- Submissions

Full Text
Environmental Analysis & Ecology Studies
Natural Infection of Preimaginal Stages of Flies of the Sarcophagidae Family with Brachymeria Podagrica and Dirhinus Himalayanus (Hymenoptera: Chalcididae) in the United Arab Emirates
Rolf K Schuster* and Saritha Sivakumar
Central Veterinary Research Laboratory Dubai, United Arab Emirates
*Corresponding author: Rolf K Schuster, Central Veterinary Research Laboratory Dubai, United Arab Emirates
Submission: October 27, 2022; Published: December 05, 2022

ISSN 2578-0336 Volume10 Issue2
Abstract
Two species of parasitoid wasps, Brachymeria podagrica (Fabricius, 1787) and Dirhinus himalayanus Westwood, 1836 of the Chalcididae family, hatched from pupae maggots which were deposited by sarcophagid flies on a camel meat bait. While B. podagrica infects maggots, D. himalayanus lay eggs into fly pupae. The preimaginal development of both wasp species under laboratory conditions at 26 to 28 oC lasted 25-28 and 24-33d, respectively. The yield of wasps in four different samples varied between 31 and 83% and the development of Sarcophaga dux and Wohlfahrtia nuba was impeded considerably compared to an unexposed control group. The calliphorid species, Chrysomya albiceps, remained uninfected. B. podagrica and D. himalayanus are potential candidates for a biological control of flies.
Keywords:Parasitoid wasps; Brachymeria podagrica; Dirhinus himalayanus; Sarcophagidae; United Arab Emirates
Introduction
Parasitoid wasps of the family Chalcididae show a high biodiversity and a cosmopolitan distribution. According to Aguiar AP et al. [1] the species inventory consists of 1469 species in 96 genera. Chalcididae species are pupal (idiobionts) or larvo-pupal (koinobionts) endoparasitoids in holometabolous insects. Some species are ectoparasitoids within the puparia of their host. Chalcidid wasps have the potential to be used for the biological control of horticultural pests [2,3] as well as for the control of filth flies in animal farms [4-6]. Already at the end of last century, experiments with Muscidifurax raptor (Hymenoptera: Pteromalidae), a wasp with cosmopolitan distribution and pupal parasitoid of house and stable flies were carried out to reduce fly population in chicken houses [7] and in pig farms [8]. Since some chalcidids use carrion flies as hosts, these wasps also have an importance in forensic entomology [9-12]. The composition of Chalcididae species in the Middle East was investigated by Delvare G et al. [13], Delvare G [14], Gul MA et al. [15] and Falahatpisheh A et al. [16]. An attempt to study the development of necrophagous flies under the hot summer conditions in Dubai resulted in the finding of two chalcidid wasps, Brachymeria podagrica (Fabricius, 1787) and Dirhinus himalayanus Westwood, 1836 (Hymenoptera: Chalcididae). Since little was known about the development of these parasitoids a few experiments were carried out. The purpose of this study was to establish data on the preimaginal development of these two parasitoid wasps and their impact on their hosts.
Materials and Methods
In late August 2021, a plastic container containing 500g of fresh camel meat as a bait to attract flies was deposited at a shaded place in the garden of the Central Veterinary Laboratory (CVRL) in Dubai. In order to collect 3rd stage fly larvae, the plastic container was put into a larger Petri dish with a layer of dry fine-grained sand on its bottom. The temperature during exposure varied between night and day from 29 to 39 °C and relative humidity fluctuated between 60 and 90%. Already three hours after deposition of the bait, filth flies (Sarcophagidae, Calliphoridae and Muscidae) were attracted. Since the smell of decaying meat attracted carpenter ants (Camponotus sp.) that tried to take away fly larvae, the breeding container had to be placed on a tray filled with water to avoid disturbances by ants.
On the 3rd day after deposition of the meat, the first batch of white truncate 3rd fly instars crawled out and fell into the sand. Of these larvae (day 4: sample 1, n=51) were separated into a small plastic box with dry sand and were allowed to stay outside for another two days. In the following two days (D5 and D6), 253 and 75 larvae left the bait, respectively. Amongst the 253 larvae of day 5, there was a single grey hairy maggot, the larval stage of Chrysomya albiceps. On day 7, the majority of larvae that had moved out from the bait were grayish hairy maggots and further observations were concentrated on 73 larvae of this type. Pupation started always on the subsequent day. After the larvae underwent pupation, pupae were taken into the laboratory and stored in closed Petri dishes at a temperature of 24 to 26 °C. At daily examination of the content of Petri dishes, the number of insects hatched from fly pupae was noted. For the species determination, insects were immobilized by cooling the Petri dishes for 5min. in the deep freezer. All flies and parasitoid wasps were killed with acetic ether. Unhatched pupariae were opened by preparation needles one week after the last insect had hatched.
In a second experiment, another camel meat bait was placed in Mid October 2021 to produce fly offspring and parasitoid wasps. With the occurrence of the first fly larval stages, the container was transferred into a bucket that was covered with a finely woven (mm) net to avoid an overcrowding and to impede access for parasitoid wasps. Sample 5 consisted of 70 freshly emerged from the bait 3rd fly instars, that were exposed for 24h to parasitoids in the garden and prior to pupation, these maggots were collected and stored in a Petri dish at 24 to 26 °C in the laboratory. Another 120 freshly emerged maggots from the same camel meat bait were collected and kept for pupation in an incubator at 38 °C. Sixty fresh pupae (sample 6) were then exposed for three days in an open petri dish in the garden and then kept in the laboratory and 60 unexposed pupae served as uninfected controls. The software Quantitative Parasitology (QPweb) Reizigel J et al. [17] was used to generate the percentage of fly and wasp development as well as their 95% confidence intervals.
Results
The meat sample attracted flesh flies (Sarcophagidae), blow flies (Calliphoridae), houseflies (Musca domestica) and necrophoerous beetles. Third stage fly larvae of the creamy colored truncated type moved out from the container on three subsequent days (Table 1). On day 7, a larger number of grey hairy larvae of C. albiceps left the bait and imagines of this species eclosed already on days 15 and 16 after deposition of the bait. Of 70 hairy maggots, 67 (=95.71%) pupated and developed into adults of C. albiceps (Table 2). Contrary to this, the number of eclosed sarcophagid imagines was unexpectedly low. Of 379 3rd stage larvae in samples 1 to 3, 372 pupated but only 40 flies reached the imago stage (Table 2). A total of 34 Sarcophaga dux enclosed 15-23 days after the bait was put, while the development of 5 Wohlfahrtia nuba took 20-21 days to develop.
Table 1:Duration of preimaginal fly development stages under late summer conditions in Dubai (T: 29-39 °C, rel. hum.: 60-90%. Samples 1-3: Sarcophagidae; sample 4: Chrysomya albiceps.
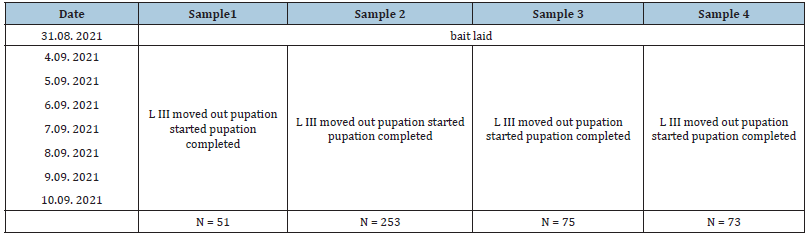
Table 2:Development of parasitoids wasps, Brachymeria podagrica and Dirhinus himalayanus in preimaginal stages of naturally infected sarcophagid flies (samples 1-3). Sample 4 consisted of larvae and pupae of Chrysomya albiceps.
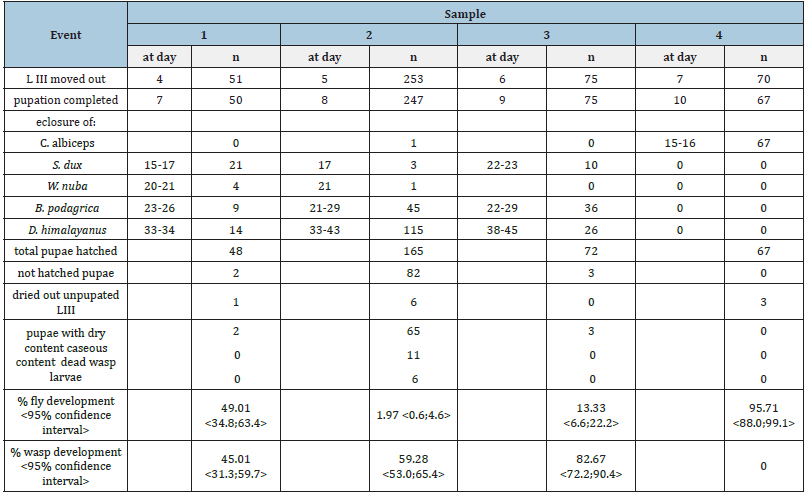
Soon after W. nuba enclosure in sample 1, small dark coloured, winged insects belonging to the order Hymenoptera occurred in the Petri dishes. These insects were 5.1-6.5mm long, with black coloured body. The basic colour of legs was brown with yellow patches on extremities. This species was determined as Brachymeria podagrica (Figure 1). In samples 1-3, B. podagrica emerged from pupae 23-29 days after the start of the experiment. Days later, a second wasp species, Dirhinus himalayanus (Figure 2) with different habitus emerged. These insects had a black body, were smaller (4.5- 6.0mm) and slender and the head showed two projecting horn like structures when looked at from dorsal side. Both wasp species had strong hind femurs with combs of small teeth at ventral side. D. himalayanus appeared between day 33 and 45 days after the start of the experiment. As a result of this trial, 80 B. podagrica and 155 D. himalayanus were harvested. The development rate of wasps in these observations ranged from 45.01 to 82.67% and eclosure rate of flies varied between 1.97 to 49.01 %. C. albiceps showed the highest development proportion of 95.71% and was not influenced by the two wasp species.
Figure 1: Brachymeria podagrica, lateral view. The body is shiny black and the brown legs have creamy white patches. The tegula on the base of the forewings is of the same colour.

Figure 2: Dirhinus himalayanus, dorsal view. The genus Dirhinus is recognizable by the frontal horns. The body of D. himalayanus is black, antennae, 1st and 2nd pair and the distal 3rd of legs are dark brown.

Eighty-seven of 372 pupae remained uneclosed in samples 1 to 3 and were mechanically opened. The majority (n=70) contained a brownish dry content with remnants of the fly larval respiratory system (anterior spiracles). Another 11 pupae comprised a smelly caseous content while dried out wasps or their larvae were present in six pupariae.
The second experiment aimed at what development stage of the hosts was targeted by the parasitoids. The exposition of 3rd Sarcophagidae instars resulted in emergence of B. podagrica 25 to 28 days later (Table 3). Ten out of 70 (=14.29%) sarcophagid larvae exposed to parasitoid wasps developed into adult W. nuba while 43 (=61.43%) B. podagrica emerged from pupae in sample 5. From sample 6 where 60 Sarcophagidae pupae were exposed, a total of 19 D. himalayanus 24-33 d after exposure and only a single W. nuba eclosed from this sample. Contrary to the two exposed groups, 58 (=96.67%) flies (54 S. dux and 4 W. nuba) of the not exposed control group eclosed on day 13-15 d and 17-18 d, respectively.
Table 3:Natural exposition of Sarcophagidae larvae (sample 6) and pupae (sample 7) to two species of chalcidid wasps. A further sample served as unexposed control. * after L3 emerged from bait; ** after pupation completed.

Discussion
The geographical distribution of both parasitoid species is well investigated but little experimental work was done on their life cycle. Iqbal T [18] reviewed the occurrence of chalcidoid wasps in Pakistan and recorded the distribution of Pakistani species in other parts of the world. According to this list, B. podagrica has a cosmopolitan distribution and occurs in seven biogeographic realms in temperate, subtropical and tropical zones on all continents except Antarctica. Delvare G [14] classified D. himalayanus as initially Indo Pacific species that is also found in Arabia and Africa and was most probably introduced there. Our observation showed that the odor of decaying meat even under the conditions of hot summer temperatures relatively quickly attracts necrophagous flies and motivated them to deposit eggs or larvae. Although imagines of C. albiceps were seen already at the day of the deposition of the bait, a large number of its greyish hairy maggot occurred only at day 7, completed pupation at day 10 and adult flies eclosed at day 15 to 16. Since C. albiceps is of forensic importance, a number of experiments were carried out under different temperature regimes in order to establish the duration of the life cycle. Carvalho Queiroz [19], Al-Shareef et al. [20] and El-Kassem Bossly [21] calculated 4 to 5 days for the larval development at 30-35 °C and another 5 to 5.5 days at 25-27 °C for the pupal period. The reason for the prolonged development in our observation is probably that the larval stages of C. albiceps were suppressed by those of sarcophagid flies. Wells JD et al. [22] who monitored the presence of insects and their development stages on exposed rat carcasses saw adults of C. albiceps on the deposited baits but time for the colonization of the rat carcasses with their larvae took longer compared to S. dux and W. nuba.
Although kept at the same conditions, C. albiceps development stages were not attacked by parasitoid wasps and 95.71% flies eclosed from pupae. This is in contrast to observations by Marchiori CH [23] in Brazil who reported a 50.4% of parasitism in C. albiceps caused by B. podagrica. S. dux and W. nuba eclosed at day 15 to 17 and at day 20 to 21, respectively. This development time is comparable to observations of other authors [24-28]. Both flies and wasps hatched from the anterior site of the pupae but the mechanism for this is different. Sarcophagid flies and a large number of other muscoid flies belong to the section of Schizophora. When hatching from puparium, these flies inflate a membranous sac (ptilinum) that protrudes from the face above the antennae and due to this pressure, the puparium brakes at the line of weakness in the puparium. Contrary to this, both examined wasp species gnawed with their mandibles the puparial shell
Both parasitoids reduced the progeny of sarcophagid flies, W. nuba and S. dux. This reduction was less distinct in sample 1 but more obvious in later samples. Imagines of chalcidid wasps feed on nectar and thus were present in the garden of CVRL. The smell of rotting meat attracted them to the place where the bait was kept. This might explain the rising percentage of wasp development in samples 1 to 3. In a relative large proportion of fly pupae in sample 2, but also in samples 5 and 6 remained unhatched. Most of these pupae had a dry light brownish content with remnants of the respiratory tract (spiracle plates and tracheae). It is possible that freshly pupated larvae died due to the impact of a heavy infestation. According to Delvare G [14] and Gul MA et al. [15] D. himalayanus is an ectoparasitoid of muscomorphous Diptera. Contrary to this, B. podagrica could only be obtained when fly larvae were exposed and for this reason B. podagrica is considered an endoparasitoid. Further experiments are planned to study the development of these two parasitoids in detail, to try to expand the host range and to breed them on a larger scale in order to use both species for biological control of fly population on agricultural farms.
Conclusion
B. podagrica and D. himalayanus reduced the progeny of their hosts and may become important under the aspect of rising insecticide resistance. Since both species of parasitoids emerge after the eclosure of their host flies, these wasps can have an application in criminal forensic investigations to determine the postmortem interval in cases when the corps was detected in decaying conditions. Further experiments to study the preimaginal development of these parasitoids under different temperature regimes are necessary.
References
- Aguiar AP, Deans AR, Engel, MS, Forshage, M, Hubers JT, et al. (2013) Order Hymenoptera. In: Zhang ZQ (Ed.), Animal Biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa 3703(1): 51-62.
- Barbuceanu D, Andriescu I (2012) The parasitoid complex of Eupoecilia ambiguella (Lepidoptera: Tortricidae) in a vineyard of southern Romania. Studies and communications. the sciences of Nature 28(2): 99-104.
- Husni H, Kainoh Y, Honda H (2001) Effects of host pupal age on host preference and host suitability in Brachymeria lasus (Walker) (Hymenoptera: Chalcididae). Appl Entomol Zool 36(1): 97-102.
- Floate K, Khan B, Gibson G (1999) Hymenopterous parasitoids of filth fly (Diptera: Muscidae) pupae in cattle feedlots. Can Entomol 131(3): 347-362.
- Gibson GAP, Floate KD (2004) Filth fly parasitoids on dairy farms in Ontario and Quebec, Canada. Can Entomol 136(3): 407-417.
- Pitzer JB, Kaufman PE, Geden CJ, Hogsette JA (2011) The ability of selected pupal parasitoids (Hymenoptera: Pteromalidae) to locate stable fly hosts in a soiled equine bedding substrate. Environ Entomol 40(1): 88-93.
- Rutz D, Axtel R (1979) Sustained release of Muscidivorax raptor (Hymenoptera: Pteromalidae) for housefly (Musca domestica) control in two types of caged-layer poultry houses. Environ Entomol 8(6): 1105-1110.
- Coch F (1981) The seasonal appearance of the Musca domestica parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae) in a swine production facility. Angew Parasitol 22: 217-221.
- Grassberger M, Frank C (2003) Temperature related development of the parasitoid wasp nasonia vitripennis as forensic indicator. Med Vet Entomol 17(3): 257-262.
- Magni PA, Jourdan S (2006) Presence of the parasite Brachymeria sp. (Hymenoptera: Chalcididae) in the entomofauna of a corpse. 4th Meeting European Association for Forensic Entomology (EAFE), Bari, Italy, pp. 26-29.
- Marchiori CH (2010) Parasitoids of Diptera of forensic interest collected in Goiás, Brazil. In: Gomes L (Ed.), Forensic Entomology: New Trends and Technologies in Criminal Sciences, Technical Books Publisher, Rio de Janeiro, Brazil, pp. 270-281.
- Rivers DB (2016) Parasitic Hymenoptera as forensic indicator species. In: Shetty BSK, Padubidri JR (Eds.), Forensic Analysis-From Death to Justice, Intech Open, London, UK.
- Delvare G, Talaee L, Goldansaz SH (2011) New Chalcididae (Hymenoptera: Chalcidoidea) of economic importance from Iran. Ann Zool 61(4): 789-801.
- Delvare G (2017) Order Hymenoptera, family Chalcididae. In: Harten A (Ed.), Arthropod fauna of the UAE 6, Department of the President’s Affairs, Abu Dhabi, pp. 225-274.
- Gul MA, Soliman AM, Al Dhafer HM, Gadallah NS (2018) Species of Dirhinus Dalman, 1818 (Hymenoptera: Chalcididae, Dirhininae) from Saudi Arabia: New species and a new record. Zootaxa 4483(3): 455-479.
- Falahatpisheh A, Fallahzadeh M, Dousti AF, Delvare G (2018) Review of Iranian Chalcididae (Hymenoptera, Chalcidoidea). Zootaxa 4394(2): 251-269.
- Reiczigel J, Marozzi M, Fabian I, Rozsa L (2019) Biostatistics for parasitologists-a primer to quantitative parasitology. Trends Parasitol 35(4): 277-281.
- Iqbal T (2015) Taxonomic study of Chalcidididae (Chalcidoidea: Hymenoptera) of Khyber Pakhtunkhwa, PhD thesis University of Peshewar, Pakistan, pp.132.
- Queiroz MM (1996) Temperature requirements of Chrysomya albiceps (Wiedemann, 1819) (Diptera, Calliphoridae) under laboratory conditions. Mem Inst Oswaldo Cruz 91(6): 785-788.
- Al-Shareef LAH, Al Qurashi, SID (2916) Study of some biological aspects of the blowfly Chrysomya albiceps (Wiedemann 1819) (Diptera: Calliphoridae) in Jeddah, Saudi Arabia. Egypt J Forensic Sci 6(1): 11-16.
- El-Kassem Bossly HA (2021) Development of Chrysomya albiceps (Wiedemann, 1819) (Diptera: Calliphoridae) from the Jazan region of southwest Saudi Arabia under different laboratory temperatures: Applications in forensic entomology. Egypt J Forensic Sci 11: 30.
- Wells JD, MacInnis AE, Dsouza MA, Ulabdin Z, Al Mughawi S, et al. (2021) Forensic entomology when the evidence is “no insect.” Best carrion fly species for predicting maximum postmortem interval in the United Arab Emirates. Forensic Sci Int 328: 110999.
- Marchiori CH (2019) Brachymeria podagrica (Hymenoptera: Chalcididae) (Fabricius) collected in Brazil.
- Amoudi MA (1993) Effect of temperature on the developmental stages of Wohlfahrtia nuba (Diptera: Sarcophagidae). J Egypt Soc Parasitol 23(3): 697-705.
- Villet MH, MacKenzie B, Muller WJ (2006) Larval development of the carrion-breeding flesh fly, Sarcophaga (Liosarcophaga) tibialis Macquart (Diptera: Sarcophagidae), at constant temperatures. Afr Entomol 14: 357-366.
- Sukontason KL, Sanit S, Klong-klaew T, Tomberlin JK, Sukontason K (2014) Sarcophaga (Liosarcophaga) dux (Diptera: Sarcophagidae): A flesh fly species of medical importance. Biol Res 47(1): 1-9.
- Zhang X, Li Y, Shang Y, Ren L, Chen W, Wang S, et al. (2020) Development of Sarcophaga dux (Diptera: Sarcophagidae) at constant temperatures and differential gene expression for age. J Therm Biol 93: 102735.
- Salimi M, Rassi Y, Oshaghi M, Chatrabgoun O, Limoee M, et al. (2018) Temperature requirements for the growth of immature stages of blowflies species, Chrysomya albiceps and Calliphora vicina, (Diptera: Calliphoridae) under laboratory conditions. Egypt J Forensic Sci 8: 2-6.
© 2022 © Rolf K Schuster. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






