to the under-canopy environment.
Materials and Methods
Study area description
Figure 1:Monthly variations in microclimatic condition across the four study sites along an altitudinal gradient..
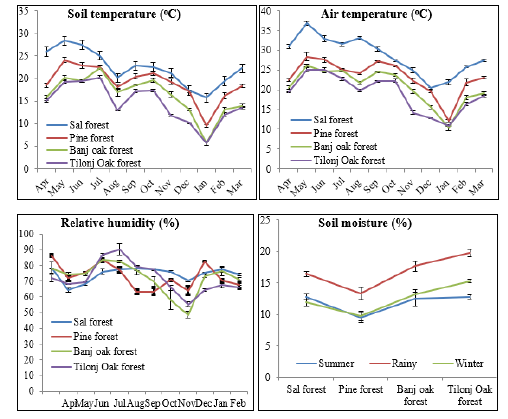
The study area is located between 29o 17’ N latitudes and 79o 26’ E longitudes along an altitudinal transect of 300-2200m altitude in Nainital district, Uttarakhand (Central Himalaya) (Figure 1 & Table 1). Altitudinally, Nainital district is located in temperate zone, although latitudinally it falls within the sub-tropical belt. Because of sharp rise in altitude, the temperature falls within the range reported for temperate zone; but because of relatively lower latitude, day length in winters is not as short as in typical temperate zone of the globe. In general, the mean atmospheric temperature falls by 3.7 °C with a rise of 1000m altitude in this region, which is more rapid in higher altitudes [21]. In addition to difference in day length, the influence of monsoon rainfall pattern renders the climate of this region different from that of temperate zone. In typical temperate climate the latter half of summer (July-August) is generally dry [22], while it is the most-wet part of the year in the present study area. In this region long-term (1980-2012) dataset of temperature and rainfall indicated linear trends for both maximum and minimum temperature, which were found to be significantly (P<0.05) increasing (0.01 °C and 0.02 °C /decade), respectively, and rainfall was found to be significantly (P<0.05) increasing during this period. Mean daily sunshine hours in the mid altitude belt of the study area varied seasonally and gradually increased from 2-3.3hrs in rainy season, 5.6-7.8hrs in winter season and 8-9hrs in summer season [23].
Table 1:Location of the study sites and dominant tree species in the forests along an altitudinal gradient in western Himalaya.
Forest vegetation and dominant tree species
This region is broadly divided into three ecological zones, namely the subtropical foothill zone (the low laying land <800m asl; with high water table), the temperate forest zone (extending between the foothill zone and timber line around 2800masl.), and alpine and sub alpine zone (above the timber line with bushes, grasses and herbs as the main vegetation) [24-25]. In general, from lower to higher elevations the following forests prevail: Sal (Shorea robusta) forests below 1000m; Chir Pine (Pinus roxburghii) forest between 1000 and 1600m and; Banj Oak (Quercus leucotrichophora) forests between 1500-2200m asl. The higher limit of this altitudinal zone is represented by forests of tree species such as Tilonj Oak (Q. floribunda), Kharsu Oak (Q. semecarpifolia), Blue Pine (Pinus wallichiana), Deodar (Cedrus deodara) etc.
Micro-climatic data collection
In the above mentioned four dominant forest types across the elevational gradient of 300-2200m altitude intact and undisturbed stands of 1ha area were selected for micro-climatic data collection. Micro-climatic data of each of the selected forest stands (both at N and S aspects) was recorded at monthly intervals. On each visit to the study sites of about one week every month micro-climatic data was recorded within the marked forest stands (beneath the forest canopy) three times a day from morning till evening (10am-5pm). Micro-climatic data (air temperature and relative humidity, RH) were collected with the help of Pocket Weather Meter (Kestrel 4000 NV) and soil temperature was determined using a soil thermometer upto 30cm soil depth. To determine the soil moisture about 10g fresh composite soil sample (in triplicate) was collected from 30cm soil depth, dried in an oven at 103 ˚C for 24hrs or till constant weight and weighed. The moisture content was calculated on oven dry weight basis following [26]:
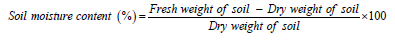
Data was treated statistically (Statistica 8.0) for linear regression between microclimatic parameters and altitude of the forest sites, and ANOVA between microclimatic parameters across the aspects (N and S), altitude and months [27]. All the data for various study parameters given in the results section is mean across two growth cycles of trees and forests (April 2014-March 2015 and April 2015-March 2016).
Results
In the low altitude Sal forests, the mean annual air temperature (˚C) across the two study years (2014-15 and 2015-16; unless referred otherwise) was recorded 28.6oC (Table 2). It was 1 ˚C higher at S aspect (29.1±2.8) as compared to N aspect (28.1±2.2). The peak value was recorded in May, and it declined to a minimum in December, and slightly increased thereafter (Figure 1). Mean annual soil temperature (30cm depth) followed a similar trend as recorded for atmospheric temperature. It was recorded 22.9 ˚C at S aspect and 21.8 ˚C at N aspect. Peak value of soil temperature was recorded during summer (May-June) that declined until January (Figure 1). The mean value of RH was recorded 74.5% that was marginally higher at N aspect (75.6±7.8) as compared to 73.4±7.8 at S aspect (Figure 1). Across the annual cycle the RH was recorded maximum during rainy season and minimum during summer. Mean annual soil moisture was recorded 13.7%; it was marginally lower at S aspect (13.2%) than at the N aspect (14.2%). Soil moisture was found to be maximum during rainy season and minimum during winter season (Table 3). In the mid altitude Pine forests, the mean annual air temperature was recorded 23.1 ˚C (Table 2). It was slightly higher at S aspect (23.8±3.1) as compared to N aspect (22.4±3.5). The peak value of atmospheric temperature was recorded in May that declined to a minimum in December, and slightly increased thereafter (Figure 1). Mean annual soil temperature (19.0oC) followed a similar trend as recorded for atmospheric temperature. It was recorded 19.5 ˚C at S aspect and 18.5 ˚C at N aspect. Peak value of soil temperature was recorded during summer that declined until January (Figure 1). The mean value of relative humidity was recorded 73.0%; it was (73.3±8.1) at N aspect as compared to 72.7±8.5 at S aspect (Figure 1). The mean annual soil moisture was recorded at 10.9%. It was slightly lower (10.0%) at S aspect than at the N aspect (11.7%). It was found maximum during rainy season and minimum during summer season (Figure 1). In the mid-altitude Oak forests, the mean value of air temperature was recorded 20.7 ˚C, which was slightly higher at S aspect (21.1±1.9) as compared to N aspect (20.3±2.8) (Table 2). The peak air temperature was recorded in May, and then it declined to a minimum in December, and slightly increased thereafter (Figure 1). Mean annual soil temperature (16.2 ˚C) exhibited a similar trend as recorded for atmospheric temperature. It was recorded 16.7 ˚C at S aspect and 15.8 ˚C at N aspect. Peak value of soil temperature was recorded during summer that declined until January and increased thereafter (Figure 1). The mean value of RH (72.3%) was higher at N aspect (73.0±8.8) as compared to 71.5±9.5 at S aspect (Figure 1). Mean annual soil moisture was recorded 14.5%. It was found 13.5% at S aspect and 15.4% at N aspect. Soil moisture was recorded at maximum during rainy season and minimum during summer season (Figure 1).
Table 2:Various micro-climate parameters recorded under the canopy of four forests in Nainital district, Central Himalaya.
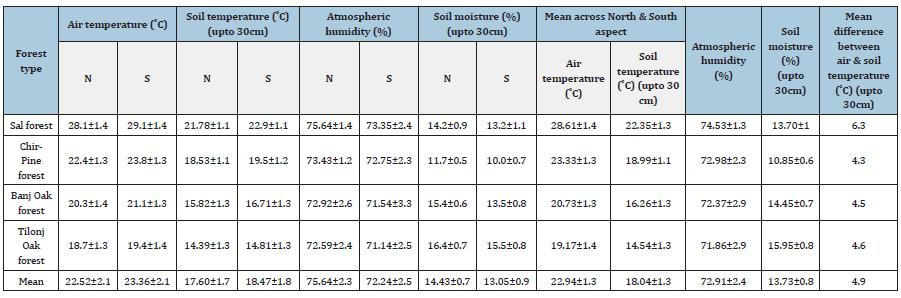
Table 3:Statistical relationship between microclimate and altitudinal gradient of the trees in Western Himalaya.
In the high-altitude Tilonj Oak forests the mean value of air temperature (19.2 ˚C) was slightly higher at S aspect (19.4±2.9) as compared to N aspect (18.7±3.0) (Table 2). The peak value was recorded in May, and then it declined to a minimum in December and slightly increased thereafter (Figure 1). Mean annual soil temperature (14.6 ˚C) followed a similar trend as recorded for air temperature. It was recorded 14.8 ˚C at S aspect and 14.3 ˚C at N aspect. Peak value of soil temperature was recorded during summer that declined until January (Figure 1). The mean value of RH (71.9%) was higher at N aspect (72.6±8.8) as compared to 71.1±6.5 at S aspect (Figure 1). Mean annual soil moisture (14.5%) was slightly lower (15.5%) at S aspect than at the N aspect (16.4%). It was found maximum during rainy season and minimum during summer season (Figure 1).
Discussion
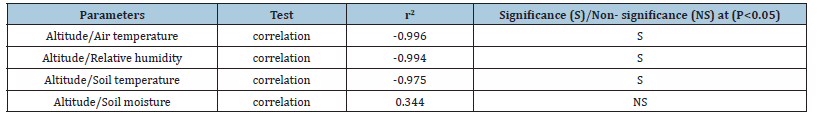
Forest canopies buffer climate extremes and promote microclimates that may function as refugia for understory species under changing climate [28]. It is well known that tree canopies serve to buffer understory environments from climate extremes and can produce moderating effect on regional warming [6,29]. This buffering may promote microclimate that function as microrefugia, locations that provide favourable local climatic conditions amidst unfavorable regional conditions [30]. It has been suggested that forest canopies, in combination with topography, can create conditions that are decoupled from regional warming [31]. However, the buffering capacity of forests is poorly understood as most studies have taken meteorological data from local stations that provide in overall climatic condition of the region [21]. In this study the microclimatic conditions across the four forests varied significantly. The air temperature decreased significantly with increasing altitude (P<0.001) (Table 3 & Figure 2). In the study area May was the warmest month (mean across the four study sites=29.1 ˚C) and January was the coldest month (mean=11.0 ˚C). The only noticeable trend was found in case of Sal forest (300m asl) in which minimum temperature was recorded during December, and for rest of the three forest sites located towards higher altitudes it was recorded during January. The difference between mean annual temperature at the site lowest and highest altitude was 9.1 ˚C. With respect to air temperature the S aspects were slightly warmer than the N aspects. Heasen et al. [2] across European forests reported that sub-canopy air temperatures differ substantially from free air temperatures, being on average 2.1±1.6 ˚C lower in summer and 2.0±0.7 ˚C higher in winter. In our study area this difference between outside and inside the canopy was about 4 ˚C lower from free air temperature (27 ˚C) reported by [32].
Figure 2:Altitudinal pattern of air temperature and relative humidity of central Himalaya.
Forests function as a thermal insulator, cooling the understory when ambient temperatures are hot, and warming the understory when ambient temperatures are cold [9] that has a cooling effect on soil. The mean annual soil temperature we found was about 5 ˚C lower than the mean annual air temperature within forest canopies (Table 2). This difference (4-5 ˚C) was almost similar across the study sites except for the low altitude Sal Forest, where it was recorded 6.3 ˚C. Similar to the air temperature, soil temperature also declined with altitude significantly (p<0.01) (Table 3 & Figure 2). Here again, the difference in mean soil temperature between the low and high-altitude sites was 7.8 ˚C. In a similar study in the old-growth Douglas-fir forests, air temperature (maximumminimum) varied by 2.7 °C along a 200m transect in southern Washington, whereas soil temperature varied by 5.9 °C [11]. There existed a significant negative relationship between air temperature and altitude (r=0.996; p<0.004), relative humidity and altitude (r= -0.993; p<0.007), and soil temperature and altitude (r=0.975; p<0.025). In our study area the mean annual RH was recorded in a narrow range of 72-75% across the forests that was found decreasing significantly (p<0.01) with increasing altitude (Table 3). Interestingly, the RH was recorded maximum during April for Sal and Pine forests, and during July-August for the high altitude Banj Oak and Tilonj Oak forests (Figure 1).
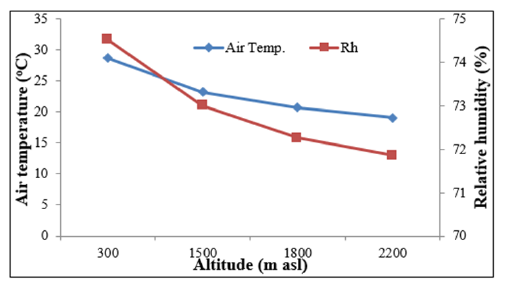
The minimum RH was recorded during December for most of the forests. As the RH is a function of rain fall and temperature it was recorded minimum in May in Sal Forest and during November and December in the Oak forests. Maximum RH was recorded during August in all the forests, except for April in Pine Forest. The N aspects recorded markedly higher RH than the S aspects for all the study sites round the year. Across the seasons and aspects of the forests the soil moisture was recorded maximum during rainy season (mean=16.4%) and minimum during summer (mean=11.7%). Both air temperature, soil temperature and RH decreased significantly with increasing altitude (Table 3 & Figure 2). Soil moisture, however, did not show a definite trend with altitude as in the Pine forest it was recorded lowest (10.9%) across all the forests, which experienced severe forest fire during the study years (Table 2 & Figure 2). In general, the soil moisture was recorded greater in the high-altitude Oak forests (Table 2). The mean soil moisture was found slightly greater at N aspect (14.4%) than at the S aspect (13.1%). The greater soil moisture in Oak forests may be due to the evergreen dense and multi-layer canopy of that permits low sun light to the forest floor hence lower evaporative loss of soil water21. ANOVA indicates that both air temperature (p<0.026) and soil temperature (p<0.001) were significantly different across the forests and sampling months (Table 3). Soil moisture was also found significantly different across the forest sites and sampling months (p<0.05) (Table 3).
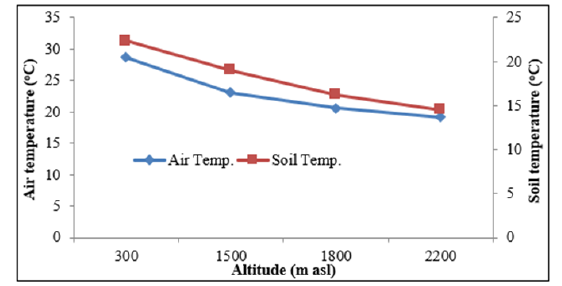
Physico-chemical properties of forest soils vary in space and time because of variation in topography, climate, weathering process, vegetation cover and microbial activities [33] and several other biotic and abiotic factors. Oak forests having closed canopy [34] are found conducive for soil water storage compared to Pine forests [35]. Joshi and Negi [36] reported significantly greater (p<0.001) soil moisture in Oak forests (range=15.0-33.0%) compared to Pine forests (range=7.0-17.0%) of this region during both winter and rainy seasons. It has been pointed out that deep soil rich in organic matter and detritus layer in the Oak forests might have resulted into higher water retention capacity [37] despite almost equal litter fall in the Oak (5.8 t ha-1 yr-1) and Pine forests (6.5 t ha-1 yr-1) [38]. Role of temperature and sunshine duration on phenological responses of plants is extensively reported [39-40]. In this study the Sal forests located at lower altitude exhibited earlier leafing and rapid leaf expansion due to early rise in air and soil temperature [41] (Figure 3). For example, in April 2014 when leafing occurred in S. robusta the mean monthly temperature was (31.3 °C) at N aspect, and (33.2 °C) at S aspect, as compared to (22.8 °C) at N aspect, and (23.3 °C) at the high altitude forest sites. Also, soil temperature in April 2014 was recorded distinctly higher at lower altitude than at the higher altitudes (28.2 °C vs. 18.9 °C). Soil temperature is known to affect leaf expansion through its influence on rates of water and mineral absorption by roots and through its effect on leaf temperature [42-43]. Rai et al. [44] reported that in Betula utilis the greater leaf area was associated with higher soil temperature. The rising air temperature during spring through summer resulted in full leaf expansion well before the onset of rainy season to utilize the favourable warm and wet rainy season, when the environment is predictable but more competitive [21] (Figure 4).
Figure 3:Altitudinal pattern of soil temperature and soil moisture (upto 30cm soil depth) of central Himalaya.
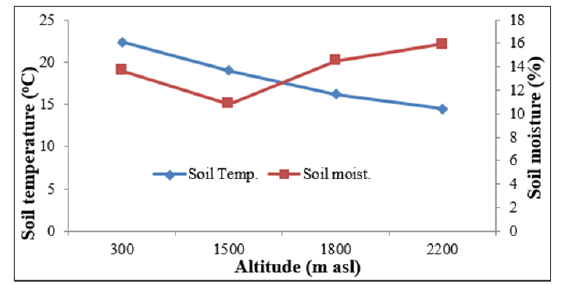
Figure 4:Altitudinal pattern of air temperature and soil temperature (upto 30cm soil depth) of central Himalaya.
To conclude, the dampening of air temperature, higher humidity and soil moisture inside these forest canopies has several advantages to the plants and the fauna dependent upon them. These forests support over 220 species of plants (trees, shrubs and herbs) and many of them are economically important such as medicinal and aromatic plants and wild edibles. These forests with distinct microclimate also keep away the obnoxious weeds such as Lantana camara, Eupatorium spp., Parthenium spp. etc. those are found quite extensively outside these forests [45]. Thus, these forests provide congenial air temperature, soil moisture and humidity and contribute to biodiversity conservation in the face of global warming in this region.
Acknowledgement
Thanks to Director, GBPNIHE, Kosi-Katarmal, Almora for providing facilities.
References
- Geiger R (1965) The climate near the ground. Harvard University Press, Cambridge, Massachusetts, USA.
- Haesen S, Lembrechts JJ, De Frenne P, Lenoir J, Aalto J, et al. (2021) Forest temp-sub‐canopy microclimate temperatures of European forests. Glob Change Biol 27(23): 6307-6319.
- Geiger R, Aron RH, Todhunter P (2009) The climate near the ground. Rowman & Littlefield, Washington DC, USA.
- Perry DA, Oren R, Hart SC, (1994) Forest ecosystems. The Johns Hopkins University Press, Baltimore, Maryland, USA.
- Grubb PJ (1977) The maintenance of species‐richness in plant communities: The importance of the regeneration niche. Biol Rev 52(1): 107-145.
- Chen JM, Liu J, Cihlar J, Goulden ML (1999) Daily canopy photosynthesis model through temporal and spatial scaling for remote sensing applications. Ecol Model 124(2-3): 99-119.
- Ewers RM, Banks-Leite C (2013) Fragmentation impairs the microclimate buffering effect of tropical forests. PLOS one 8(3): e58093.
- Von Arx G, Graf Pannatier E, Thimonier A, Rebetez M (2013) Microclimate in forests with varying leaf area index and soil moisture: Potential implications for seedling establishment in a changing climate. J Ecol 101(5): 1201-1213.
- De Frenne P, Zellweger F, Rodríguez-Sánchez F, Scheffers BR, Hylander K, et al. (2019) Global buffering of temperatures under forest canopies. Nat Ecol Evol 3(5): 744-749.
- Reifsnyder WE, Furnival GM, Horowitz JL (1971) Spatial and temporal distribution of solar radiation beneath forest canopies. J Agric Meteorol 9: 21-37.
- Chen J, Franklin JF, (1997) Growing-season microclimate variability within an old-growth Douglas-fir forest. Clim Res 8(1): 21-34.
- Holland PG, Steyn DG (1975) Vegetational responses to latitudinal variations in slope angle and aspect. J Biogeogr 2(3): 179-183.
- Ghimire B, Rogan J, Miller J (2010) Contextual land-cover classification: Incorporating spatial dependence in land-cover classification models using random forests and the getis statistic. Remote Sens Lett 1: 45-54.
- Paudel S, Vetaas OR (2014) Effects of topography and land use on woody plant species composition and beta diversity in an arid trans-Himalayan landscape, Nepal. J Mt Sci 11: 1112-1122.
- Pook EW, Costin AB (1966) Water stress on native vegetation during the drought of 1965. Aust J Bot 14(2): 257-267.
- De Frenne P, Lenoir J, Luoto M, Scheffers BR, Zellweger F, et al. (2021) Forest microclimates and climate change: Importance, drivers and future research agenda. Glob Chang Biol 27(11): 2279-2297.
- Zellweger F, De Frenne P, Lenoir J, Vangansbeke P, Verheyen K, et al. (2020) Forest microclimate dynamics drive plant responses to warming. Science 368(6492): 772-775.
- Waring RH, Running SW (2010) Forest ecosystems: Analysis at multiple scales, (3rd edn), Elsevier, Amsterdam, Netherlands.
- Closa I, Irigoyen JJ, Goicoechea N (2010) Microclimatic conditions determined by stem density influence leaf anatomy and leaf physiology of beech (Fagus sylvatica L.) growing within stands that naturally regenerate from clear-cutting. Trees 24: 1029-1043.
- Harper J, White J (1974) The demography of plants. Annu Rev Ecol Evol Syst 5(1): 419-463.
- Singh G, Rawat GS (2012) Depletion of oak (Quercus spp.) forests in the western Himalaya: Grazing, fuelwood and fodder collection. J Sustain For Journal 29: 36-43.
- Kozlowski TT (1971) Growth and development of trees. Cambial growth, root growth, and reproduction growth, Volume II, Academic Press Inc, Cambridge, Massachusetts, USA.
- Negi GCS (1989) Phenology and nutrient dynamics of tree leaves in Kumaun Himalayan forests. PhD Thesis.
- Champion HG, Seth SK (1968) A revised survey of the forest types of India. Manager of publications, Delhi, India.
- Singh JS, Singh SP (1987) Forest vegetation of the Himalaya. The Botanical Review 53: 80-192.
- Jackson ML (1962) Soil chemical analysis. Prentice Hall of India Pvt Ltd, New Delhi, India, p. 498.
- Snedecor GW, Cochran WG (1994) Statistical methods. (8th edn), Oxford & IBH Pub, New Delhi, India, pp. 397-400.
- Davis KT, Dobrowski SZ, Holden ZA, Higuera PE, Abatzoglou JT (2019) Microclimatic buffering in forests of the future: The role of local water balance. Ecography 42(1): 1-11.
- De Frenne P, Rodríguez-Sánchez F, Coomes DA, Baeten L, Verstraeten G, et al. (2010) Microclimate moderates plant responses to macroclimate warming. Proc Natl Acad Sci 110: 18561-18565.
- McLaughlin BC, Ackerly DD, Zion Klos P, Natali J, Dawson TE, et al. (2017) Hydrologic refugia, plants, and climate change. Glob Chang Biol 23(8): 2941-2961.
- Lenoir J, Hattab T, Pierre G (2017) Climatic microrefugia under anthropogenic climate change: Implications for species redistribution. Ecography 40(2): 253-266.
- Rawat PK, Tiwari PC, Pant CC (2011) Climate change accelerating hydrological hazards and risks in Himalaya: A case study through remote sensing and GIS modeling. Int J Geomat Geosci 1(4): 678.
- Paudel S, Sah JP (2003) Physiochemical characteristics of soil in tropical sal (Shorea robusta Gaertn) forests in eastern Nepal. Himal J Sci 1(2): 107-110.
- Saxena AK (1979) Ecology of vegetation complex of north western catchment of river Gola, PhD Thesis, Kumaun University, Uttarakhand, India
- Tyagi JV, Qazi N, Rai SP, Singh MP (2013) Analysis of soil moisture variation by forest cover structure in lower western Himalayas, India. J For Res 24: 317-324.
- Joshi G, Negi GCS (2015) Physico-chemical properties along soil profiles of two dominant forest types in Western Himalaya. Curr Sci 109(4): 798-803.
- Sheikh MA, Kumar M, (2010) Nutrient status and economic analysis of soils in oak and pine forests in Garhwal Himalaya. J Am Sci 6(2): 117-122.
- Negi GCS, Rikhari HC, Garkoti SC (1988) The hydrology of three high‐altitude forests in central Himalaya, India: A reconnaissance study. Hydrol Process 12(2): 343-350.
- Adams SR, Langton FA (2005) Photoperiod and plant growth: A review. J Horti Sci and Biotech 80: 2-10.
- Robert F, Risser G, Pétel G (1999) Photoperiod and temperature effect on growth of strawberry plant (Fragaria X ananassa Duch): Development of a morphological test to assess the dormancy induction. Sci Hort 82: 217-226.
- Singh P (2019) Climate change impacts on phenological responses of dominant tree species of central Himalayan forests. PhD Thesis, Kumaun University, Uttarakhand, India.
- Chapin FS, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of differing growth form in an Alaskan tundra ecosystem: Implications for herbivory. J Ecol 68(1): 189-209.
- Van Cleve K, Viereck LA (1981) Forest succession in relation to nutrient cycling in the boreal forest of Alaska. Forest succession. Springer, New York, USA, pp. 185-211.
- Rai ID, Adhikari BS, Rawat GS, Bargali K (2012) Community structure along timberline ecotone in relation to micro-topography and disturbances in western Himalaya. Not Sci Biol 4(2): 41-52.
- Negi VS, Pathak R, Rawal RS (2019) Long-term ecological monitoring on forest ecosystems in Indian Himalayan region: criteria and indicator approach. Ecol Ind 102: 374-381.
© 2023 © Pradeep Singh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






