- Submissions

Full Text
Environmental Analysis & Ecology Studies
Plant Mediated Green Synthesis of Nanoparticles and their Role in Mitigation of Abiotic Stress in Crop Plants: A Review
Savita1, Anju Srivastava2, Reena Jain2, Pratap Kumar Pati3 and Kuldeep Kumar Koul1*
1Department of Botany, Hindu College, University of Delhi, India
1Department of Chemistry, Hindu College, University of Delhi, India
1Department of Biotechnology, Guru Nanak Dev University, India
*Corresponding author: Kuldeep Kumar Koul, Department of Botany, Hindu College, University of Delhi, Delhi, India
Submission: April 21, 2022; Published: May 18, 2022

ISSN 2578-0336 Volume10 Issue1
Abstract
In a developing country like India, with significant number of people living under poverty line, agriculture is the mainstay for the livelihood of more than 50 per cent population. To meet the food requirement of increasing population, although a large acreage of land is under cultivation of a range of crop species, the average yield every year, however, has not been as anticipated. Plants have failed to realize their full growth potential owing to varying abiotic stress conditions like high salinity, drought, extreme temperatures, presence of heavy metals, water logging etc. Efforts have been made and are underway to devise strategies/methodologies that could evoke defensive response in plants when exposed to stress conditions. In the recent past, the use of nanoparticles has emerged as a ray of hope in providing solution for combating this obstacle that hamper crop plants from realizing their full growth potential. Nanoparticles are small particles with dimensions satisfying the nanometer scale. Although nanoparticles have been synthesized using physical and chemical methods, these methods are not only expensive, but also are non-eco-friendly and hazardous to health. Of late, a safer approach (green approach) of synthesizing nanoparticles, using plant extracts, have been adopted and has found wide acceptance among the agricultural scientists. Nanoparticles have large surface area, show quick absorbance/penetration with precision delivery to the target site. Use of nanoparticles like Fe, Cu, Zn, Ag, Au, silica etc. have shown positive response in plants manifested in increased germination, high chlorophyll content, increased proline synthesis, photosynthesis etc. The encouraging response seen in plants has led to the resurgence in efforts to try a range of nanoparticles synthesized from myriad plant species. This communication reviews the efforts made by various scientific groups using nanoparticles and the results achieved
Keywords: Green synthesis; Plant, Nanoparticles, Biotic and abiotic stress
Introduction
The ever-increasing population in the world and particularly in the developing countrylike India, has intensified the demand for enhanced food production. With the population expected to escalate by 34 percent by 2050, The global crop production will have to rise from the present 2.1 billion tons to about 3 billion tons [1]. Although to cater to the needs of ever-increasing population, a vast area of land has been brought under cultivation of various food crops, the agricultural yield, every year, has not been up to the mark due to varying biotic and abiotic stresses. Among the common natural abiotic stresses, that has despicably thwarted efforts to have a bumper harvest, have been the soil salinity, drought, and extreme temperatures.
Although to overcome these obstacles, efforts have been and are being made to devise and/or strategize scientific methods that could ameliorate the growth conditions of the crop plants, the results obtained, have been far from satisfactory. Of late, Nanotechnology has emerged as a potent tool that has helped in resolving a range of problems hither- to-fore unresolved [2]. It has offered an eco-friendly mechanism that has proved pivotal in overcoming some of the problems confronting the agricultural scientist. It uses the physio-chemical properties of a substance at molecular level to explore biological and material world at nano meter scale. Nanoparticles, being tiny in size (1-100nm), show unique physio-chemical characters such as large surface area, improved/enhanced reactivity, increased solubility and penetrance via the membranes that enable them target any cell organelle and diverse morphology as compared to bulk particles [3,4].
Owing to all these attributes that the small size bestows on a chemical component, the nanoparticles are suitable for chemical delivery that has the quality of reaching the target site with precision. For example, the chemical fertilizers, which are applied in large doses to enhance the productivity of crop, usually are not target specific and/or are not absorbed completely by plants resulting in their accumulation in soil making it toxic. Use of nanoparticles has given a fillip to the plant growth and productivity by enhancing the availability and absorption/penetration of nutrients into the plants. Nanotechnology holds promise in overcoming the stress related problem faced by the crop plants. However, it can be effectively used once its full potential is identified and particularly, their role in mitigation of various types of stresses faced by plants and their mechanism of action [5]. Realizing the pivotal role that plant based nanoparticles can play in alleviating the problems confronting the mankind, it was considered worthwhile to write this review in order to summarize and address the advancements in the field of green synthesis of nanoparticles.
Plant Mediated Green Synthesis of Nanoparticles
Nanoparticles, on account of its unique physiochemical properties, have wide applications in agriculture, engineering, environmental remediation, biotechnology, microbiology, medicine, electronics, mechanics, optics, and material science [6]. Methods employed thus far, to synthesize nanoparticles, can be broadly categorised into two. The first category includes the topdown approach and the second includes the bottom-up approach. While in top-down approach, bulk materials are reduced to the nano-dimensions by employing lithographic techniques or etching, mechanical processes such as high energy ball milling, laser pyrolysis method, machining, grinding, etc., in the bottom-up approach, the chemical methods such as gas phase method, hydrothermal method, sol-gel method etc. are used for the synthesis of nanoparticles from simple atoms or molecules [7,8]. The bottom-up approach seems more promising and effective method for synthesis of nanoparticles, as it provides the options to form nanoparticles of desired shape and size depending upon the subsequent application by controlling the precursor concentrations, pH, temperature etc. [6,7].
Need for green synthesis
Various approaches adopted for the synthesis of nanoparticles have its limitations. The mechanical and physical methods are not able to give nanoparticles of expected size. Moreover, the maintenance of high temperature and pressure required during the synthesis incurs heavy expenditure. The chemical methods involve use of organic solvents and a range of other chemicals as capping and reducing agents which are non-ecofriendly, toxic health hazard and hard to degrade. Commonly used chemicals in the synthesis process include sodium borohydride and hydrazine hydrate. This apart, while chemicals like ammonium ions, citric acid, carbon monoxide, formaldehyde, hydrogen, hydrogen peroxide, hydroxylamine hydrochloride, sodium carbonate etc. are used as reducing agents, Cetyltrimethylammonium bromide (CTAB), Dodecyl amine (DDA), Ethylene diamine tetra acetic acid (EDTA), Oleic acid, Polyetherimide (PEI), Polyethylene glycol (PEG), Polyamidation (PAMAM), Polyacrylic acid (PAA) etc. work as capping agents.
Realizing the serious consequences, the continuous use of nanoparticles synthesized using physical and chemical methods can lead to, it became imperative to invent an alternative method(s) which was least invasive. Of late there has been a resurgence in efforts in adopting a greener approach toward nanoparticle synthesis [8,9]. The greener methods (Green chemistry) involve the use of natural products (plant extracts/ bacterial- fungal extracts) restricting the use of toxic chemicals to the minimum.
The principles of green chemistry were established in 1998 considering the main objectives to reduce environmental hazards, and risk to human health with enhanced next generation applications. Environmentally benign, green method of nanoparticle synthesis is simple and cost effective with high rate of reproducibility of more stable material concomitant with low risk of contamination of the product [8,10]. Furthermore, it is very straightforward to scale up. Although the green methods of nanoparticle synthesis encompass the use of various microorganisms (bacteria, fungi, yeast, etc.) besides the plants, the methods involving exclusively the microbes (bio-assisted methods) is slow with less reproducibility and offers limited number of size and shape when compared to routes that use plant-based active ingredients [8].
The use of plant extracts as the production assembly of various types of nanoparticles have drawn attention, due to the active ingredients of plant metabolites like polyphenols, flavonoids, tannins, saponins, resins, essential oils, terpenes, terpenoids etc., which help in conversion of metal ions into nanoparticles by behaving as reducing and capping agents [11]. The plant extracts provide a rapid, non-toxic, non-pathogenic, biodegradable and economical single step technique for the biosynthetic processes involved in making of various metallic and non-metallic nanoparticles. (Table 1) lists the plants that have facilitated nanoparticle synthesis.
Table 1: Plant mediated green synthesis of silver, gold, iron and copper nanoparticles.

Limitations of plant mediated green synthesis
Although the use of plant extracts as source of nanoparticles holds promise, there is no clarity about the exact mechanism of nanoparticle synthesis using plant extracts [8,11]. Here it will be apt to mention that the plants of the same species occupying varying habitats vary in their chemical compositions that may lead to different results and interpretations in different laboratories. This can be considered as a major limitation associated with plant based biogenic methods. However, the green method of nanoparticle synthesis has far more advantages than these minor limitations.
Abiotic Stress Interactions and Nanoparticles
The effect of nanoparticles, whether harmful or advantageous, depends upon the type and concentration of nanoparticles used as well as the plant species [11-200].
Uptake and translocation mechanism of nanoparticles in crops
Figure 1: Shows the mechanism of absorption, uptake, transport and penetration of nanoparticles in plants. (A) Treatment methods of nanoparticles: Foliar spray (Flowers, leaves, hydathodes etc.) and irrigation through roots. (B) Uptake of nanoparticles is hampered by many barriers such as cuticle, epidermis, endodermis, casparian strips. Roots hairs on roots, stomata, hydathods and lenticels provide passage to nanoparticles into the plant system. (C) Nanomaterials can follow the apoplastic,symplastic and or apo-symplastic pathways for moving up and down the plant (D) Nanoparticles penetrate into the plant cell through several mechanisms such as endocytosis, pore formation, through plasmodesmata and can be mediated by carrier proteins. Source: Modified after Pérez-de-Luque [208].
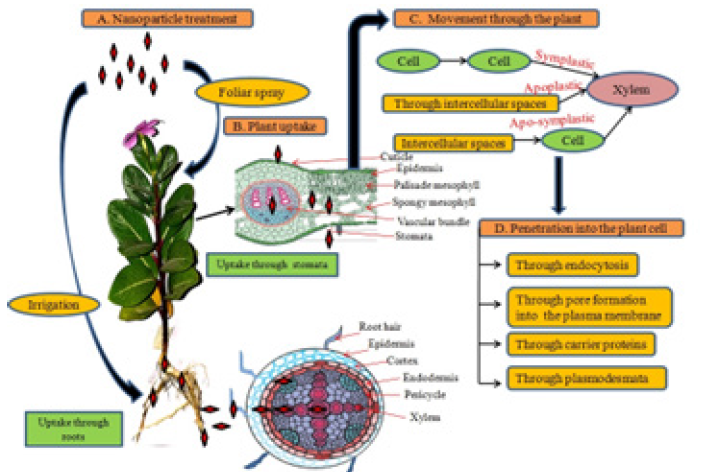
Generally, lateral roots of the plant absorb the nanoparticles from the soil from where they enter into the vascular system through the cortex and pericycle of the roots [201]. While the nanoparticles that are smaller than the pore of the cell wall passeasily through it [202,203], the larger nanoparticles fail to penetrate the cell wall of root cells. Instead, the cell wall opening of flowers, stigmas, hydathodes, and stomata provide an easy passage to the larger nanoparticles which interfere with the metabolic pathways of these cells (Figure 1) [204]. A body of data is available which suggest that nanoparticles move within the plant body by 1) Binding with ion channel proteins, (2) endocytosis through newly formed pores, (3) binding with some organic molecule, and/or by aligning with membrane transporters [205-207]. Nevertheless, further investigation is required to explain the selectivity and uptake mechanism of nanoparticles among types of plants, which is still not very clear.
Mechanistic Interaction of Nanoparticles under abiotic stress in plants
Plants, owing to their rooted and sedentary nature, cannot escape unpredictable changes happening within and outside the lithosphere. Any exposure to stress conditions result in an outburst of Reactive Oxygen Species (ROS) that cause the oxidative breakdown of myriad biomolecules central to the structural integrity of the cell and its photosynthetic machinery/metabolism [209]. Although plants are fortified with an inbuilt defence mechanism to overcome the negative effects of stress conditions, by way of inducing a battery of genes/ proteins into action and/ or by stimulating the production of stress busting molecules like proline, glycerol, inositol, glycine, trehalose etc., the decline in overall growth and productivity despite all the defences, more often than not, get accentuated [210]. While soil salinity and drought lead to osmotic stress in plants, waterlogging creates hypoxia conditions for roots which is usually overcome by a shift in starch metabolism to fermentation that manifests in reduced growth. Similarly, the presence of heavy metals in soil stimulates the accumulation of metal chelates, polyphosphates and organic acids which extenuates the severity of impact, the growth, nevertheless, is affected.
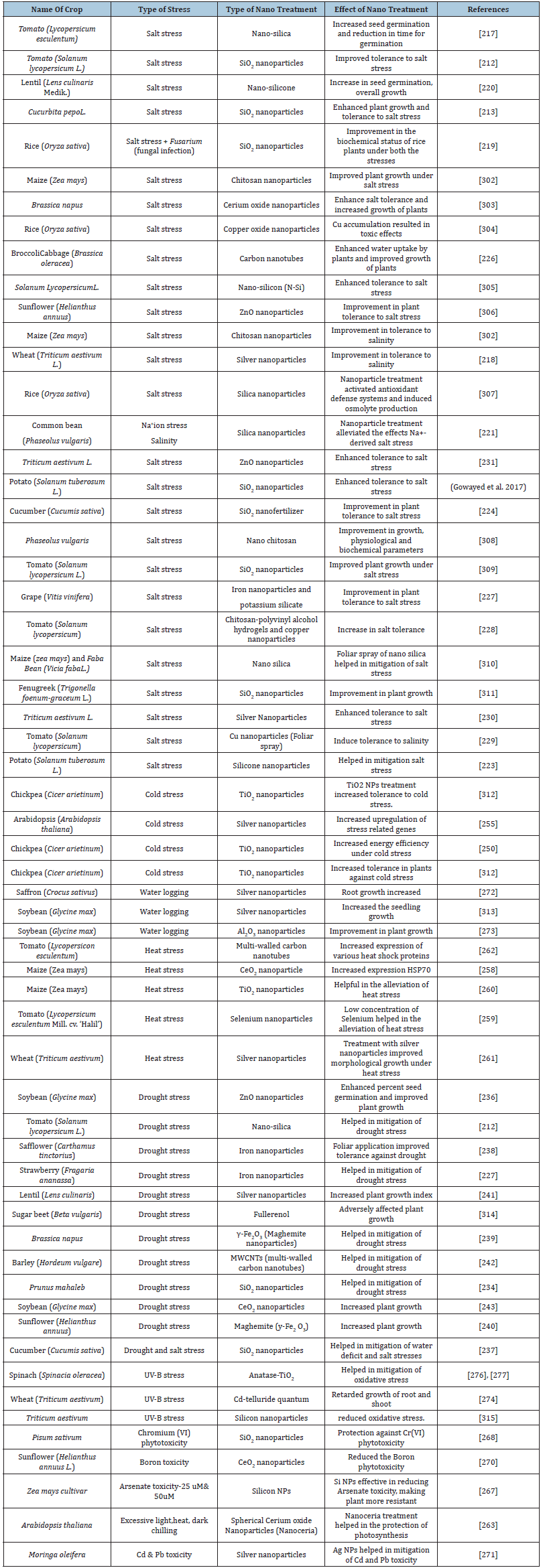
Nanoparticles, owing to various structural/functional attributes, have the potential to mitigate the disruptive effects of abiotic stresses on plants by 1) imitating the activities of antioxidant enzymes that scavenge the ROS [211], 2) by binding to the heavy metals thereby preventing it from getting incorporated into the plant system, 3) by improving the rate of photosynthesis by protecting the photosystems [212], [213], and 4) by alteration of expression of the genes involved in plant defence responses. However, the response of plants to nanoparticle types and the concentration used varied from species to species [214]. This has opened many avenues to explore a range of nanoparticles that could help plants overcome the abiotic stress induced barriers. In the recent times there has been a spate of reports which prove the potential of nanoparticles in mitigation of abiotic stresses in plants. (Table 2) sum-up the role of nanoparticles in circumventing abiotic stresses.
Table 2:Role of nanoparticles in mitigation of abiotic stresses in crop plants.
Salinity stress:Saline soils are physiologically dry soils. High concentration of salt in soil makes the soil water potential more negative that reduces the potential gradient between the soil and the roots. This hampers the uptake by roots that culminates in the build-up of ionic toxicity and nutritional imbalance in plants. Besides, vital processes like lipid metabolisms, protein synthesis, photosynthesis are also affected [215]. Reports are available on the potential of Si and SiO2 nanoparticles and silicon fertilizer in the alleviation of salt stress in several plants such as Basil [216]; Tomato [212,217]; Wheat [218]; Rice [219]; Lentil [220]; Common Bean [221]; Potato [222,223]; Cucumber [224] etc. A significant increase in the plant growth, chlorophyll content, proline level, antioxidant enzyme system, photosynthetic rate and other vital processes, subsequent to nanoparticle treatment, have been observed.
Silica nanoparticles help in the mitigation of salinity stress by decreasing the Na+ absorption by different plant tissues. Decreased levels of Na+ concentration in tissues maintain the osmotic potential of plants which improves the absorption of water and minerals from the soil, that stimulates the plant growth and development under salt stress [225]. In broccoli, use of multiwalled carbon nanotubes helped in alleviation of salt stress by slight alteration in plasma membrane properties and aquaporin transduction, transportation that increased water uptake and net assimilation of CO2 [226]. Other nanoparticles used effectively in mitigation of salt stress includes iron nanoparticles in Grapes [227], chitosan in Tomato [228], Copper oxide in Tomato [229], silver in Wheat [230], ZnO in Wheat [231], Maghnemite in peppermint [232] etc. which showed the similar response like silica nanoparticle.
Drought stress:Drought causes wilting of plants. With little or no water available in the surroundings although some plant species undergo rolling of leaves to prevent the loss of water by exposing less leaf surface to dry air, this nevertheless, reduces growth, vigour and the rate of photosynthesis [233]. Treatment of silicon in Sorghum and silica nanoparticles in hawthorn (Crataegus sp.) showed improved drought tolerance. The seedlings of hawthorn treated with different concentrations of silica nanoparticles, showed a positive response on photosynthesis, water, proline, carbohydrate, Malondialdehyde (MDA) and chlorophyll content [234]. Similarly, treatment of two cultivars of Sorghum with silicon nanoparticles improved the water uptake by improving shoot to root ratio in both the cultivars irrespective of their susceptibility for drought stress [235,236]. The use of silica nanoparticles have also helped in mitigating the adverse effects of drought stress in Tomato, Prunus and Cucumis by improving the water uptake and mineral absorption [212,234,237]. In drought stressed soybean seeds enhancement in seed germination and shoot to root ratio was observed upon treatment with ZnO nanoparticles [236].
Due to drought and salinity stress, it becomes difficult to absorb iron from the soil which is an important micronutrient for the plants. The deficiency of iron besides causing significant damage to metabolism, leads to chlorosis in plants. Several studies have been conducted to reveal the mitigating effects of Fe nanoparticles under drought stress. Foliar application of iron nanoparticles in safflower and irrigation of strawberry with solution containing iron nanoparticles improved the resistance against drought in both the plants [227,238]. Similar results were obtained when Brassica napus and sunflower seeds were treated with γ-Fe2O3 (Maghemite nanoparticles) [239,240]. Promising role of nanoparticles in the improvement of physicochemical attributes and agronomic traits under drought conditions have also been recorded in Lentil, Barley, Soybean and Wheat upon treatment with silver nanoparticles, multiwalled carbon nanotubes, CeO2 and TiO2 nanoparticles, respectively [241-244]
Chilling stress:Exposures to very cold temperatures have had varying effects on the functioning of plants. Freezing temperature damages the plant cell by distortion of permeability and leakage of ions from the membrane which reduces the germination of seeds and the growth of plants [245]. Chilling stress also adversely affects photosynthesis in plants by damaging rubisco enzyme and by reducing chlorophyll content, CO2 assimilation and rate of transpiration [247,248]. It is only the cold tolerant species that show less damaging effect of chilling [246]. Application of nanoparticles helps in the alleviation of damaging effects of chilling stress by (1) decreasing the membrane damage and reducing the ion leakage [249]; (2) Enhanced production of Rubisco enzyme and gene expression of chlorophyll binding gene [250], (3) Increasing ability of chloroplast to immerse light [251], (4) Increasing the antioxidant enzyme activity [252] and by (5) inhibiting the ROS production [253]. Application of TiO2 in Chickpea [250,254] and silver nanoparticles in Arabidopsis [255] increased upregulation of stress related genes that increased tolerance against cold stress.
Heat Stress:Exposure to high temperatures cause denaturation of proteins that hinder metabolic processes. Heat stress increases oxidative stress, which causes degradation of membrane lipids, leakage of ions, reduced rate of photosynthesis, decreased chlorophyll content and protein degradation [256,257]. Although, plants have overcome the harmful effects of elevated temperatures by the production of molecular chaperones and heat shock proteins (HSPs), the adverse effects of heat stress persist still in some plants. The use of nanoparticles like Cerium oxide and TiO2 in Maize [258,260], Selenium in Tomato [259] and silver nanoparticles in Wheat [261] improved the tolerance of plants against heat stress. Application of Selenium, CeO2, TiO2 and multiwalled carbon nanotubes have been reported to enhance the upregulation of heat shock proteins such as HSP70 and HSP90 [258,260,262]. Treatment with Spherical Cerium oxide nanoparticles (Nanoceria) in Arabidopsis reduced the stress caused by light, heat, dark and chilling conditions [263].
Heavy metal stress and other stresses:Heavy metal stress disturbs the uptake of nutrients and vital supplements by the plants. This apart, besides suppressing the activity of the enzyme, it disturbs the uptake causing reduced plant growth due to the deficiency of essential nutrients [264]. Presence of heavy metals in environment cause oxidative stress in plants which degrade the plant metabolism. Treatment of TiO2 and Al2O3 nanoparticles in tobacco plants increased the upregulation of miRNA expression which in result improved the plant tolerance against heavy metal stress [265,266]. Similarly, while application of silicon nanoparticles in maize reduced the arsenate toxicity [267], in Pea and bamboo it reduced chromium toxicity and lead toxicity respectively [268,269]; SeO2 reduced boron toxicity in Sunflower [270]; Silver nanoparticles reduced lead and cadmium toxicity in Moringa [271].
Waterlogging chokes the root system by depleting the oxygen content of soil. This causes accumulation of carbon dioxide which ceases germination and growth. Application of silver nanoparticles in Saffron and Soybean and Al2O3 nanoparticles in Soybean has improved the corm and seed germination, respectively, and increased the plant growth under water logging stress [272,273]. Treatment of Cd-telluride quantum dots and silicon nanoparticles in wheat proved successful in alleviating the UV-B stress by redu oxidative stress [274,275]. A similar response was observed in spinach with the treatment of Anatase-TiO2 nanoparticles under UV-B stress [276,277].
Mechanism of Action of Nanoparticles under Abiotic Stress
The chemical and physical interaction of nanoparticles with biological systems such as plants is mainly due to their intrinsic catalytic reactivity, nano size and large surface area. Generation of reactive oxygen species (ROS), during the interaction of nanoparticles with plants under abiotic stress, is a common phenomenon [260,278]. Besides inducing ROS, nanoparticles have triggered the upregulation of a number of stress related genes [211]. Furthermore, it can imitate the antioxidant enzymes and other signalling molecules that result in transcriptional changes and alteration in secondary metabolite production. Changes in the metabolic pathway subsequent to nanoparticle treatment has improved the tolerance of plants against the stress conditions [279]. There are very few studies on the effect of nanoparticles on antioxidant enzymes and their interaction at the molecular level. Antioxidant enzymes play an important role in the defence mechanism of plants against all stresses. It helps in the scavenging of ROS and reactive nitrogen species (RNS) which cause oxidative stress in biological systems. The commonly present antioxidant enzymesinclude catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol and glutathione reductase (GR), glutathione peroxidase (GPX) etc. Application of gold nanoparticles in Brassica juncea [280]; CeO2 nanoparticles in kidney bean [281] and silver nanoparticles in Spirodela polyrhiza have enhanced the production of these enzymes [282].
Under stress conditions, the defence system is activated by signalling network. Calcium ions play a major role in signal transduction pathway and are called the second messenger. Stimulus of any kind of stress elevates the level of cytosolic Ca2+ concentration through calcium ion channels which eventually bind with Ca2+ -binding protein (CaBP) and activate them (Figure 2). The activated CaBPs directly bind to promoters of specific stress related genes and causes the repression or induction of their expression and accordingly, induce tolerance against the stress. Nanoparticles can imitate Ca2+ ions and bind with the CaBPs. It can cause overexpression of CaBPs that triggers the activation of plant defence system through expression of stress related genes [283]. Silver nanoparticles bind with Ca+/Na+ ion pumps via calcium ion receptors or calcium ion channels and instigate the signaling cascade in plants [284]. In addition, C60 nanocrystals activates the Ca+/calmodulin-dependent protein kinase II [285]; cadmium sulfide QDs induced overexpression of calcium-binding protein CML45 as well as calcium-dependent protein kinase 23 [286]. These CaBPs play an important role in the development of resistance in plants against abiotic stresses [287,288].
Figure 2: Shows the mechanism of action of nanoparticles in plants under stress stimulus. NP- Nanoparticles; CCa2+ ions; CaBPs- Calcium binding proteins; ROS- Reactive oxygen species. Source: Modified after Khan et al. [283].
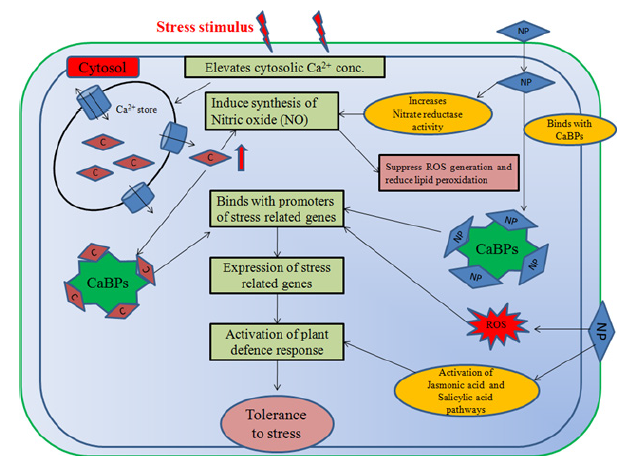
Figure 3: Percentage of salinity in coastal water samples.
It has been observed that Nitric Oxide (NO) modulates the antioxidant gene expression and suppresses the generation of ROS and eventually reduces lipid peroxidation. Treatment of nanoparticles can elevate the level of Ca2+ in the cytosol which induces the synthesis of Nitric oxide (NO). In addition, nanoparticles can increase the nitrate reductase enzyme activity in plants which enhances the concentration of NO to activate the immune response in plants under biotic and abiotic stress conditions [289,290]
Under biotic stress conditions plant induces two types of resistance (1) Acquired systemic resistance (ASR) via the Salicylic acid (SA) signaling pathway and (2) induced systemic resistance (ISR) through the Jasmonic acid (JA) and/or ethylene signaling pathways [291]. Jasmonates and salicylic acid interact with hormones such as Auxins, Ethylene, and Gibberellins that regulate plant growth as well as defense responses to various stresses [292,293]. Application of Chitosan nanoparticles induces plant defense response through the SA pathway under biotic stress [294] and the JA pathway under abiotic stress conditions [295]. In a study the activation of ISR in wheat through JA pathways with the treatment of TiO2 nanoparticles was reported [296]. In another study, Chitosan-PVA and Chitosan-PVA + Cu NPs promote the expression of SOD genes and JA in tomato under salinity stress [228].
The mechanism of action of nanoparticles is still not well known. However, it is envisaged that the genomic and proteomic studies can help in elucidation of the exhaustive mechanism of nanoparticles under abiotic stress conditions. In a study the modification in the proteins involved in the metabolism of sulphur in roots of Eruca sativa following the treatment with silver nanoparticles was reported [297]. Application of polyvinylpyrrolidone (PVP) coated silver nanoparticles induced the expression of stress related genes in Arabidopsis thaliana [298]. The transcriptional response of this model plant was analyzed by cDNA expression microarrays which showed the upregulation of 286 genes including metal and oxidative stress related genes and downregulation of 81 genes including ethylene signalling pathway. Treatment of TiO2 and Al2O3 nanoparticles on tobacco plants showed the upregulation of miRNA which playsa significant role in abiotic stresses [265,266].
The proteomic study of rice with the treatment of silver nanoparticles showed 1) The upregulation of stress related genes, 2) Ca2+ regulation and signalling, 3) Induction of oxidative stress response pathway, 4) Cell division and cell wall synthesis, 5) Protein degradation, and 6) Apoptosis [284]. The exposure of ZnO, TiO2, and fullerene soot in the roots of Arabidopsis thaliana and multiwalled carbon nanotubes in Tomato resulted in the upregulation of biotic and abiotic stress related genes [299,300]. Sores et al. reported that NiO nanoparticles induced oxidative stress in Hordeum while the treatment of NiO in combination with SiO2 nanoparticles helped in mitigation of oxidative stress indicating the protective role of SiO2 nanoparticles in abiotic stress [301].
Conclusion
Nanoparticles have emerged as potent tool endowed with qualities of eliciting the defensive response in plants under stress conditions. Its small size, large surface area, easy absorbance/ penetration and the ease of finding the target site has given it an edge over the other conventional methods employed to overcome stress related problems. The positive response shown by a range of crop species under varying stress conditions like high salinity [302- 315], presence of heavy metals, extreme temperatures, and drought conditions, subsequent upon nanoparticle treatment, underpins the positive role played by the nanoparticles in alleviating stress induced complications. Although nanoparticles are being widely used, its mechanism of action is still not yet clear. It is surmised that any insight gained into the mechanism of nanoparticle action will be pivotal realizing that agriculture in India contribute about 18 per cent of the GDP while providing employment to more than 50 per cent workforce. A thorough understanding of the mechanism of nanoparticle action will pave the way for developing new strategies to tackle a range of stress related problems confronting the agriculturists.
Author Contribution Statement
Dr. Savita- Searching of literature and compilation of data in the
form of tables.
Dr. Anju Srivastava: Editing and expert inputs.
Dr. Reena Jain: Editing and expert inputs.
Dr. Pratap Kumar Pati: Editing and expert inputs.
Dr. K.K. Koul: Editing and expert inputs.
References
- FAO (2009) High level expert forum-how to feed the world in 2050. Economic and Social Development, Food and Agricultural Organization of the United Nations, Italy.
- Pérez-de-Luque A, Rubiales D (2009) Nanotechnology for parasitic plant control. Pest Manag Sci 65(5): 540-545.
- Roco MC (2003) Broader societal issues of nanotechnology. J Nanoparticle Res 5: 181-189.
- Dubchak S, Ogar A, Mietelski JW, Turnau K (2010) Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Spanish J Agric Res 8(1): 103-108.
- Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. Nanotechnology and Plant Sciences, p. 19-35.
- Virkutyte J, Varma RS (2012) Environmentally friendly preparation of metal nanoparticles, p. 7-33.
- Christian P, Kammer FV, Baalousha M, Hofmann T (2008) Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17(5): 326-343.
- Thalla CP, Devatha A (2018) Synthesis of inorganic nanomaterials. Micro Nano Technol, pp. 169-184.
- Saif S, Tahir A, Chen Y (2016) Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 6(11): 209.
- Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P (2006) The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol 69(5): 485-492.
- Kozma G, Rónavári A, Kónya Z, Kukovecz A (2016) Environmentally benign synthesis methods of zero-valent iron nanoparticles. ACS Sustain Chem Eng 4(1): 291-297.
- Vilchis-Nestor AR, Sánchez-Mendieta V, Camacho-López MA, Gómez-Espinosa RM, Camacho-López MA, et al. (2008) Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis Mater Lett 62(17-18): 3103-3105.
- Aswathy Aromal S, Philip D (2012) Facile one-pot synthesis of gold nanoparticles using tannic acid and its application in catalysis. Phys E Low-dimensional Syst Nanostructures 44(7-8): 1692-1696.
- Sun Q, Cai X, Li J, Zheng M, Chen Z, et al. (2014) Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surfaces A Physicochem Eng Asp 444: 226-231.
- Ajitha B, Ashok Kumar Reddy Y, Sreedhara Reddy P (2015) Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater Sci Eng C 49: 373-381.
- Huang L, Weng X, Chen Z, Megharaj M, Naidu R (2014) Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity. Spectrochim Acta Part A Mol Biomol Spectrosc 130: 295-301.
- Wang T, Lin J, Chen Z, Megharaj M, Naidu R (2014) Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J Clean Prod 83: 413-419.
- Wang Z, Xu C, Li X, Liu Z (2015) In situ green synthesis of Ag nanoparticles on tea polyphenols-modified graphene and their catalytic reduction activity of 4-nitrophenol. Colloids Surfaces A Physicochem Eng Asp 485: 102-110.
- Weng X, Huang L, Chen Z, Megharaj M, Naidu R (2013) Synthesis of iron-based nanoparticles by green tea extract and their degradation of malachite. Ind Crops Prod 51: 342-347.
- Shahwan T, Abu Sirriaha S, Nairata M, Boyacı E, Eroğlu AE, et al. (2011) Green synthesis of iron nanoparticles and their application as a fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J 172(1): 258-266.
- Smuleac V, Varma R, Sikdar S, Bhattacharyya D (2011) Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J Memb Sci 379(1-2): 131-137.
- Xin H, Yang X, Liu X, Tang X, Weng L, et al. (2016) Biosynthesis of iron nanoparticles using tie guanyin tea extract for degradation of bromothymol blue. J Nanotechnol, p. 1-8.
- Plachtová P, Medříková Z, Zbořil R, Tuček J, Varma RS, et al. (2018) Iron and iron oxide nanoparticles synthesized with green tea extract: Differences in ecotoxicological profile and ability to degrade malachite green. ACS Sustain Chem Eng 6(7): 8679-8687.
- Lourenço IM, Pieretti JC, Nascimento MHM, Lombello CB, Seabra AB (2019) Eco-friendly synthesis of iron nanoparticles by green tea extract and cytotoxicity effects on tumoral and non-tumoral cell lines. Energy Ecol Environ 4(6): 261-270.
- Hoag GE, Collins JB, Holcomb JL, Hoag JR, Nadagouda MN, et al. (2009) Degradation of bromothymol blue by greener nano-scale zero-valent iron synthesized using tea polyphenols. J Mater Chem 19(45): 8671.
- Markova Z, Novak P, Kaslik J, Plachtova P, Brazdova M, et al. (2014) Iron(II,III)–polyphenol complex nanoparticles derived from green tea with remarkable ecotoxicological impact. ACS Sustain Chem Eng 2(7): 1674-1680.
- KSV G, Harika Reddy P, Zamare D (2017) Green synthesis of iron nanoparticles using green tea leaves extract. J Nanomedine Biotherapeutic Discov 7(1).
- Nadagouda MN, Castle AB, Murdock RC, Hussain SM, Varma RS (2010) In vitro biocompatibility of Nanoscale Zerovalent Iron Particles (NZVI) synthesized using tea polyphenols. Green Chem 12(1): 114-122.
- Kuang Y, Wang Q, Chen Z, Megharaj M, Naidu R (2013) Heterogeneous fenton-like oxidation of monochlorobenzene using green synthesis of iron nanoparticles. J Colloid Interface Sci 410: 67-73.
- Sutradhar P, Saha M, Maiti D (2014) Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J Nanostructure Chem 4(1): 86.
- Asghar MA, Zahir E, Shahid SM, Khan MN, Asghar MA, et al. (2018) Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 90: 98-107.
- Ankamwar B, Damle C, Ahmad A, Sastry M (2005) Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5(10): 1665-1671.
- Ramesh PS, Kokila T, Geetha D (2015) Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim Acta Part A Mol Biomol Spectrosc 142: 339-343.
- Ramanujam K, Sundrarajan M (2014) Antibacterial effects of biosynthesized MgO nanoparticles using ethanolic fruit extract of Emblica officinalis. J Photochem Photobiol B Biol 141: 296-300.
- Anbukkarasi V, Srinivasan R, Elangovan N (2015) Antimicrobial activity of green synthesized zinc oxide nanoparticles from Emblica Officinalis. International Journal of Pharmaceutical Sciences Review and Research 33(2): 110-115.
- Jagajjanani Rao K, Paria S (2013) Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater Res Bull 48(2): 628-634.
- Rao KJ, Paria S (2015) Aegle marmelos leaf extract and plant surfactants mediated green synthesis of Au and Ag nanoparticles by optimizing process parameters using taguchi method. ACS Sustain Chem Eng 3(3): 483-491.
- Jha AK, Prasad K (2011) Biosynthesis of gold nanoparticles using bael (Aegle marmelos) leaf: Mythology meets technology. Int J Green Nanotechnol 3(2): 92-97.
- Tripathy A, Raichur AM, Chandrasekaran N, Prathna TC, Mukherjee A (2010) Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanoparticle Res 12(1): 237-246.
- TC P, Mathew L, Chandrasekaran N, Ashok M, Mukherjee A (2010) Biomimetic synthesis of nanoparticles: Science, technology applicability. Biomimetics Learning from Nature.
- Lalitha A, Subbaiya R, Ponmurugan P (2013) Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol App Sci 2(6): 228-235.
- Jadhav MS, Kulkarni S, Raikar P, Barretto DA, Vootla SK, et al. (2018) Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J Chem 42(1): 204-213.
- Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275(2): 496-502.
- Kumar N, Vazhacharickal P, Mathew J, Joy J (2015) Synthesis of silver nano particles from neem leaf (Azadirachta indica) extract and its antibacterial activity CIB tech. J Biotechnol 4: 20-31.
- Velusamy P, Das J, Pachaiappan R, Vaseeharan B, Pandian K (2015) Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L). Ind Crops Prod 66: 103-109.
- Oudhia A, Kulkarni P, Sharma S (2015) Green synthesis of Zno nanotubes for bioapplications. Int J Adv Eng Res Stud 4(2): 280-281.
- Elumalai K, Velmurugan S (2015) Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl Surf Sci 345: 329-336.
- Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32: 55-61.
- Madan HR, Sharm SC, Udayabhanu, Suresh D, Vidya YS, et al. (2016) Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim Acta Part A Mol Biomol Spectrosc 152: 404-416.
- Moorthy T, Ashok C, Rao KV, Viswanathan C (2015) Syntheis and characterization of MgO nanoparticles by neem leaves through green method. Mater Today Proc 2(9): 4360-4368.
- Rajendaran K, Muthuramalingam R, Ayyadurai S (2019) Green synthesis of Ag-Mo/CuO nanoparticles using Azadirachta indica leaf extracts to study its solar photocatalytic and antimicrobial activities. Mater Sci Semicond Process 91: 230-238.
- Sharma DK, Shah DV, Roy DR (2018) Green synthesis of CuO nanoparticles using Azadirachta indica and its antibacterial activity for medicinal applications. Mater Res Express 5(9).
- Ansilin S, Nair JK, Awasthy C, Rama V, Peter J, et al. (2016) Green synthesis and characterisation of copper oxide nanoparticles using Azadirachta indica (Neem) leaf aqueous extract. J Nanosci Technol 2(5): 221-223.
- Dey A, Manna S, Chattopadhyaya S, Mondal D, Chattopadhyay D, et al. (2019) Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J Saudi Chem Soc 23(2): 222-238.
- Rafique M, Shaikh AJ, Rasheed R, Tahir MB, Gillani SSA, et al. (2018) Aquatic biodegradation of methylene blue by copper oxide nanoparticles synthesized from Azadirachta indica leaves extract. J Inorg Organomet Polym Mater 28(6): 2455-2462.
- Rehana D, Mahendiran D, Kumar RS, Rahiman AK (2017) Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed Pharmacother 89: 1067-1077.
- Nagar N, Devra V (2018) Green synthesis and characterization of copper nanoparticles using Azadirachta indica Mater Chem Phys 213: 44-51.
- Hamadneh I, Alhayek H, Al-Mobydeen A, Jaber AA, Albuqain R, et al. (2019) Green synthesis and characterization of yttrium oxide, copper oxide and barium carbonate nanoparticles using Azadirachta Indica (the neem tree) fruit aqueous extract. Egypt J Chem 62(4): 573-581.
- Thirumurugan A, Harshini E, Marakathanandhini BD, Kannan SR, Muthukumaran P (2017) Catalytic degradation of reactive red 120 by copper oxide nanoparticles synthesized by Azadirachta indica. Bioremediation and Sustainable Technologies for Cleaner Environment, pp. 95-102.
- Jassal V, Shanker U, Kaith BS, Shankar S (2015) Green synthesis of potassium zinc hexacyanoferrate nanocubes and their potential application in photocatalytic degradation of organic dyes. RSC Adv 5(33): 26141-26149.
- Reddy V, Torati RS, Oh S, Kim C (2013) Biosynthesis of gold nanoparticles assisted by Sapindus mukorossi fruit pericarp and their catalytic application for the reduction of p-nitroaniline. Ind Eng Chem Res 52(2): 556-564.
- Sharma P, Babu PJ, Bora U (2012) Sapindus mukorossi aqueous fruit extract as reducing, capping and dispersing agents in synthesis of gold nanoparticles. Micro Nano Lett 7(12): 1296-1299.
- Elumalai S, Devika R (2014) Biosynthesis of silver nanoparticles using Curcuma longa and their antibacterial activity. Int J Pharm Res Sci 2(1): 98-103.
- Prabhu SN (2015) Green route synthesis of stable isotropic gold nanoparticles using leaf extract of Curcuma longa and their characterization. Adv Appl Sci Res 6(8): 167-169.
- Mihir H, Siddhivinayak B (2015) Calcination and microwave assisted biological synthesis of iron oxide nanoparticles and comparative efficiency studies for domestic wastewater treatment. Int Res J Environ Sci 4(6): 28-36.
- Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, et al. (2011) Green synthesis and antibacterial effect of silver nanoparticles using vitex negundo L. Molecules 16(8): 6667-6676.
- Fatimah I, Yudha SP, Mutiara NAL (2016) Green synthesis of ZnO nanoparticles via complex formation by using Curcuma longa AIP Conf Proc 1710(1).
- Naghikhani R, Nabiyouni G, Ghanbari D (2018) Simple and green synthesis of CuFe2O4-CuO nanocomposite using some natural extracts: photo-degradation and magnetic study of nanoparticles. J Mater Sci Mater Electron 29(6): 4689-4703.
- Das RK, Sharma P, Nahar P, Bora U (2011) Synthesis of gold nanoparticles using aqueous extract of Calotropis procera Mater Lett 65(4): 610-613.
- Poovizhi J, Krishnaveni B (2015) Synthesis, characterization and antimicrobial activity of zinc oxide nanoparticles synthesized from Calotropis Procera. Int J Pharm Sci Drug Res 7(5): 425-431.
- Singh RP, Shukla VK, Yadav RS, Sharma PK, Singh PK, et al. (2011) Biological approach of zinc oxide nanoparticles formation and its characterization. Adv Mater Lett 2(4): 313-317.
- Dubey S, Sharma YC (2017) Calotropis procera mediated one pot green synthesis of cupric oxide nanoparticles (CuO-NPs) for adsorptive removal of Cr(VI) from aqueous solutions. Appl Organomet Chem 31(12): e3849.
- Reddy KR (2017) Green synthesis, morphological and optical studies of CuO nanoparticles. J Mol Struct 1150: 553-557.
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 79(3): 594-598.
- Ijaz F, Shahid S, Khan SA, Ahmad W, Zaman S (2017) Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial , antioxidant and photocatalytic dye degradation activities. Trop J Pharm Res 16(4): 743-753.
- Edison TNJI, Sethuraman MG (2013) Electrocatalytic reduction of benzyl chloride by green synthesized silver nanoparticles using pod extract of Acacia Nilotica. ACS Sustain Chem Eng 1(10): 1326-1332.
- Awwad AM, Amer AW (2020) Biosynthesis of copper oxide nanoparticles using Ailanthus altissima leaf extract and antibacterial activity. Chem Int 6(4): 210-217.
- Laokul P, Maensiri S (2009) Aloe vera solution synthesis and magnetic properties of Ni-Cu-Zn ferrite nanopowders. J Optoelectron Adv Mater 11: 857-862.
- Gunalan S, Sivaraj R, Venckatesh R (2012) Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: Optical properties. Spectrochim Acta Part A Mol Biomol Spectrosc 97: 1140-1144.
- Maensiri S, Laokul P, Klinkaewnarong J, Phokha S, Promarak V, et al. (2008) Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. Optoelectron Adv Mater Rapid Commun 2(3): 161-165.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22(2): 577-583.
- Qian Y, Yao J, Russel M, Chen K, Wang X (2015) Characterization of green synthesized nano-formulation (ZnO-A. vera) and their antibacterial activity against pathogens. Environ Toxicol Pharmacol 39(2): 736-746.
- Ali K, Dwivedi S, Azam A, Saquib Q, Al-Said MS, et al. (2016) Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J Colloid Interface Sci 472: 145-156.
- Kumar PPNV, Shameem U, Kollu P, Kalyani RL, Pammi SVN (2015) Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. Bionanoscience 5(3): 135-139.
- Sangeetha G, Rajeshwari S, Venckatesh R (2011) Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: Structure and optical properties. Mater Res Bull 46(12): 2560-2566.
- Kumar DA, Palanichamy V, Roopan SM (2014) Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 127: 168-171.
- Anbuvannan M, Ramesh M, Viruthagiri G, Shanmugam N, Kannadasan N (2015) Synthesis, characterization and photocatalytic activity of ZnO nanoparticles prepared by biological method. Spectrochim Acta Part A Mol Biomol Spectrosc 143: 304-308.
- Ramesh C, Prasad MH, Ragunathan V (2011) Effect of Arachis hypogaea L. leaf extract on barfoeds solution; Green synthesis of Cu2O nanoparticles and its antibacterial effect. Curr Nanosci 7(6): 995-999.
- Forough M, Farhadi K (2010) Biological and green synthesis of silver nanoparticles. Turkish J Eng Environ Sci 34(4): 281-287.
- Thema FT, Manikandan E, Dhlamini MS, Maaza M (2015) Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater Lett 161: 124-127.
- Manjari G, Saran S, Arun T, Vijaya Bhaskara Rao A, Devipriya SP (2017) Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. Journal of Saudi Chemical Society 21(5): 610-618.
- Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, et al. (2012) Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem 47(12): 2405-2410.
- Nasrollahzadeh M, Mohammad Sajadi S, Rostami-Vartooni A (2015) Green synthesis of CuO nanoparticles by aqueous extract of Anthemis nobilis flowers and their catalytic activity for the A3 coupling reaction. Journal of Colloid and Interface Science 459: 183-188.
- Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma H (2010) Green synthesis of silver nanoparticles using argemone mexicana leaf extract and evaluation of their antimicrobial activities. Digest Journal of Nanomaterials and Biostructures 5(2): 483-489.
- Thakur S, Sharma S, Thakur S, Radheshyam R (2018) Green synthesis of copper nano-particles using asparagus adscendens root and leaf extract and their antimicrobial activities. Int J Curr Microbiol Appl Sci 7(4): 683-694.
- Fardood ST, Ramazani A (2016) Green synthesis and characterization of copper oxide nanoparticles using coffee powder extract. J Nanostructures 6(2): 167-171.
- Sharmila G, Sakthi Pradeep R, Sandiya K, Santhiya S, MuthukumaranC, et al. (2018) Biogenic synthesis of cuo nanoparticles using bauhinia tomentosa leaves extract: Characterization and its antibacterial application. J Mol Struct 1165: 288-292.
- Maruthupandy M, Yong Zuo, Chen JS, Song JM, Niu HL, et al. (2017) Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications. Appl Surf Sci 397: 167-174.
- Yang X, Li Q, Wang H, Huang J, Lin L, et al. (2010) Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora Journal of Nanoparticle Research 12: 1589-1598.
- Huang J, Lin L, Qingbiao L, Daohua S, Wang Y, et al. (2008) Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried cinnamomum camphora leaf in tubular microreactors. Ind Eng Chem Res 47(16): 6081-6090.
- Zhan G, Huang J, Du M, Abdul Rauf I, Ma Y, et al. (2011) Green synthesis of Au-Pd bimetallic nanoparticles: Single-step bioreduction method with plant extract. Mater Lett 65(19-20): 2989-2991.
- Saranyaadevi K, Subha V, Sachidanandan ERR, Renganathan S (2014) Synthesis and characterization of copper nanoparticle using capparis zeylanica leaf extract. Int J ChemTech Res 6(10): 4533-4541.
- Li S, Shen Y, Xie A, Xuerong Y, Qiu L, et al. (2007) Green synthesis of silver nanoparticles using capsicum annuum L. Green Chem 9(8): 852-858.
- Sankar R, Manikandan P, Malarvizhi V, Fathima T, Shivashangari KS, et al. (2014) Green synthesis of colloidal copper oxide nanoparticles using carica papaya and its application in photocatalytic dye degradation. Spectrochim Acta Part A Mol Biomol Spectrosc 121: 746-750.
- Nasrollahzadeh M, Sajjadi M, Sajadi SM (2018) Biosynthesis of copper nanoparticles supported on manganese dioxide nanoparticles using centella asiatica leaf extract for the efficient catalytic reduction of organic dyes and nitroarenes. Chinese J Catal 39(1): 109-117.
- Nagajyothi PC, Sreekanth TVM, Tettey CO, Jun YI, Mook SH (2014) Characterization, antibacterial, antioxidant, and cytotoxic activities of zno nanoparticles using coptidis rhizoma. Bioorg Med Chem Lett 24(17): 4298-4303.
- Shende S, Ingle AP, Gade A, Rai M (2015) Green synthesis of copper nanoparticles by citrus medica linn. (idilimbu) juice and its antimicrobial activity. World J Microbiol Biotechnol 31(6): 865-873.
- Satyavani K, Ramanathan T, Gurudeeban S (2011) Plant mediated synthesis of biomedical silver nanoparticles by using leaf extract of citrullus colocynthis. Res J Nanosci Nanotechnol 1(2): 95-101.
- Arunachalam R, Dhanasingh S, Kalimuthu B, Uthirappan M, Rose C, et al. (2012) Phytosynthesis of silver nanoparticles using coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surfaces B Biointerfaces 94: 226-230.
- Krupa AND, Vimala R (2016) Evaluation of Tetraethoxysilane (TEOS) sol-gel coatings, modified with green synthesized zinc oxide nanoparticles for combating microfouling. Mater Sci Eng C Mater Biol Appl 61: 728-735.
- Sathyavathi R, Krishna MB, Rao SV, Saritha R, Rao DN (2010) Biosynthesis of silver nanoparticles using coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett 3(2): 138-143.
- Roy N, Alam MDN, Mondal S, Ismail Sk, Laskar RA, et al. (2012) Exploring indian rosewood as a promising biogenic tool for the synthesis of metal nanoparticles with tailor-made morphologies. Process Biochem 47(9): 1371-1380.
- Singh C, Baboota R, Naik PK, Harvinder S (2012) Biocompatible synthesis of silver and gold nanoparticles using leaf extract of dalbergia sissoo. Adv Mater Lett 3(4): 279-285.
- Muniyappan N, Nagarajan NS (2014) Green synthesis of silver nanoparticles with dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem 49(6): 1054-1061.
- Narasaiah P, Mandal BK, Sarada NC (2017) Biosynthesis of copper oxide nanoparticles from drypetes sepiaria leaf extract and their catalytic activity to dye degradation. IOP Conf Ser Mater Sci Eng 263(2).
- Chung I, Rahuman A, Marimuthu S, Kirthi AV, Anbarasan K, et al. (2017) Green synthesis of copper nanoparticles using eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med May 14(1): 18-24.
- Vanathi P, Rajiv P, Narendhran S, Rajeshwari S, Rahman PKSM, et al. (2014) Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater Lett 134: 13-15.
- Madhavi V, Prasad TNVKV, Reddy AVB, Ravindra BR, Madhavi G (2013) Application of phytogenic zerovalent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim Acta Part A Mol Biomol. Spectrosc 116: 17-25.
- Wang Z, Fang C, Megharaj M (2014) Characterization of iron-polyphenol nanoparticles synthesized by three plant extracts and their fenton oxidation of azo dye. ACS Sustain Chem Eng 2(4): 1022-1025.
- Wang T, Jin X, Chen Z, Megharaj M, Naidu R (2014) Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ 466-467: 210-213.
- Shanan ZJ, Hadi SM, Shanshool SK (2018) Structural analysis of chemical and green synthesis of cuo nanoparticles and their effect on biofilm formation. Baghdad Sci J 15(2): 211-216.
- Maham M, Sajadi SM, Kharimkhani MM, Nasrollahzadeh M (2017) Biosynthesis of the cuo nanoparticles using euphorbia chamaesyce leaf extract and investigation of their catalytic activity for the reduction of 4-nitrophenol. IET Nanobiotechnology 11(7): 766-772.
- Nasrollahzadeh M, Sajadi SM, Khalaj M (2014) Green synthesis of copper nanoparticles using aqueous extract of the leaves of euphorbia esula l and their catalytic activity for ligand-free ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv 4(88): 47313-47318.
- Zangeneh MM, Ghaneialvar H, Akbaribazm M, Ghanimatdan M, Abbasi N, et al. (2019) Novel synthesis of falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo J Photochem Photobiol B Biol 197.
- Mehr ES, Sorbiun M, Ramazani A, Fardood ST (2018) Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using ferulago angulata (schlecht) boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. J Mater Sci Mater Electron 29(2): 1333-1340.
- Nasrollahzadeh M, Sajadi SM (2015) Green synthesis of copper nanoparticles using ginkgo biloba leaf extract and their catalytic activity for the huisgen [3+2] cycloaddition of azides and alkynes at room temperature. J Colloid Interface Sci 457: 141-147.
- Naika HR, Lingaraju K, Manjunath K, Danith K, Nagaraju G, et al. (2015) Green synthesis of cuo nanoparticles using gloriosa superba extract and their antibacterial activity. J Taibah Univ Sci 9(1): 7-12.
- Petla RK, Vivekanandhan S, Misra M, Mohanty AK, Satyanarayana N (2012) Soybean (glycine max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol 3(1): 14-19.
- Aladpoosh R, Montazer M (2015) The role of cellulosic chains of cotton in biosynthesis of zno nanorods producing multifunctional properties: Mechanism, characterizations and features. Carbohydr Polym 126: 122-129.
- Murthy HCA, Desalegn T, Kassa M, Abebe B, Assefa T (2020) Synthesis of green copper nanoparticles using medicinal plant hagenia abyssinica (brace) jf. gmel. leaf extract: antimicrobial properties. J Nanomater 2020: 1-12.
- Philip D (2010) Green synthesis of gold and silver nanoparticles using hibiscus rosa sinensis. Phys E: Low-dimensional Syst and Nanostructures 42(5): 1417-1424.
- Vishveshvar K, Aravind Krishnan MV, Haribabu K, Vishnuprasad S (2018) Green synthesis of copper oxide nanoparticles using ixiro coccinea plant leaves and its characterization. Bionanoscience 8(2): 554-558.
- Hudlikar M, Joglekar S, Dhaygude M, Kodam K (2012) Green synthesis of Tio2 nanoparticles by using aqueous extract of jatropha curcas Latex Mater Lett 75: 196-199.
- Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, et al. (2009) Green synthesis of silver nanoparticles using seed extract of jatropha curcas. Colloids Surfaces A Physicochem Eng Asp 348: 1-3.
- Cheirmadurai K, Biswas S, Murali R, Thanikaivelan P (2014) Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv 4(37): 19507-19511.
- Yogesh BA, Keshav KD, Gotan HJ, Ganesh EP, Suresh KG, et al. (2017) Biosynthesis of copper oxide nanoparticles using leaves extract of Leucaena leucocephala and their promising upshot against the selected human pathogens. Int J Mol Clin Microbiol 7(1): 776-786.
- Kathiravan V, Ravi S, Ashokkumar S (2014) Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta Part A Mol Biomol Spectrosc 130: 116-121.
- Elavazhagan T, Kantha DA (2011) Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine 6: 1265-1278.
- Savitha R, Saraswathi U (2016) A study on the preventive effect of silver nano particles synthesized from millingtonia hortensis in isoproterenol induced cardio toxicity in male wistar rats. World J Pharm Pharm Sci 5(8): 1442-1450.
- Kiran Kumar HA, Badal KM, Mohan Kumar K, Sireesh Babu M, Sai Kumar T, et al. (2014) Antimicrobial and antioxidant activities of Mimusops elengi seed extract mediated isotropic silver nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc 130: 13-18.
- Sathish Kumar G, Gobinath C, Karpagam K, Hemamalini V, Premkumar K, et al. (2012) Phyto-synthesis of silver nanoscale particles using Morinda citrifolia and its inhibitory activity against human pathogens. Colloids Surfaces B Biointerfaces 95: 235-240.
- Nasrollahzadeh M, Atarod M, Sajadi SM (2016) Green synthesis of the Cu/Fe3O4 nanoparticles using Morinda morindoides leaf aqueous extract: A highly efficient magnetically separable catalyst for the reduction of organic dyes in aqueous medium at room temperature. Appl Surf Sci 364: 636-644.
- Mubayi A, Chatterji S, Prasant KR, Geeta W (2012) Evidence based green synthesis of nanoparticles. Adv Mater Lett 3(6): 519-525.
- Vasanth K, Ilango K, Mohan Kumar R, Agrawal A, Dubey GP (2014) Anticancer activity of Moringa Oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces 117: 354-359.
- Elumalai K, Velmurugan S, Ravi S, Kathiravan V, Ashokkumar S (2015) Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 143: 158-164.
- Galan CR, Silva MF, Mantovani D, Bergamasco R, Vieira MF (2018) Green synthesis of copper oxide nanoparticles impregnated on activated carbon using Moringa Oleifera leaves extract for the removal of nitrates from water. Can J Chem Eng 96(11): 2378-2386.
- Chitra G, Balasubramani G, Ramkumar R, Sowmiya R, Perumal P (2015) Mukia maderaspatana (Cucurbitaceae) extract-mediated synthesis of silver nanoparticles to control Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Parasitol Res 114(4): 1407-1415.
- Aminuzzaman M, Kei LM, Liang WH (2017) Green synthesis of copper oxide (CuO) nanoparticles using banana peel extract and their photocatalytic activities. AIP Conference Proceedings 1828(1): 020016.
- Santhoshkumar T, Abdul AR, Raj Kumar G, Sampath M, Asokan B, et al. (2011) Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 108(3): 693-702.
- Yuvakkumar R, Suresh J, Nathanael AJ, Sundrarajan M, Hong SI (2014) Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum) peel extract and its antibacterial applications. Mater Sci Eng C Mater Biol Appl 41: 17-27.
- Sundrarajan M, Gowri S (2011) Green synthesis of titanium dioxide nanoparticles by nyctanthes arbor-tristis leaves extract. Chalcogenide Lett 8(8): 447-451.
- Mallikarjunaa K, Narasimha G, Dillipa GR, Praveen B, Shreedhar B, et al. (2011) Green synthesis of Silver nanoparticles using Ocimum leaf extract and their characterization. Dig J Nanomater Biostruct 6(1): 181-186.
- Yadav A, Kaushik A, Joshi A (2018) Green synthesis of silver nanoparticles using Ocimum sanctum and Ocimum americanum L. for their antibacterial potential. Int J Pharma Res 8(1): 42-49.
- Abdul Salam H, Sivaraj R, Venckatesh R (2014) Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum var. purpurascens Benth. Lamiaceae leaf extract. Mater Lett 131: 16-18.
- Sulaiman GM, Tawfeeq AT, Jaaffer MD (2018) Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro Biotechnol Prog 34(1): 218-230.
- Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, et al. (2013) Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces 108: 80-84.
- Rajiv P, Rajeshwari S, Venckatesh R (2013) Bio-fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc 112: 384-387.
- Nagajyothi PC, Muthuraman P, Sreekanth TVM, Kim DH, Shim J (2017) Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab J Chem 10(2): 215-225.
- Reddy NJ, Nagoor Vali D, Rani M, Rani SS (2014) Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater Sci Eng C Mater Biol Appl 34: 115-122.
- Nasrollahzadeh M, Momeni SS, Sajadi SM (2017) Green synthesis of copper nanoparticles using Plantago asiatica leaf extract and their application for the cyanation of aldehydes using K4Fe(CN)6. J Colloid Interface Sci 506: 471-477.
- Fu L, Fu Z (2015) Plectranthus amboinicus leaf extract-assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int 41(2): 2492-2496.
- Sundrarajan M, Ambika S, Bharathi K (2015) Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv Powder Technol 26(5): 1294-1299.
- Gopinath K, Gowri S, Arumugam A (2013) Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J Nanostructure Chem 3(1): 68.
- Saif S, Tahir A, Asim T, Chen Y (2016) Plant mediated green synthesis of cuo nanoparticles: comparison of toxicity of engineered and plant mediated cuo nanoparticles towards daphnia magna. Nanomaterials 6(11): 205.
- Kaur P, Thakur R, Chaudhury A (2016) Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem Lett Rev 9(1): 33-38.
- Nazar N, Bibi I, Kamal S, Iqbal M, Shazia N, et al. (2018) Cu nanoparticles synthesis using biological molecule of granatum seeds extract as reducing and capping agent: Growth mechanism and photo-catalytic activity. Int J Biol Macromol 106: 1203-1210.
- Sorbiun M, Shayegan Mehr E, Ramazani A, Taghavi Fardood S (2018) Green synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak fruit hull (jaft) and comparing their photocatalytic degradation of basic violet 3. Int J Environ Res 12(1): 29-37.
- Gnanadesigan M, Anand M, Ravi Kumar S, Maruthupandy M, Vijaya Kumar V, et al. (2011) Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac J Trop Med 4(10): 799-803.
- Bordbar M, Sharifi-Zarchi Z, Khodadadi B (2017) Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum root extract: High catalytic activity for reduction of 4-nitro phenol, rhodamine B and methylene blue. J Sol-Gel Sci Technol 81(3): 724-733.
- Jafarirad S, Mehrabi M, Divband B, Kosari Nasab M (2016) Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater Sci Eng C Mater Biol Appl 59: 296-302.
- Vasantharaj S, Selvam S, Mythili S, Palanisamy S, Kavitha G, et al. (2019) Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B Biol 191: 143-149.
- David L, Bianca M, Adriana V, Liliana O, Maria PS, et al. (2014) Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surfaces B Biointerfaces 122: 767-777.
- Kavithaa K, Paulpandi M, Ponraj T, Murugan K, Sumathi S (2016) Induction of intrinsic apoptotic pathway in human breast cancer (MCF-7) cells through facile biosynthesized zinc oxide nanorods. Karbala Int J Mod Sci 2(1): 46-55.
- Prasad KS, Patra A, Shruthi G, Chandan S (2017) Aqueous Extract of Saraca indica leaves in the synthesis of copper oxide nanoparticles: Finding a way towards going green. J Nanotechnol, p.1-6.
- Ramesh M, Anbuvannan M, Viruthagiri G (2015) Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 136: 864-870.
- Sharma RK, Yadav S, Gupta R, Arora G (2019) Synthesis of magnetic nanoparticles using potato extract for dye degradation: A green chemistry experiment. J Chem Educ 96(12): 3038-3044.
- Mallikarjuna K, Balasubramanyam K, Narasimha G, Kim H (2018) Phyto-synthesis and antibacterial studies of bio-based silver nanoparticles using Sesbania grandiflora (Avisa) leaf tea extract. Mater Res Express 5(1).
- Das J, Paul Das M, Velusamy P (2013) Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc 104: 265-270.
- Nabikhan A, Kandasamy K, Raj A, Alikunhi NM (2010) Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum Colloids Surfaces B Biointerfaces 79(2): 488-493.
- Njagi EC, Huang H, Lisa S, Homer G, Hugo MG, et al. (2011) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 27(1): 264-271.
- Ochieng PE, Iwuoha E, Michira I, Masikini M, Ondiek J, et al. (2015) Green route synthesis and characterization of ZnO nanoparticles using Spathodea campanulata. Int J Biochem Phys 23: 53-61.
- Khatami M, Heli H, Mohammadzadeh Jahani P, Azizi H, Lima Nobre MA (2017) Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnology 11(6): 709-713.
- Shahriary M, Veisi H, Hekmati M, Hemmati S (2018) In situ green synthesis of Ag nanoparticles on herbal tea extract (Stachys lavandulifolia)-modified magnetic iron oxide nanoparticles as antibacterial agent and their 4-nitrophenol catalytic reduction activity. Mater Sci Eng 90: 57-66.
- Atale N, Saxena S, Nirmala JG, Narendhirakannan R, Mohanty S, et al. (2017 ) Synthesis and characterization of Sygyzium cumini nanoparticles for its protective potential in high glucose-induced cardiac stress: A green approach. Appl Biochem Biotechnol 181(3): 1140-1154.
- Rajesh KM, Ajitha B, Kumar YA, Suneetha Y, Reddy P (2018) Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical , optical and antimicrobial properties. Opt Int J Light Electron Opt 154: 593-600.
- Yugandhar P, Vasavi T, Jayavardhana Rao Y, Uma Maheswari Devi P, Narasimha G, et al. ( 2018 ) Cost effective, green synthesis of copper oxide nanoparticles using fruit extract of Syzygium alternifolium (Wt.) walp., characterization and evaluation of antiviral activity. J Clust Sci 29(4): 743-755.
- Sivaraj R, Rahman KSM, Rajiv P, Abdul H, Venckatesh R (2014 ) Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim. Acta Part A Mol Biomol Spectrosc 133: 178-181.
- Muthulakshmi L, Rajini N, Nellaiah H, Kathiresan T, Jawaid M, et al. ( 2017) Preparation and properties of cellulose nanocomposite films with in situ generated copper nanoparticles using Terminalia catappa leaf extract. Int J Biol Macromol 95: 1064-1071.
- Issaabadi Z, Nasrollahzadeh M, Sajadi SM (2017) Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity. J Clean Prod 142: 3584-3591.
- Nasrollahzadeh M, Sajadi SM, Rostami Vartooni A, Hussin SM (2016) Green synthesis of CuO nanoparticles using aqueous extract of Thymus vulgaris leaves and their catalytic performance for N-arylation of indoles and amines. J Colloid Interface Sci 466: 113-119.
- Sharma P, Pant S, Poonia P, Kumari S, Dave V, et al. (2018) Green synthesis of colloidal copper nanoparticles capped with Tinospora cordifolia and its application in catalytic degradation in textile dye: An ecologically sound approach. J Inorg Organomet Polym Mater 28(6): 2463-2472.
- Udayabhanu, Netravathi PC, Pavan Kumar, Suresh D, Lingaraju K, et al. (2015 ) Tinospora cordifolia mediated facile green synthesis of cupric oxide nanoparticles and their photocatalytic, antioxidant and antibacterial properties. Mater Sci Semicond Process 33: 81-88.
- Rajaram K, Aiswarya DC, Sureshkumar P (2015) Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater Lett 138: 251-254.
- Veisi H, Hemmati S, Javaheri H (2017) N -Arylation of indole and aniline by a green synthesized CuO nanoparticles mediated by Thymbra spicata leaves extract as a recyclable and heterogeneous nanocatalyst. Tetrahedron Lett 58(32): 3155-3159.
- Dobrucka R, Długaszewska J (2016 ) Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci 23(4): 517-523.
- Prabhu D, Arulvasu C, Babu G, Manikandan R, Srinivasan P (2013 ) Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo induce growth-inhibitory effect on human colon cancer cell line HCT15. Process Biochem 48(2): 317-324.
- Shaikh RR, Lettre DP (2016) Biosynthesis of copper nanoparticles using Vitis vinifera leaf extract and its antimicrobial activity. Sch Res Libr 8: 265-272.
- Delma MBT, Vijila MB, Rajan MJ (2016) Green Synthesis of copper and lead nanoparticles using Zingiber Officinale stem extract. Int J Sci Res Publ 6(11): 134-137.
- Khani R, Roostaei B, Bagherzade G, Moudi M (2018 ) Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina-christi (L.) Willd.: Applic ation for adsorption of triphenylmethane dye and antibacterial assay. J Mol Liq 255: 541-549.
- Venkatpurwar V, Pokharkar V (2011 ) Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater Lett 65(6): 999-1002.
- Dietz KJ, Herth S (2011 ) Plant nanotoxicology. Trends Plant Sci 16(11): 582-589.
- Fleischer A, O’Neill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol 121(3): 829-838.
- Navarro E, Anders B, Renata B, Nana B, Juliane F, et al. (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17(5): 372-386.
- Verano Braga T, Rona M, Katarzyan W, Adelina R, Jonthan R, et al. (2014). Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano 8(3): 2161-2175.
- Maine MA, Duarte MV, Suñé NL ( 2001) Cadmium uptake by floating macrophytes. Water Res 35(11): 2629-2634.
- Kurepa J, Tatjana P, Stefan V, Hans A, Bryan M, et al. (2010 ) Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett 10(7): 2296-2302.
- Tani FH, Barrington S (2005 ) Zinc and copper uptake by plants under two transpiration rates. Part II. Buckwheat (Fagopyrum esculentum). Environ Pollut 138(3): 548-558.
- Pérez-de Luque A (2017) Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front Environ Sci 5.
- Khan MN, Mobin M, Abbas ZK, AlMutairi KA, Siddiqui ZH (2017) Role of nanomaterials in plants under challenging environments. Plant Physiol Biochem 110: 194-209.
- Burg MB, Ferraris JD (2008 ) Intracellular organic osmolytes: function and regulation. J Biol Chem 283(12): 7309-7313.
- Wei H, Wang E (2013 ) Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem Soc Rev 42(14): 6060-6093.
- Haghighi M, Pessarakli M (2013 ) Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum) at early growth stage. Sci Hortic (Amsterdam) 161: 111-117.
- Siddiqui MH, Al Whaibi MH, Faisal M, Al Sahli AA (2014) Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo Environ Toxicol Chem 33(11): 2429-2437.
- Lin D, Xing B (2007 ) Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ Pollut 150(2): 243-250.
- Parida AK, Das AB (2005 ) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60(3): 324-349.
- Manizheh K, Alipour ZT, Ashraf S, Marshi A (2014) Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J Chem Heal Risks 4(3): 49-55.
- Maryam M, Zhara A, Maryam H (2012 ) The effect of N_Si on tomato seed germination under salinity levels. Intern J Environ Sci 6(16): 87-90.
- Mohamed AKSH, Qayyum MF, Abdel-Hadi AM, Rehman RA, Ali S, et al. (2017 ) Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch Agron Soil Sci 63(12): 1736-1747.
- Hegazy HS, Hassan N, Abdel Haliem MEF, Naguib M (2015 ) Biochemical response of rice plant to biotic and abiotic stress under silica ions and nanoparticles application. Egypt J Bot 55(1): 79-103.
- Sabaghnia NJM (2014) Graphic analysis of nano-silicon by salinity stress interaction on germination properties of lentil using the biplot method. J Agriculture For 60(3): 29-40.
- Alsaeedi AH, El Ramady H, Alshaal T, El Garawani M, Elhawat N, et al. ( 2017 ) Engineered silica nanoparticles alleviate the detrimental effects of Na+ stress on germination and growth of common bean (Phaseolus vulgaris). Environ Sci Pollut Res 24(27): 21917-21928.
- Gowayed MH, Al Zahrani HSM, Metwali EMR (2017 ) Improving the salinity tolerance in potato (Solanum tuberosum) by exogenous application of silicon dioxide nanoparticles. Int J Agric Biol 19(1): 183-192.
- Kafi M, Nabati J, Saadatian B, Oskoueian A, Shabahang J (2019) Potato response to silicone compounds (micro and nanoparticles) and potassium as affected by salinity stress. Ital J Agron 14(3): 162-169.
- Yassen A, Abdallah E, Gaballah M, Zaghloul S (2017) Role of silicon dioxide nano fertilizer in mitigating salt stress on growth, yield and chemical composition of cucumber (Cucumis sativus). Int J Agric Res 12(3): 130-135.
- Acosta Motos J, Ortuño M, Bernal Vicente A, Diaz Vivancos P, Sanchez Blanco M, et al. (2017 ) Plant responses to salt stress: Adaptive mechanisms. Agronomy 7(1): 18.
- Martínez MC, Zapata L, Chalbi, Carvajal M (2016) Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J Nanobiotechnology 14(42): 1-14.
- Mozafari A, Ghadakchiasl A, Ghaderi N (2018) Grape response to salinity stress and role of iron nanoparticle and potassium silicate to mitigate salt induced damage under in vitro Physiol Mol Biol Plants 24(1): 25-35.
- Hernández H (2018) Chitosan-PVA and copper nanoparticles improve growth and overexpress the SOD and JA genes in tomato plants under salt stress. Agronomy 175(8): 1-11.
- Pérez-Labrada F, López-Vargas ER, Ortega-Ortiz, Cadenas-Pliego, Benavides-Mendoza, et al. (2019) Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 8(6): 151.
- Abou-Zeid H, Ismail G (2018) The role of priming with biosynthesized silver nanoparticles in the response of triticum aestivum l to salt stress. Egypt J Bot 58(1): 73-85.
- Fathi A, Zahedi M, Torabian S, Khoshgoftar A (2017) Response of wheat genotypes to foliar spray of ZnO and Fe2O3 nanoparticles under salt stress. J Plant Nutr 40(10): 1376-1385.
- Askary M, Talebi SM, Amini F, Bangan ADB (2017) Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 63(1): 65-75.
- Martı́nez-Vilalta, Piñol J (2002) Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. For Ecol Manage 161(1-3):247-256.
- Ashkavand P (2018) Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb (Rosaceae). Boletín la Soc Argentina Botánica 53(2): 207-219.
- Hattori T (2005) Application of silicon enhanced drought tolerance in sorghum bicolor. Physiol Plant 123(4): 459-466.
- Alsaeedia A, HassanEl-Ramady, Alshaal T, El-Garawany M, Elhawat N, et al. (2019) Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol Biochem 139: 1-10.
- Sedghi M (2013) Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann West Uni Timisoara 16(2): 73-78.
- Zareii D, Roozbahani A, Hosnamidi A (2014) Evaluation the effect of water stress and foliar application of Fe nanoparticles on yield, yield components and oil percentage of safflower (Carthamus Tinctorious L). Int J Adv Biol Biomed Res 2(4): 1150-1159.
- Palmqvist NGM, Seisenbaeva GA, Svedlindh P, Kessler VG (2017) Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res Lett 12(1): 631.
- Martínez-Fernández, Vítková M, Bernal MP, Komárek M (2015) Effects of nano-maghemite on trace element accumulation and drought response of Helianthus annuus L. in a contaminated mine soil. Water Air Soil Pollut 226(4).
- Hojjat SS (2016) The effect of silver nanoparticle on lentil seed germination under drought stress. Intl J Farm Alli Sci 5(3): 208-212.
- Karami A (2017) Multiwalled carbon nanotubes and nitric oxide modulate the germination and early seedling growth of barley under drought and salinity. Agric Conspec Sci cus 82(4): 331-339.
- Cao Z, Rossi L, Stowers C, Zhang W, Lombardini L, et al. (2018) The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L) Merr) under different soil moisture conditions. Environ Sci Pollut Res 25(1): 930-939.
- Jaberzadeh A, Moaveni P, Moghadam HRT, Zahedi H (2013) Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not Bot Horti Agrobot Cluj-Napoca 41(1): 201.
- Suzuki K, Nagasuga K, Okada M (2008) The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49(3): 433-442.
- Yordanova RY, Popova LP (2007) Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol Plant 29(6): 535-541.
- Liu TD, Song FB (2012) Maize photosynthesis and microclimate within the canopies at grain-filling stage in response to narrow-wide row planting patterns. Photosynthetica 50(2): 215-222.
- Heidarvand L, Millar AH, Taylor NL (2017) Responses of the mitochondrial respiratory system to low temperature in plants. CRC Crit Rev Plant Sci 36(4): 217-240.
- Mohammadi H, Esmailpour M, Gheranpaye A (2016) Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L) plants. Acta Agric Slov 107(2): 385.
- Hasanpour H, Maali-Amir R, Zeinali H (2015) Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ J Plant Physiol 62(6): 779-787.
- Ze Y, Liu C, Wang L, Hong M, Hong F (2011) The regulation of TiO2 nanoparticles on the expression of light-harvesting complex ii and photosynthesis of chloroplasts of arabidopsis thaliana. Biol Trace Elem Res 143(2): 1131-1141.
- Mohammadi R, Maali-Amiri R, Mantri NL (2014 ) Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ J Plant Physiol 61(6): 768-775.
- Giraldo JP (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13(4): 400-408.
- Amini S, Maali-Amiri, Mohammadi R, Kazemi- Shahandashti (2016) cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol Biochem 111: 39-49.
- Kohan-Baghkheirati, Geisler-Lee (2015) Gene expression, protein function and pathways of arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions cold salt drought and heat. Nanomaterials 5(2): 436-467
- Karuppanapandian T (2011) 2,4-dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol Biochem 49(2): 168-177.
- Prasad PVV, Pisipati SR, Momčilović I, Ristic Z (2011) Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast ef-tu expression in spring wheat. J Agron Crop Sci 197(6): 430-441.
- Zhao L (2012) Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2 , heat shock protein, and lipid peroxidation. ACS Nano 6(11): 9615-9622.
- Qi M, Liu Y, Li T (2013) Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res 156(1-3):323-328.
- Haghighi M, Abolghasemi R, Silva T (2014) Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci Hortic 178: 231-240.
- Iqbal M, Raja NI, Mashwani Z, Hussain M, Ejaz M, et al. (2019) Effect of silver nanoparticles on growth of wheat under heat stress. Iran J Sci Technol Trans A Sci 43(2): 387-395.
- Khodakovskaya MV, Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6(3): 2128-2135.
- Wu H, Tito N, Giraldo JP (2017) Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 11(11): 11283-11297.
- Capuana M (2011) Heavy metals and woody plants biotechnologies for phytoremediation. iForest-Biogeosciences For 4 (1): 7-15.
- Burklew CE, Ashlock J, Winfrey WB, Zhang B (2012) Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). Plos One 7(5): 34783.
- Frazier TP, Burklew CE, Zhang B (2014) Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Funct Integr Genomics 14(1): 75-83.
- Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK, et al. (2016) Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front Environ Sci 4: 46.
- Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L) seedlings. Plant Physiol Biochem 96: 189-198.
- Emamverdian A, Ding Y, Mokhberdoran F, Xie Y, Zheng X, et al. (2020) Silicon dioxide nanoparticles improve plant growth by enhancing antioxidant enzyme capacity in bamboo (Pleioblastus pygmaeus) under lead toxicity. Trees 34(2): 469-481.
- Tassi E, Giorgetti L, Morelli E, Peralta-Videa JR, Gardea-Torresdey JL, et al. (2017) Physiological and biochemical responses of sunflower (Helianthus annuus L) exposed to nano-CeO2 and excess boron: Modulation of boron phytotoxicity. Plant Physiol. Biochem 110: 50-58.
- Luqmon A, Ayoade LA, Agbaje L, Segun AA, Rasheed OA, et al. (2019) Zero-valent silver nanoparticles attenuate Cd and Pb toxicities on moringa oleifera via immobilization and induction of phytochemicals. Plant Physiol Biochem 139: 283-292.
- Rezvani N, Sorooshzadeh A, Farhadi N (2012) Effect of nano-silver on growth of saffron in flooding stress. Int J Biol Biomol Agri Food Biotechnol Engr 6: 11-16.
- Mustafa G, Sakata K, Komatsu S (2015) Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J Proteomics 128: 280-297.
- Chen H, Zhai JR, Mei TD, Han R (2011) Influence of enhanced UV-B radiation on F-actin in wheat division cells. Plant Diversity 33(3): 306-310.
- Durgesh KT, Shweta, Shweta S, Swati S, Rishikesh P, et al. (2017) An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem 110: 2-12.
- Zheng L, Mingyu S, Chao L, Liang C, Huang H, et al. (2007) Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol Trace Elem Res 119(1): 68-76.
- Zheng L, Mingyu S, Xiao W, Chao L, Chunxiang Q, et al. (2008) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res 121(1): 69-79.
- Simon DF, Domingos RF, Hauser C, Hutchins CM, Zerges W, et al. (2013) Transcriptome sequencing (RNA-seq) analysis of the effects of metal nanoparticle exposure on the transcriptome of chlamydomonas reinhardtii. Appl Environ Microbiol 79(16): 4774-4785.
- Jalil SU, Ansari MI (2019) Nanoparticles and abiotic stress tolerance in plants. Plant Signaling Molecules, pp. 549-561.
- Gunjan B, Zaidi MGH, Sandeep A (2014) Impact of gold nanoparticles on physiological and biochemical characteristics of brassica juncea. J Plant Biochem Physiol 2(3).
- Sanghamitra M, Jose RPV, Jesica TR, Youping Sun, Ana C Barrios (2016) Soil organic matter influences cerium translocation and physiological processes in kidney bean plants exposed to cerium oxide nanoparticles. Sci Total Environ 569-570: 201-211.
- Jiang HS, Li M, Chang FY, Li W, Yin LY (2012) Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem 31(8):1880-1886.
- Khan MIR, Khan NA, Masood A, Per TS, Asgher M (2016) Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-efficiency of nitrogen and sulfur, and glutathione production in mustard. Front Plant Sci 7.
- Fateme M, Hossein Askari, Sara H, Yvonne S, Andreas R, et al. (2014) Proteomics study of silver nanoparticles toxicity on oryza sativa L. Ecotoxicol Environ Saf 108: 335-339.
- Yanyan M, Jing X, Shen Y, Liang C, Yunpeng B, et al. (2014) Nanoparticle as signaling protein mimic: robust structural and functional modulation of CaMKII upon specific binding to fullerene C60 nanocrystals. ACS Nano 8(6): 6131-6144.
- Marmiroli M, Imperiale D, Pagano L, Villani M, Zappettini A, et al. (2015) The proteomic response of arabidopsis thaliana to cadmium sulfide quantum dots, and its correlation with the transcriptomic response. Front Plant Sci 6.
- Guo YX, Pedro RSCF, Man LW, Meng LX, Yan CC, et al. (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234(1): 47-59.
- Boudsocq M, Sheen J (2013) CDPKs in immune and stress signaling. Trends Plant Sci 18(1): 30-40.
- Juan C, Xiang L, Chao W, Shan SY, Xiu LL, et al. (2015) Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. J Hazard Mater 297: 173-182.
- Chandra S, Chakraborty N, Dasgupta A, Sarkar J, Panda K, et al. (2015) Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci Rep 5(1): 15195.
- Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 48(1): 21-43.
- Zhu F, Xi DH, Yuan S, Xu F, Zhang DW, et al. (2014) Salicylic acid and jasmonic acid are essential for systemic resistance against Tobacco mosaic virus in Nicotiana benthamiana. Mol Plant Microbe Interact 27(6): 567-577.
- Jing Y, Guihua D, Chunqin L, Lin L, Guangyu H, et al. (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic Stresses. Front Plant Sci 10: 1349.
- Jia X, Meng Q, Zeng H, Wang W, Yin H (2016) Chitosan oligosaccharide induces resistance to tobacco mosaic virus in arabidopsis via the salicylic acid-mediated signalling pathway. Sci Rep 6: 26144.
- Doares SH, Narvaez JV, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108(4): 1741-1746.
- Jiang F, Shen Y, Ma C, Zhang X, Cao W, et al. (2017) Effects of TiO2 nanoparticles on wheat (Triticum aestivum L.) seedlings cultivated under super-elevated and normal CO2 PLoS One 12(5): e0178088.
- Candida V, Guido D, Elisabetta O, Bhakti P, Milena M, et al. (2013) Morphological and proteomic responses of eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS One 8(7).
- Kaveh R, Li S, Ranjbar S,Tehrani R, Brueck CL, et al. (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47(18): 10637-10644.
- Premysl L, Radomira V, Jana A, Jan H, Petr M, et al. (2012) Nanoparticle-specific changes in arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J Hazard Mater 241-242: 55-62.
- Mariya K, Enkeleda D, Meena M, Yang X, Zhongrui L, et al. (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3(10): 3221-3227.
- Cristiano S, Simão BN, Alexandrade S, Manuel A, Ana Cunha, et al. (2018) SiO2 nanomaterial as a tool to improve Hordeum vulgare L. tolerance to nano-NiO stress. Sci Total Environ 622-623: 517-525.
- Oliveira HC, Gomes BCR, Pelegrino MT, Seabra AB (2016) Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 61: 10-19.
- Rossi L, Zhang W, Lombardini L, Ma X(2016) The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ Pollut 219: 28-36.
- Costa MVJ, Sharma PK (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54(1): 110-119.
- Almutairi ZM (2016) Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. POJ 9(1): 106-114.
- Torabian S, Zahedi M, Khoshgoftar AH (2016) Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J Plant Nutr 39(2): 72-180.
- Abdel MEFH, Hegazy HS, Hassan NS, Naguib DM (2017) Effect of silica ions and nano silica on rice plants under salinity stress. Ecol Eng 99: 282-289.
- Zayed M, Elkafafi S, Zedan A, Dawoud S (2017) Effect of nano chitosan on growth, physiological and biochemical parameters of phaseolus vulgaris under Salt Stress. J Plant Prod 8(5): 577-585.
- Moshabaki FI, Tahmourespour A, Hoodaji M, Ataabadi M, Mohammadi A (2018) Influence of exopolysaccharide-producing bacteria and SiO2 nanoparticles on proline content and antioxidant enzyme activities of tomato seedlings (Solanum lycopersicum L.) under salinity stress. Polish J Environ Stud 28(1): 153-163.
- Emary FE, Amer M(2018) Role of nano-silica in amelioration salt stress effect on some soil properties, anatomical structure and productivity of faba bean (Vicia faba L.) and maize (Zea mays L.) Plants. J Plant Prod 9(11): 955-964.
- Ivani R, Sanaei SHN, Ghahraman B, Astaraei AR, Feizi H (2018) Role of bulk and Nanosized SiO2 to overcome salt stress during fenugreek germination (Trigonella foenum-graceum L.). Plant Signal Behav 13(7): e1044190.
- Saeed A, Reza MA, Rahmat M, Seyyedeh SKS(2017) cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol Biochem 111: 39-49.
- Mustafa G, Sakata K, Hossain Z, Komatsu S (2015) Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J Proteomics 122: 100-118.
- Milan B, Ivana B, Milan Z, Danijela A, Slobodanka P, et al. (2016) Drought impact is alleviated in sugar beets (Beta vulgaris L.) by foliar application of fullerenol nanoparticles. PLoS One 11(11): e0166248.
- Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, et al. (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110: 70-81.
© 2022 © Kuldeep Kumar Koul. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






