- Submissions

Full Text
Environmental Analysis & Ecology Studies
Plant Growth-Promoting Effects of Azotobacter as a Biofertilizer During the Acclimatization Process of Plantains Cultivars
Beovides-García Y1*, Simó-González JE1, Pérez-Peñaranda MC2, González-Marrero I2, López-Torres J1, Basail-Pérez M1, Rayas-Cabrera A1 and Santos-Pino A1
1Directorate of Plant Biotechnology, Research Institute of Tropical Roots and Tuber Crops (INIVIT), Cuba
2Directorate of Science and Innovation, Business Group of Biological and Pharmaceutical Laboratories (LABIOFAM), Cuba
*Corresponding author: Beovides-García Y, Directorate of Plant Biotechnology, Research Institute of Tropical Roots and Tuber Crops (INIVIT), Cuba
Submission: September 28, 2022; Published: November 28, 2022

ISSN 2578-0336 Volume10 Issue2
Abstract
The objective of this work was to determine the effect of a new Azotobacter-based biofertilizer on the development of plantain plantlets obtained by in vitro culture, during their acclimatization. Application were made weekly according to a completely randomized design (in 20x12cm polyethylene bags) with five treatments: 0 (absolute control), 1,2,3 and four applications of the biofertilizer. After one week of acclimatization, survival percentage was determined on the two commercial cultivars studied: ‘INIVIT PB-2012’ (ABB) and ‘INIVIT PV 06-30’ (AAB). Measurements were made in 20 randomly selected plantlets per treatment at 50 and 60 days after planting (dap): plant height, number of leaves, petiole length, width and length of the developed leaf and its leaf area was calculated. The survival plantlets at seven dap always behaved above 98 %. Evaluations at 60 dap, demonstrated that Azotobacter enhanced transplant vigor and stimulated the morphological development of both cultivars. A substantial increment of the size and health of surviving plantlets (height, longest and widest leaves) was determined, which were significantly different to the control (without biofertilizer). Plantlets inoculated with the new biofertilizer for three weeks were ready for transplanting, at least, nine days before to the control, and that has a favorable economic value.
Keywords: Biofertilizer; Biological nitrogen fixation; in vitro culture; Plant growth-promoting rhizobacteria; Sustainable agriculture
Introduction
The negative effects of the climatic change, especially on the agriculture, have gotten the attention about the necessity to promote environmentally friendly agricultural practices. Humanity needs an alternative agricultural development paradigm, able to produce enough healthy foods for millions of people. According to Ranjan S et al. [1], due to the exorbitant cost of nitrogen fertilizers and the environmental damages on use of chemical fertilizers, biofertilizers could be the best alternative to chemical inputs and will help in reducing the rate of ecological disturbance to a great extent. In general, the high cost of chemical fertilizers and difficulties to access them are very common problem for farmers around the world. At the same time, Nicholls CI et al. [2] considered that the ecological resilience of agroecosystems is closely associated to social resilience; because of that, appropriate government and academic strategies should be integrated to fulfill the objectives of sustainable development outlined by the Food and Agriculture Organization of the United Nations.
In current agricultural ecosystems, the poor soil fertility is one of the major threats to crops productivity. The application of beneficial soil microorganisms offers an important alternative for restoring, maintaining and improving soil health and fertility, and at the same time, crop health and productivity for a sustainable agriculture [3,4]. Among of them, biofertilizers such as micorhizas, Azospirillum, Pseudomonas, Bacillus or Azotobacter can add and compensate the nutrient loss from soil [5,6]. Two recent papers [1,7], explained some reasons why biofertilizers have become in a novel tool for enhancing soil fertility and crop productivity. In general, a wide group of plant growth-promoting rhizobacteria has shown an important role in the sustainable agriculture [8,9]. Among the most important beneficial microorganisms, Azotobacter chroococcum has been recognized for its positive activity as a freeliving nitrogen fixing bacterium; this bacteria also produce plant growth regulators, increase the solubility of mineral phosphates. Azotobacter is the most commonly isolated and researched free living nitrogen fixer contributing to the natural vegetation and soil fertility [1]; this highly aerobic and heterotrophic bacterium has an interesting anti-fungi activity [10] and produce antibiotics [11]. Among the most important crops around the world, plantains have a high demand on the global food market, but their yield depends on several factors. For example, fertilization, watering and the quality of the planting material are critical inputs for a successful production of this crop. Inputs may represent 70% of the production cost and the plantlets alone account for 36.49% of those costs [12].
In this sense, plant biotechnology has many advantages, as compared to the traditional method to produce good planting material (higher multiplication rate and better phytosanitary quality [13], but the last stage of the in vitro process is technically so complex because it determines when the plantlets are ready to their transplantation to the field. In this important phase, usually defined as acclimatization, the plantlets are grown in a controlled environment (greenhouse) with high demand for fertilizer, water and labor [14] before they reach the appropriate characteristics to their successful planting at farm. Recently, researchers from the Cuban Business Group LABIOFAM developed a biofertilizer based on an A. chroococcum strain nitrogen solubilizer [15]; the new product is in the process of technical validation for its future commercial use in the agriculture. However, the impact of this bioproduct during the acclimatization phase of most important crops, including plantains and bananas, is not known. For that reason, the objective of this work was to determine the effect of the Azotobacter-based biofertilizer on the development of plantain plantlets obtained by in vitro culture, during their acclimatization.
Materials and Methods
All the experiments were carried out in the greenhouse area from the Biotechnology Unit at the Research Institute of Tropical Roots and Tubers Crops (INIVIT), in Santo Domingo, Cuba.
Plant material and the experimental design
Two commercial plantains cultivars from the germplasm bank of INIVIT were chosen as the initial plant material: ‘INIVIT PB- 2012’ (ABB) and ‘INIVIT PV 06-30’ (AAB); both were obtained in the Cuban breeding program of Musa spp. Plantlets produced by in vitro culture were obtained from elite and healthy plants selected on farm and grown under controlled conditions at the greenhouse (phase 0 for in vitro culture). A relative humidity of 85-90 % was guaranteed into the greenhouse; it was covered by an agricultural anti-aphids mesh and a plastic mesh that allowed to reduce sunlight by up to 70%. In vitro plantlets were transplanted, at the stage of 2-3 leaves and 3-5cm plant height, into polyethylene bags (20 x 12.5cm) with a conventional mixed substrate (70 % organic matter (filter cake) and 30 % soil). The novel biofertilizer, produced from an Azotobacter strain isolated and studied by Pérez MC et al. [15], was used at a recommended unique doses of 20L ha-1. Its effect was evaluated by the aspersion on the substrate and plantlets. The experimental design include five treatments according with the number of bioproduct applications every seven days.
Fifty plantlets for each treatment were grown in the greenhouse
conditions and experiments were conducted in a completely
randomized design with five treatments (replicated two times):
T1. Absolute control (without biofertilizer)
T2. One application
T3. Two applications
T4. Three applications
T5. Four applications
Measurements and statistical analysis/
After one week of acclimatization, survival percentage was determined. At 50 and 60 days after planting, (dap) the measurements were recorded on twenty randomly selected plantlets from each treatments. At data collection, the following variables were evaluated: plant height (cm), number of leaves, petiole length (cm), width and length of the developed leaf (cm) and its leaf area (cm2) was calculated. The collected data were subjected to the analysis of variance (ANOVA) appropriate to the completely randomized design. All statistical procedure was according to the experimental design and using the tools from the IBM.SPSS/PC+ statistical package version 23.0 for Windows® [16]. Whenever differences existed among means values, the comparison of them was carried out with the test of Tukey for P≤ 0.05.
Results
The results showed that Azotobacter mostly enhanced transplant vigor and stimulated vegetative growth. Observations of the survival plantlets percentage seven days after planting always behaved above 98 %. All plants, regardless of the treatment received, maintained the morphological characteristics of each cultivar during the experimental period. Evaluations at 60 dap allowed to quantify the stimulating effect of the new biofertilizer on the morphological development of both cultivars. Table 1 show the observed effect on the evaluated variables in the cultivar ‘INIVIT PB-2012’.
Table 1:Effect of an Azotobacter-based biofertilizer on the general plant development during the acclimatization phase of plantain plantlets cv. ‘INIVIT PB-2012’.
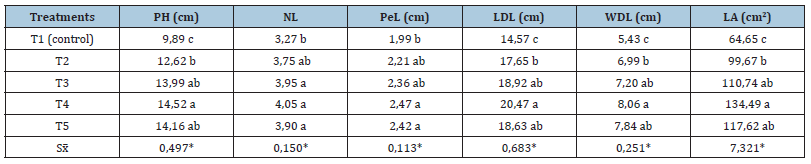
*Means followed by the same letter in a same column are not significantly different according to Tukey’s HSD test (P≤
0.05).
Legend: PH: Plant height, NL: Number of leaves, PeL: Petiole length, length (LDL) and width (WDL) of the developed leaf,
LA: Leaf area.
T1-absolute control (without biofertilizer); T2-with one application of Azotobacter; T3-with two applications; T4- with
three applications; T5- with four applications.
With more than two applications of the new biofertilizer most of the evaluated variables were significantly different from the absolute control, but without statistical differences among the treatments with some application of the bioproduct. After three applications, plant height increased in 4.63cm (46.81%), but petiole and leaves improved their size too. Due to an increment in the leaves size (length and width of the developed leaf): 5.9cm (40.49%) and 2.63cm (48.43%), respectively, the leaf area grew significantly regarding to the control, in more than 69cm2 (108%) when the Azotobacter solution was applied three times each seven days. During the experiments with the cultivar ‘INIVIT PV 06-30’ (a cooking plantain called as “plátano macho” in Cuba, AAB group) weekly applications induced the same stimulant effect on the plantlets with lightly superior values to those of cultivar ‘INIVIT PB- 2012’, which was positive in some essential variables related with their quality for farm planting: plant height, length and width of the developed leaf, and in consequence, for its leaf area (Table 2).
Table 2:Effect of an Azotobacter-based biofertilizer on the general development during the acclimatization phase of plantain plantlets cv. ‘INIVIT PV 06-30’.
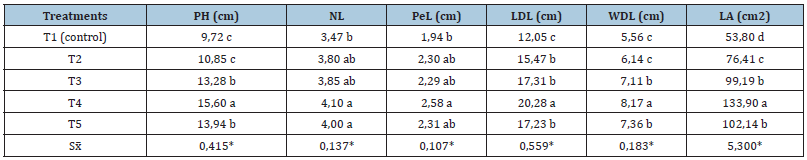
*Means followed by the same letter in a same column are not significantly different according to Tukey’s HSD test (P≤
0.05).
Legend: PH: Plant height, NL: Number of leaves, PeL: Petiole length, length (LDL) and width (WDL) of the developed leaf,
LA: Leaf area
According to measurements, the optimum treatment was T4 (three applications) for both cultivars, with significant differences observed with the control but without statistical differences with two or four applications. Despite this, had no significant differences in number of leaves and petiole length for all treatments with the bioproduct. When three applications were made, increments were significantly bigger than the absolute control for plant height, length and width of the developed leaf and leaf area, was 60.49, 68.29, 46.94 and 148.88 %, respectively. For the leaf area, there were not statistical differences among the treatments with two (110,74cm2), three (134,49cm2) or four applications (117,62cm2) of Azotobacterbased biofertilizer in ‘INIVIT PB-2012’ cultivar, but in ‘INIVIT PV 06-30’ the fourth combination of three inoculations each seven days was clearly the best treatments. General observations showed that plantlets inoculated with the new biofertilizer for three weeks (every seven days) were ready for transplanting, at least, nine days before to the control, and that has a favorable economic value (data not shown). It means that size and health of surviving plantlets (height, longest and widest leaves) was significantly different to the absolute control (without biofertilizer) and it demonstrated that three applications were enough to achieve the ideal plants height (12-15cm) in 50 dap, and other desirable characteristics for planting in farm: three true developed leaves or more, pseudostem diameter superior to 1.0-1.5cm.
Discussion
Plantlets with three applications of the Azotobacter-based biofertilizer, 60 days after planting (dap) exceeded the control with significant differences in most of the evaluated variables. According to Zhou J et al. [17], the dynamic of plants growth is related with genetic and environmental factors, in that sense, the phenotypic analysis of plant growth variables is an important approach to understand how plants interact with environmental changes as well as respond to different treatments. During a recent research, to evaluate three organic inputs during the acclimatization of banana (cv. Williams) micro propagated seedlings, authors informed that best results were found with a bioproducts combination increasing the height by 29.6 % and the pseudostem diameter by 19.9 %, while in the leaf area the best treatment overcame in 84.7 % the remain treatments [18]. In all cases, values were smaller than the ones obtained in the present research, but both bioproducts stimulated the plantlets development.
Among the causes of these results, in absence of others possible reasons associated to the observed differences, the applications of the new biofertilizer were responsible of them. A few years ago, the exact mode of action behind the growth promoting activity of Azotobacter was not very clear, but there is a coincidence about several probable mechanisms related with growth hormone production (gibberellins, Indole Acetic Acid (IAA) and cytokinins) or the presence of siderophores [19] due to a bigger access to the sparingly soluble Fe in the environment. At the same time, this microorganism is one of the most important a non-symbiotic N2- fixing bacteria with a high diversity and dispersion in soils, and therefore, significant improvements in crop productivity and soil fertility [7].
One of the most important finding was the significantly increment on the leaf area in those treatments with two or three applications of the biofertilizer, in both cultivars. This variable determines the light interception (photosynthesis process efficiency) and is an important parameter in determining plant productivity [20], especially in its biomass production [21]. Higher photosynthesis provides better growth of plants because around 90% of plant biomass is a consequence of the CO2 assimilation through photosynthesis [22]. The reduction of acclimatization phase in around nine days was economically important, because in less time the plantlets were ready for farm transplant. Theoretically, plantains and bananas plantlets require between 45 and 60 days to be ready to transplant to the farm, it depend of many factors related with the acclimatization conditions, the cultivar, etc. Nevertheless, when some microorganisms are using as a biofertilizer, due to several causes, it should be expected that its effect could not be observed before 50-60 days after planting.
Evaluations at 60 dap are justified because after the microorganisms exogenous application and they enter in contact with the root, they should colonize and adhere to the radicular tissue before they can materialize their action. Some researchers found that this kind of colonization requires time and it only happens when there is compatibility between the microorganisms and intrinsic factors of the plant, as those perspired of root [23]. All those factors could influence in a lack of inoculant’s effect during the first 45-50ddp. These results coincided with previous findings published by Beovides Y et al. [24], which didn’t find any effect of the natural bioestimulant VIUSID Agro®, before 45-50 days of its application, during the acclimatization of taro (Colocasia esculenta Schott) plantlets.
The differences observed in the intensity of the answer in some variables for each cultivar could be associated to significant specificity between microbe and plant genotype already observed by Anbi AA et al. [25] for endophytes like Azotobacter sp. In general, many aspects are related with the complex process of colonization by those kind of beneficial microorganisms. In an interesting review, Trivedi P et al. [23] referred recently about interactions among the host, environment and microbes that take place both, above and below ground; they mentioned too, some findings associated to the requirements of common adaptation mechanisms for an effective colonization. Probable, there are genes involved in motility, adhesion and biofilm production help to the plant colonization. Studies based on comparative genomics [26] informed about the identification of some homologs of known bacterial genes involved in colonization, pathogenesis or provision of nutrients to plants. On the other hand, Huang AC et al. [27] affirmed that root metabolome changes could determine a specialized microbial communities that alter plant performance. In this sense, studies made by Leach JE et al. [28] and Trivedi P et al. [23] showed that metabolomics could contribute with information for the detection and quantification of small molecules, such as benzoxazinoids and strigolactones that impact on plant-microbiome interactions.
Results of this research showed that the evaluated new biofertilizer promoted the growth of plantlets and the used number of applications had a direct effect on the development of acclimatized plantain plants. Azotobacter is said to contribute in a substantially way to the nitrogen content of soil, a number of beneficial characters are present in this organism such as higher nitrogen fixation, ammonia excretion, production of vitamins and growth promoters, production of siderophores, production of antifungal antibiotics. It was reported that Azotobacter secretes substances that inhibit the growth of certain root pathogens and improve root growth and uptake of plant nutrients [29].
Additionally, these positive traits offer promising possibilities to ecologically engineer Azotobacter species likely providing significant N inputs, while reducing reliance to N-containing fertilizers such as urea [30,31]. All that is in correspondence with the special interest of advancing applied research on Azotobacter species as both agriculturally important plant growth promoting N2-fixing rhizobacterium that can be used for improving plant N nutrition and a biofertilizer based products at large scale, having significant improvements in crop productivity and soil fertility. They are able to synthetized plant growth hormones and these hormones can not only improve plant growth and nutrient uptake, but can also indirectly protect host plants from phytopathogens and stimulate other beneficial rhizosphere microorganisms [32,33].
Azotobacter species are able to influence directly plant growth by synthesizing plant growth hormones. These hormones can not only enhance plant growth and nutrient uptake but can also indirectly protect host plants from phytopathogens and stimulate other beneficial rhizosphere microorganisms [33]. Several works on drought stress tolerance using Azotobacter species as a solution demonstrated the efficacy of their use [34]. Maybe, it is caused because they acts as antagonists and suppress the incidence of soil borne plant pathogens and usually, bio-fertilizer liberates growth promoting substances and vitamins and helps in maintaining the soil fertility [35].
On the other hand, in case of biofertilizers, is important to consider possible interactions among the contained physiologically active compounds, and because of that, their effects on plants may depend on dose, time of treatment, general or specific growth conditions, and even, plant species. Some researchers [1,7] pointed out Azotobacter based-biofertilizers, possess unique characteristics such as cyst formation containing novel liquid (more than one type of nitrogenase, extreme tolerance to oxygen) conferring resistance to environmental stresses, an idea mentioned before by [36]. Moreover, the abundance of Azotobacter species in the soil could improve the availability not only of N through the biological nitrogen fixation processes [37], but also P as well [38].
In general, results confirmed the possibility to reduce the dependency on synthetic fertilizers with the use of effective biological-based alternatives like Azotobacter-based biofertilizers, as an effective component of integrated plant nutrition strategy, which contributes positively to sustainable agricultural production. In this sense, Azotobacter-based biofertilizer is a new input for the plantain acclimatization that can reduce the application rates of chemical fertilizers toward a more sustainable agriculture. Current findings during this research, may help to implement an applicable and cost-effective micropropagation protocol for plantains and bananas plantlets, using the new biofertilizer during the acclimatization phase.
Conclusion
Applications of the new Azotobacter-based-biofertilizer (three times, every seven days), increase significantly the plant growth (plant height, length and width of the developed leaf, and leaf area) of plantain plantlets during their acclimatization. It reduce in nine days the required period for transplanting using a conventional and non-sterile mixed substrate (70 % organic matter and 30 % soil).
Acknowledgement
The authors are thankful to the National Fund for Science and Innovation (In Spanish: FONCI) from the Ministry of Science, Technology and Environment for the financial support. Authors acknowledge the support of the BIOALI Network (117RT22), financed by the CYTED Program, to the research development.
References
- Ranjan S, Sow S, Choudhury SR, Kumar S, Ghosh M (2020) Biofertilizer as a novel tool for enhancing soil fertility and crop productivity: A review. International Journal of Current Microbiology and Applied Sciences 11: 653-665.
- Nicholls CI, Osorio LA, Altieri MA (2013) Agroecology and socioecological resilience: Adapting to climate change. Redagres and Socla. Medellin, Colombia.
- Bender SF, Wagg C, Van der Heijden MGA (2016) An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends in Ecology & Evolution 31(6): 440-452.
- De Araújo AS, Oliveira JR, Araújo RM, Gomes RL (2014) Biofertilizers on soil microbial biomass and activity. Brazilian Journal of Agricultural Sciences 9(4): 545-549.
- Sánchez D, Pérez J, Luna L, García J, Espitia A (2019) Azotobacter chroococcum and Azospirillum lipoferum as biostimulants in cultivation of Ipomoea potatoes Lam. Mesoamerican Agronomy 30(2): 563-576.
- Pérez JV, Sánchez DB (2017) Characterization and effect of Azotobacter, Azospirillum and Pseudomonas associated with Ipomoea batatas from the Colombian Caribbean. Colombian Journal of Biotechnology XIX(2): 35-46.
- Aasfar A, Bargaz A, Yaakoubi K, Hilali A, Bennis I, et al. (2021) Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Frontiers in Microbiology 12: 628379.
- Astafyeva Y, Shalabayeva K (2016) Dependence of Azotobacter chroococcum culture growth rate on salt concentrations. Engineering for Rural Development 25(27): 182-186
- Kaur P, Purewal SS (2019) Biofertilizers and their role in sustainable agriculture. In: Giri B, Prasad R, Wu QS, Varma A (Eds.), Biofertilizers for Sustainable Agriculture and Environment, Volume 55, Springer, Berlin, Germany, pp. 285-300.
- Rodriguez-Salazar J, Moreno S, Espín G (2017) LEA proteins are involved in cyst desiccation resistance and other abiotic stresses in Azotobacter vinelandii. Cell Stress Chaperones 22(3): 397-408.
- Behl RK, Sharma H, Kumar V, Narula N (2003) Interactions amongst mycorrhiza, Azotobacter chroococcum and root characteristics of wheat varieties. Journal of Agronomy and Crop Science 189(3): 151-155.
- Tiwari ILA, Verma M, Gupta S, Devi S (2019) Impact of technology adoption on production and productivity of banana. Journal of Pharmacognosy and Phytochemistry 8(3): 2032-2034.
- Ortas I, Rafique M, Akpinar C, Aka Y (2017) Growth media and mycorrhizal species effect on acclimatization and nutrient uptake of banana plantlets. Scientia Horticulturae 217(15): 55-60.
- Waman AA, Bohra P (2019) Factors governing success in shoot tip culture of bananas with special reference to mixed genomic groups: An overview. Erwerbs-Obstbau 61: 9-21.
- Pérez MC, Oramas J, Sotolongo EA, Román Y, López H (2020) Optimization of the culture medium and fermentation conditions for the production of a biofertilizer based on Azotobacter chroococcum. Vegetal Biotechnology 20(3): 189-201.
- SPSS (2016) Statistical Package for the Social Sciences (SPSS), SPSS statistical, IBM, New York, USA.
- Zhou J, Applegate C, Alonso AD, Reynolds D, Orford S, et al. (2017) Leaf‑GP: An open and automated software application for measuring growth phenotypes for arabidopsis and wheat. Plant Methods 13: 117.
- Mora GF, Naranjo MA, Albiño QA, Flores CA, Oviedo AR, et al. (2020) Optimization in the acclimatization of micro propagated banana (Musa sp.) seedlings using three organic inputs. Bionature 6(1): 1452-1461.
- Ansari RA, Rizvi R, Sumbul A, Mahmood I (2017) PGPR: Current vogue in sustainable crop production. Probiotics and Plant Health, Springer, Singapore, pp. 455-472.
- Koester RP, Skoneczka JA, Cary TR, Diers BW, Ainsworth EA (2014) Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. Journal of Experimental Botany 65: 3311-3321.
- Weraduwage SM, Chen J, Anozie FC, Morales A, Weise SE, et al. (2015) The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Frontiers in Plant Science 6: 167.
- Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant, Cell and Environment 29(3): 315-330.
- Trivedi P, Pandey A, Palni LM (2012) Bacterial inoculants for field applications under mountain ecosystems: Present initiatives and future prospects. In: Maheshwari DK (Ed.), Bacteria in Agrobiology: Plant Probiotics. Springer-Verlag, Berlin, Germany, pp. 15-44.
- Beovides Y, Rodríguez D, Peña K, Galvez D, Molina O, et al. (2017) Effect of VIUSID agro® on Colocasia taro plants INIVIT MC-2012 produced in vitro in the acclimatization phase. Agricultura Tropical 3(2): 57-61.
- Anbi AA, Mirshekari B, Eivazi A, Yarnia M, Behrouzyar EK (2020) PGPRs affected photosynthetic capacity and nutrient uptake in different Salvia species. Journal of Plant Nutrition 43: 108-121.
- Levy A, Conway JM, Dangl JL Woyke T (2018) Elucidating bacterial gene functions in the plant microbiome. Cell Host Microbe 24(4): 475-485.
- Huang AC, Jiang T, Liu YX, Bai YC, Reed J, et al. (2019) A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 364(6440): eaau6389.
- Leach JE, Triplett LR, Argueso CT, Trivedi P (2017) Communication in the phytobiome. Cell 169(4): 587-596.
- Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnology Letters 32(11): 1559-1570.
- Wani SA, Chand S, Wani MA, Ramzan M, Hakeem KR (2016) Azotobacter chroococcum-a potential biofertilizer in agriculture: An overview. In: Hakeem KR, Akhtar J, Sabir M (Eds.), Soil Science: Agricultural and Environmental Prospectives, Springer, Berlin, Germany, pp. 333-348.
- Bageshwar UK, Srivastava M, Pardha-Saradhi P, Paul S, Gothandapani S, et al. (2017) An environmentally friendly engineered Azotobacter strain that replaces a substantial amount of urea fertilizer while sustaining the same wheat yield. Applied and Environmental Microbiology 83(15): e00590-17.
- Sahoo RK, Ansari MW, Dangar TK, Mohanty S, Tuteja N (2014) Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma 251(3): 511-523.
- Arora M, Saxena P, Abdin MZ, Varma A (2018) Interaction between Piriformospora indica and Azotobacter chroococcum governs better plant physiological and biochemical parameters in Artemisia annua L. plants grown under in vitro Symbiosis 75: 103-112.
- Shirinbayan, Khosravi H, Malakouti MJ (2019) Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Applied Soil Ecology 133: 138-145.
- Singh J, Sharma MK, Singh SP, Bano R, Mahawar AK (2018) Effect of organic and inorganic sources of NPK and bio-fertilizer on enhancement of growth attributes and chlorophyll content of sweet potato. International Journal of Current Microbiology and Applied Sciences 7(9): 3659-3667.
- Sadoff HL (1975) Encystment and germination in Azotobacter vinelandii. Bacteriological reviews. 39(4): 516-539.
- Din M, Nelofer R, Salman M, Abdullah, Khan FH, et al. (2019) Production of nitrogen fixing Azotobacter (SR-4) and phosphorus solubilizing Aspergillus niger and their evaluation on Lagenaria siceraria and Abelmoschus esculentus. Biotechnology Reports. 22: e00323.
- Velmourougane K, Prasanna R, Chawla G, Nain L, Kumar A (2019) Trichoderma-Azotobacter biofilm inoculation improves soil nutrient availability and plant growth in wheat and cotton. Journal of Basic Microbiology 59: 632-644.
© 2022 © Beovides-García Y. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






