- Submissions

Full Text
Environmental Analysis & Ecology Studies
The Effect of Specific Soil Microorganisms on Soil Organic Matter and Stabilization of Quality Parameters under Drought Conditions
Jurys A* and Feizienė D
Department of Plant Nutrition and Agroecology, Lithuanian Research Centre for Agriculture and Forestry, Lithuania
*Corresponding author: Jurys A, Department of Plant Nutrition and Agroecology, Lithuanian Research Centre for Agriculture and Forestry, Institute of Agriculture, Lithuania
Submission: December 22, 2020; Published: November 16, 2021

ISSN 2578-0336 Volume9 Issue2
Abstract
Soil chemical, biological and physical properties play important role in soil quality and are related with increasing OM, plant nutrient content and availability, soil microbiologcal activity. New generation of soil amendments with specific soil microorganizms are great interest worldwide. Field experiments were carried out in 2018-2019 in Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry. The aim was to determine the effect of bio-products with Trichoderma reesei, Acinetobacter calcoaceticus and Bacillus megaterium on soil SOC, soil respiration and microbial biodiversity on loamy Cambisol. Under dry meteorological conditions, bio-products with Trichoderma reesei, Acinetobacter calcoaceticus and Bacillus megaterium caused increase in SOC content, C/N ratio, Humic/Fulvic acids ratio, increased soil respiration and microbial biodiversity. We concluded that use of mixture of three products: Trichoderma reesei + Acinetobacter calcoaceticus + Bacillus megaterium are the most promising bio-amendment under changing climate conditions.
Keywords: Soil organic carbon; PGPR; PGPF; AWCD
Abbreviations: SOC: Soil Organic Carbon; PGPR: Plant Growth-Promoting Rhizobacteria; PGPF: Plant Growth-Promoting Fungi; AWCD: Average Well Colour Development; R - Richness
Introduction
Soil is essentially a slow renewable resource with a high degree of degradation and a very
low rate of regeneration [1]. Intensive agriculture leads to soil erosion, depletion of organic
matter and other nutrients which results to permanent soil degradation and productivity
losses [2]. To maintain optimal yields and less depletion of the soil, it is necessary to protect soil
from losses of organic carbon and biodiversity, micronutrient imbalance, acidity, and salinity
[3]. The input of exogenous organic matter, such as straw or livestock manure, can enrich soil
with the necessary elements and retard SOM decomposition. Soil quality parameters can be
determined by analysis of soil chemical, biological and physical properties together [2,3]. To
determine soil quality indicators such as particulate organic matter, active C, total N, microbial
biomass, biological activities, enzymes, soil pH, cation exchange capacity, salinity, bulk density,
amino sugar, and soil aggregation should be known [2,4]. Soil microorganisms consists largely
of primary decomposers that mineralize organic materials and release nutrients and energy
for plants by enzyme-facilitated metabolic systems [2,4,5]. Plant growth and yield in natural
environments depend on a many interaction between plant roots, bacteria and fungi [4,6]. In
the sense of decomposition of organic residues microbial communities can be broken down into fungal and bacterial groups, and research indicates that these
groups functioning differently in the decomposition process [7,8].
Bacterial decomposition pathways support high turnover rates
of easily available substances, fungal-dominated decomposition
pathways are slower and includes complex organic materials
[4,7,8]. For this day it is known that main plant-beneficial
Rhizosphere Microorganisms are Mycorrhiza, Rhizobia Bacteria,
PGPR such as Pseudomonas spp., Bacillus spp. or Azospirillum spp.,
and PGPF such as Trichoderma spp. and non-pathogenic Fusarium
spp. Strains [5,8,9]. PGPR and PGPF colonize plant roots and
are responsible of plant defence not only against various foliar
pathogens but also leaf-feeding insects [8,9]. The study was aimed
to determine the influence of specific soil microorganisms (PGPR
and PGPF individually and in a complex) on SOC, soil respiration
and biodiversity on loamy Cambisol.
Materials and Methods
Site and soil description
The field experiments were conducted in 2018-2019 at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry (55°23′50′′ N and 23°51′40′′ E). The prevailing soil is Endocalcari-Epihypogleyic Cambisol (CMg-n-w-can). According to composition of soil aggregates - 52-54% sand (2,0-0,05mm), 29- 32% silt (0,05-0,002mm) and 14-19% clay (<0,002mm) - soil was loam. Research scheme and product applications described in Table 1. During 2018-2019 where were grown two crops: winter wheat (Triticum aestivum L.) cv. Ada and spring barley (Hordeum vulgare L.) cv. Luokė. A field experiment was set up in three replications. The gross plot size and net harvested area of each individual treatment was 30,0 and 22,0m2, respectively.
Table 1: Research design.

*Used doses: Trichoderma reesei - 100mL/ha, Bacillus megaterium - 100mL/ha, Acinetobacter calcoaceticus - 100mL/ ha, ammonium nitrate - 20kg N/t residues.
Soil sampling and analysis
Total nitrogen (Ntot) and total carbon (Ctot for estimation of C/N ratio) were analysed by the dry combustion method using a CNS autoanalyser Vario EL III (Elementar Analy-sensystem GmbH, Germany) [10]. Closed chamber method was applied to quantify total (autotrophic + heterotrophic) soil surface net CO2 exchange rate (NCER). CO2 fluxes were measured five times during each crop growing season, each measuring was made between 10a.m. and 12a.m. using a portable infrared CO2 analyser (IRGA) attached to a data logger (LcSRS-1000; ADC BioScientific Ltd, UK). In each treatment, the collar was inserted to 7.0cm soil depth; the chamber hood was placed on the collar for 5min. until results were recorded in the data logger. Measurements were made with three replications. Expression of soil surface net CO2 exchange rate:
Where u is molar flow of air per square metre of soil (mol m-2s-1) and Δc is difference in CO2 concentration through soil hood, dilution corrected (μmol mol-1).
Humic acid fulvic acids were determined according “Agricultural Chemical Analysis, Method 5.4. Cabi Publishing, 2002”, gravimetric and spectroscopic methods. Substrate utilization potential as average well colour development (AWCD) and R index of the soil microbial community were determined according to the community level physiological profiles method using Biolog EcoPlates [11].
Result and Discussion
Soil organic carbon
According to the SOC content, the soil of experimental fields could be classified as having low humus content. At the beginning of the experiment SOC ranged from 0,91 to 0,93%. During 2018 - 2019 seasons (Figure 1) average SOC content was higher in all treatments with microorganisms, compared to K1 and K2. In 2018, the highest SOC concentration was in treatment 3 (Trichoderma reesei). It was 9,1% higher than in K1 and 6,7% higher than in K2. In 2019 highest SOC was in treatment 5 (Bacillus megaterium), i.e., 7,0% higher compared with K1, and 5,8% compared to K2.
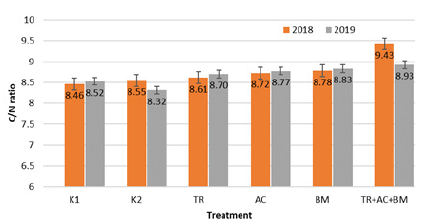
The decomposition intensity of organic matter is best determined by the C/N ratio. In 2018 period C/N ratio (Figure 2) was 10,2% higher in treatment 6 with Trichoderma reesei, Acinetobacter calcoaceticus, Bacillus megaterium compared to K1 and 9,3% with K2. In 2019 period C/N ratio (Figure 2) was highest in treatment 6 it was 4,5% higher than K1 and 6,8% than K2. An increased C/N ratio during experiment period means that the ratio of mineralization / humification processes has changed.
Figure 1: Average SOC concentration data during 2018-2019 field experiment.
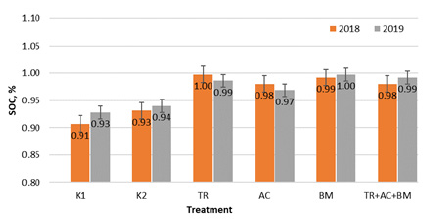
Figure 2: Average C/N ratio during 2018-2019 field experiment.
Humic/fulvic acid ratio
Soil humus, especially its stable fractions, is formed slowly, so this indicator does not fully reflect the humification processes of embedded organic matter. The high humus content and low humic/ fulvic acid ratio indicate that this humus is not long-lasting and the humification processes in such soils are weak. Humic /fulvic acid ratio was detected in 2019. Highest HA/FA (Figure 3) ratio was in treatment 6 - 51.5% higher than K1 and 48.5 % than K2.
Figure 3: Average humic/fulvic acid ratio in 2019.

Soil surface net CO2 exchange rate
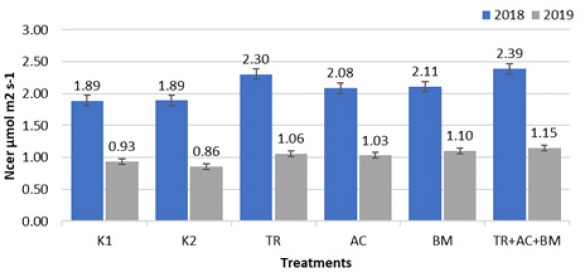
During experiment period, the mean soil NCER (Figure 4) was highest in treatment No. 6 (Trichoderma reesei + Acinetobacter calcoaceticus + Bacillus megaterium). In 2018, in this treatment, the NCER was higher by 26,5%, compared to both K1 and K2. In 2019, the mean NCER was 23,7% higher than in K1 and 33,7% higher than in K2.
Figure 4: Average soil net CO2 exchange rate.
To compare the strategy of substrate consumption by the soil microbiomes, the matters contained in a Biolog EcoPlate were classified into 6 main groups: carbohydrates, carboxylic acids, polymers, amino acids, amines and amides and miscellaneous. Data revealed that in both dry experimental years dominated carboxylic acids. In 2018 they consist 28-32%, in 2019 - 28- 31% from all substrate C sources (Figure 5 & 6). Carbohydrates amounted 21-23% and 25-28%, amino acids 19-20% and 17-20%, polymers 14-16%, amines 4-6% and 4-5%, and miscellaneous 8% and 5-8%,respectively.
Figure 5: Level of consumption of substrates by microbial communities of soil, in 2018.
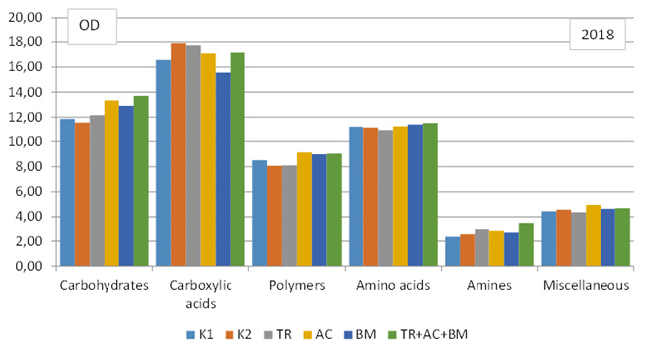
Figure 6: Level of consumption of substrates by microbial communities of soil, in 2019.
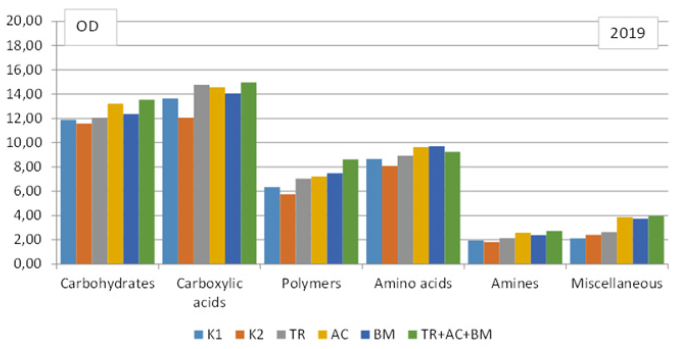
In first dry year, influence of soil microbiological amendments on C sources of soil substrate was insignificant (Figure 5). In next dry year, all products caused significantly higher OD values of each substrate category, compared ti K1 and K2. Most significant effect revealed combined application TR+AC+BM (Figure 6). The high level of carbohydrates and carboxylic acids substrate consumption is probably connected with the complexity of these substrates chemical structure and they require less time for decomposition.
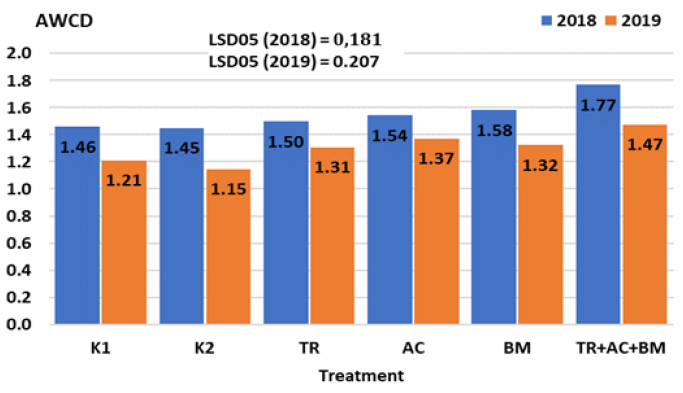
Principle Components Analysis (PCA) of average well colour development (AWCD) data revealed that during the first 4 days of the investigation average well colour development (AWCD) has grown in all samples selected and this index shows that microbial community metabolic activity in relation to carbon substrates under analysis is high. In 2018, in the 4th day of incubation, the highest AWCD index was found in treatment No.6 - it was 21-22% higher than in K1 and K2. Meanwhile, difference from controls K1 and K2 in treatments 3-5 amounted only 3-8%. In 2019, in the 4th day of incubation, the highest AWCD index also was found in treatment No.6 - it was 22-28% higher than in K1 and K2 treatments. Difference from controls K1 and K2 in treatments 3-5 reached only 8-13% (Figure 7).
Figure 7: Influence of microbiological amendments on AWCD.
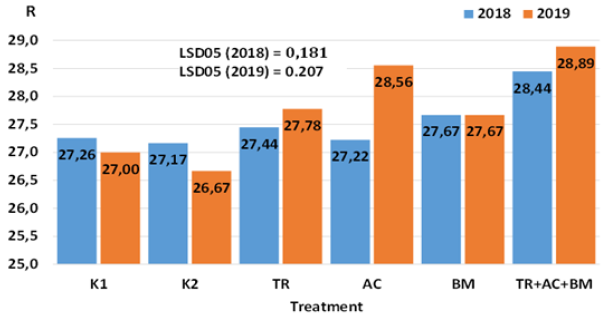
The richness (R) index showed that the microbial diversity varied with the type of soil management and generally increased with the combined application of microbiological products TR+AC+BM (Figure 8). Microbial richness varied significantly (P < 0.05) in both experimental years. In 2018 the highest R value was determined in treatment No. 6 and it was 4-5% higher than in K1 and K2 treatments. Meanwhile, differences from controls K1 and K2 and treatments No. 3-5 were insignificant. In 2019 the highest R value was determined in treatments No. 4 and No. 6. R index was 6-7% and 7-8% higher, respectively, than in K1 and K2. The correlation between AWCD and R indices was very high (r= 0.91- 0.95). The increase in values of ACWD and R is likely related with COC increase.
Figure 8: Influence of microbiological amendments on soil R index.
Climate change, production intensification conditioned great
changes of soil environment. Therefore worldwide is increased
interest in application of microbial amendments that provide
natural benefit for soil and crops. Under commercial field
conditions, microbial amendments can play an important role for
environment and human health also [12].
Securing and improving soil quality is a central challenge in face
of climate change. Considering the importance of micro-biota for
soil ecosystem the exploitation of microbial activity could provide
means to achieve soil improving. The application of microbial
amendments could improve use of plant nutrients, reduce their
release into soil and water and consequently reduce the negative
effects on the environment [13].
Our results obtained are in line with other published data.
We found positive effects of microbiological soil amendments
on Cambisol properties. Bio-products with Trichoderma reesei,
Acinetobacter calcoaceticus and Bacillus megaterium caused
increase in SOC content, C/N ratio, Humic/Fulvic acids ratio and
improved soil vitality by increasing soil respiration. We suppose
that such results were governed by changes in soil physical
properties. Pore structure, soil aggregate stability and water
retention are under investigation in our experiment. Similar results
were reported from sodic soils [14].
Bio-products act through a complex mechanisms including
plant roots, nitrogen fixation, P-solubilization, etc [16]. Therefore,
we think that microbiological product of new generation can be
advantageous under intensive farming conditions in commercial
agriculture.
Conclusion
1. Under drought conditions, bio-products with Trichoderma
reesei, Acinetobacter calcoaceticus and Bacillus megaterium caused
increase in SOC content, C/N ratio, Humic/Fulvic acids ratio.
2. All tested bio-products increased soil respiration. The
highest respiration was observed in soil after application with
mixture of three products: Trichoderma reesei + Acinetobacter
calcoaceticus + Bacillus megaterium. It was 23.7-26.5% higher,
compared to soil respiration in crop growing technologies without
bio-products.
The highest AWCD index and R index was found in treatment
after application with mixture of three products: Trichoderma
reesei + Acinetobacter calcoaceticus + Bacillus megaterium.
References
- Ruirui C, Mehmet S, Sergey B, Olga M, Klaus D, et al. (2014) Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Global Change Biology 20(7): 2356-2367.
- Elizabeth CC, Clive AK, John AK, Martin RA, Craig LS, et al. (2020) Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutrient Cycling in Agroecosystems 117: 273-298.
- Aziz I, Mahmood T, Islam KR (2013) Effect of long term no-till and conventional tillage practices on soil quality. Soil and Tillage Research 131: 28-35.
- Delgado BM, García PP, Milla R, Gallardo A, Maestre FT, et al. (2015) Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biology and Biochemistry 81: 134-142.
- Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Frontiers in Plant Science 8:
- Sasse J, Martinoia E, Northen T (2018) Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends in Plant Science 23(1): 25-41.
- de Graaff MA, Classen AT, Castro HF, Schadt CW (2010) Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytologist 188(4): 1055-1064.
- Venturi V, Keel C (2016) Signaling in the Rhizosphere. Trends in Plant Science 21(3): 187-198.
- Corné MJP, Christos Z, Roeland LB, David MW, Saskia CM Van Wees, et al. (2014) Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology 52: 347-375.
- Schmidt MWI, Skjemstad JO, Gehrt E, Kögel KI (2001) Charred organic carbon in German chernozemic soils. European Journal of Soil Science 50(2): 351-365.
- Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Applied and Environmental Microbiology 57(8): 2351-2359.
- Nuzzo A, Satpute A, Albrecht U, Strauss SL (2020) Impact of soil microbial amendments on tomato rhizosphere microbiome and plant growth in field soil. Microbial Ecology 80(2): 398-409.
- Sessitsch A, Brader G, Pfaffenbichler N, Gusenbauer D, Mitter B (2018) The contribution of plant microbiota to economy growth. Microbial biotechnology 11(5): 801-805.
- Trivedi P, Singh K, Pankaj U, Verma SK, Verma RK, et al. (2017) Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecological Engineering 102: 127-136.
- Rachel B, Stefan RJ, Gayathri I, John L, Dana P, et al. (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in Plant Science 9: 1473.
- Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, et al. (2015) Microbial interactions in the rhizosphere: Beneficial influences of plant growth promoting rhizobacteria on nutrient acquisition process. A Review Biol Fertil Soils 51: 403-415.
© 2021 © Jurys A. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






