- Submissions

Full Text
Environmental Analysis & Ecology Studies
The Relationship between Phenological Characteristics and Endogenous Hormone Contents in a Dimorphic Mixed-Mating Plant, Pseudostellaria Heterophylla
Hu Wenzhao1, Sun Qi2, Wu Lei2, Zhao Ji-Min1 and Zhang Yan-Wen1,2,3*
1Department of Biology, Changchun Normal University, China
2Institute of Grassland Science, Northeast Normal University and Key Laboratory for Vegetation Ecology, China
3Department of Biology, Eastern Liaoning University, China
*Corresponding author: Zhang Yan- Wen, Department of Biology, Institute of Grassland Science, Key Laboratory for Vegetation Ecology, Northeast Normal University, Changchun Normal University, Changchun, 130032, China
Submission: October 21, 2021; Published: October 27, 2021

ISSN 2578-0336 Volume9 Issue2
Abstract
The various developmental stages of plants are closely related to their endogenous hormone contents. Pseudostellaria heterophylla exhibits a typical dimorphic mixed-mating system. We used high performance liquid chromatography (HPLC) to determine the relative concentrations of four endogenous hormones found in the rhizomes of this medicinal plant. The results revealed significant changes in the contents of these hormones at six developmental stages in P. heterophylla. For example, the contents of gibberellic acid (GA), Abscisic acid (ABA), and Zeatin (ZT) in the Cleistogamous (CL) flower stage were higher than at previous developmental stages, and at this point only the indole-3-acetic acid (IAA) contents was low. Contents of GA and IAA at the CL seed stage were at their highest, while the contents of ABA and ZT were lower. The ratios of GA/IAA and ABA/IAA were significantly higher during the CL flower stage than at the other developmental stages. The ratios of IAA/ZT and GA/ZT were significantly lower during the CL flower stage but peaked at the CL seed stage. We speculated that the highest GA and ABA contents may promote the formation of chasmogamous (CH) flowers, whereas low IAA and high ZT contents are key to CL flowering. Moreover, high contents of GA and IAA are closely related to CL seed development and high GA/IAA and ABA/IAA ratios promote the formation of CL flowers. We discuss the relationship between endogenous hormone contents in rhizomes at different developmental stages and the flowering patterns of dimorphic flowers, providing new evidence about the physiological mechanisms that underlie the maintenance of the dimorphic mixed mating system.
Keywords: Chasmogamous (CH) flower; Cleistogamous (CL) flower; Dimorphic mixed mating system; Pseudostellaria heterophylla; Endogenous hormones
Introduction
The mating system of plants determines the genotype frequency of the offspring and
has a higher impact on the genetic structure of the population than any other life history
factor [1]. Selfing and outcrossing of plants are not completely separate. There is a transition
type between selfing and outcrossing, that is, mixing. These transition types occur within a
continuous range of variation. The adaptability of this mating system is of great significance
to plant evolution [2,3], as plants can choose the most suitable mating pattern to complete
the reproductive process. Therefore, plants with mixed mating system types may have certain
reproductive advantages in heterogeneous habitats [4].
Dimorphic mating, also known as the Chasmogamous (CH)- Cleistogamous (CL) system,
is a typical mixed mating system, which enables the production of both CH and CL flowers on
the same plant [5,6]. The two types of flowers have different functions and constitute a system
that enables sexual reproduction. That is, CH flowers can attract pollinators to visit using
bright corollas and rewards to produce outcrossed offspring, while self-pollination or crosspollination
within the same plant produces inbred offspring [7-9]. CL flowers have no corolla
or Nectaries, the number of stamens is often less than that of CH flowers, and the number of
ovules is similar. Seeds are produced by automatic selfing [5].
Under natural conditions, CH and CL flowers are produced in a
different order on plants. For example, Viola pubescens produces
CH flowers in early spring and CL flowers appear after a few weeks
[6], while Dichanthelium clandestinum produces CL flowers in
spring and CH flowers appear during the summer [6]. V. candensis
produces CH flowers first, then CL flowers for the following
flowering period, and, finally, a small number of CH flowers are
formed in autumn [6]. The relative proportions of the two types
of flowers are affected by both non-biological and biological
factors [1,7]. For example, Festuca can produce CL flowers under
low temperature conditions but only CH flowers under normal
conditions [8]. As the soil moisture decreases, the number of CH
flowers in D. clandestinum is reduced, while the appearance of CL
flowers increases significantly [9]. V. philippica produces more CH
flowers under short-day light and more CL flowers under long-light
conditions. Previous studies have focused more on the influence
of habitat on the development of plants with dimorphic mating
systems, especially their flowering patterns, but little attention
has been paid to changes in their endogenous hormone contents at
different developmental stages in these plants.
From a physiological and ecological point of view, the process of
plant flower development is regulated by its endogenous hormone
contents. For example, gibberellic acid (GA) can promote the sex
differentiation of female flowers [10]. Indole-3-acetic acid (IAA)
and zeatin (ZT) promote flower bud differentiation and flowering
[11]. Abscisic acid (ABA) is found, in some plants, to promote the
differentiation of female flowers and promote flower formation
[12], but it can also delay flowering in Arabidopsis thaliana [13].
However, research on the effects of endogenous hormones on the
flowering characteristics of CH and CL flowers in plants with a
dimorphic mixed system is extremely limited. For example, Minter
& Lord [14] found that the number of CH and CL flowers in Collomia
grandiflora is regulated by antagonistic hormones in the plant and,
when plants were sprayed with exogenous ABA and GA in vitro,
this conclusion was verified. Campos-Rivero et al. [15] also showed
that plant hormones can regulate flowering through epigenetics,
meaning that they can act as signals to coordinate flowering.
Conti [16] proposed that the transition of flowers and their
timings are controlled by a complex network, including exogenous
environmental signals and endogenous plant hormone signals, and
that GA may play a major role, because these signals may regulate
the expression of florigen genes in leaves. In view of the complexity
and diversity of plants using the dimorphic mixed mating system,
this study attempted to detect a relationship between variation in
the contents of four endogenous hormones and the phenological
characteristics of a typical dimorphic mating system plant,
Pseudostellaria heterophylla, to increase our understanding of the
maintenance mechanisms in the dimorphic mating system.
Materials and Methods
Plants and locations
P. heterophylla is a perennial herbaceous plant from the Caryophyllaceae family. This species exhibits a typical dimorphic mixed mating system [4]. Its CH flower grows on the top of the plant, is large, and has obvious entomophilous flower characteristics: five petals, white color, ten stamens, and a three-lobed stigma. The CL flower grows in the middle and lower parts of the plant, is small and closed, and has: no petals, lavender color, four sepals, and two stamens. The plant depends on the fleshy roots in the ground to survive the winter and undergo vegetative multiplication (Figure 1).
Figure 1: Dimorphic flowers and fleshy roots of P. heterophylla.
A. CH flowers
B. CL flowers
C. Fleshy roots

P. heterophylla is widely distributed in China and has attracted much attention because its underground fleshy roots can be used in medicines [4]. It is a rich resource in the eastern mountainous area of Liaoning, China, and is often used in traditional Chinese medicines or as a wild vegetable [17]. Due to the heterogeneity of the habitat under natural conditions, there are obvious differences in development stages such as flowering, fruiting, and vegetative propagation. To accurately describe its flowering and other phenological characteristics, and obtain materials for detecting hormone contents, we transplanted P. heterophylla plants from the wild to the homogenous garden at the experimental site, which was located in Liaoning, northeast China, a sunny block on the campus of Liaodong University in Dandong City, China (latitude 40° 08′ north, longitude 123° 03′ east), with an area of more than 400m2. In October of the year before the experiment, the fleshy roots of the plants were placed in pots (10cm × 10cm). Each pot held 0.5kg of native soil and one plant. During cultivation, we removed very large or small fleshy roots to maintain consistency among the materials. A total of 180 samples were equally divided into six groups, each containing 30 plants, and the planted samples were managed through the winter normally.
Sample collection for the measurement of endogenous hormone contents
When the plants began to germinate in late March 2018, we started to observe and record their phenological characteristics, including the time at which dimorphic flowers appeared, flowering period, flower number, and seed yield. Fleshy root material was removed from the plants at each developmental stage to be used for detecting hormone contents. From pilot experiments, we knew that the endogenous hormone contents in the fleshy root of these plants is relatively high and stable, while before flowering and during the late stage of fruit ripening, the leaves are absent or withered and therefore cannot be used to detect hormone contents. Therefore, fleshy root head tissue was used to extract the endogenous hormones.
We decided to sample the roots during the following six key stages:
A. The germination stage, when the plant has just emerged from the soil and the stems and leaves begin to stretch, but the CH flower has not yet appeared (April 5);
B. The CH flower stage, when more than 30% of the individuals produced CH flowers (May 5);
C. The CH fruit stage, during which the CH flowers gradually decreased and CH fruit entered a period of expansion and maturity (May 20);
D. The CL flower stage, when more than 30% of the individuals produced CL flowers (June 15);
E. The CL fruit stage, when the CL blooms gradually decreased and the fruits began to ripen (July 25); and (6) The new fleshy roots stage, wherein the stems and leaves began to wither and the new underground fleshy roots matured (September 20). Five suitable plants were randomly selected from different groups at each developmental stage and the fleshy roots were taken (the stems and leaves were removed) and washed, wrapped in tin foil, immediately placed in liquid nitrogen for quick freezing, and taken to the laboratory to be stored in the refrigerator at -80 °C for testing.
Hormone extraction
One gram of the fleshy root head sample was weighed and then mashed with dry ice in a mortar, before adding 1ml of pre-cooled 80% methanol. The samples were extracted overnight at 4 °C and centrifuged at 8000r/min. for 10min. The supernatant was removed and the residue at the bottom of the centrifuge tube was extracted with 0.5ml of 80% methanol for 2h and centrifuged again. The two supernatants were combined and the organic phase removed under nitrogen at 40 °C. A 0.5ml aliquot of petroleum ether was used to extract and decolorize the residue three times at 60-90 °C. The upper ether phase was then discarded and 0.1mol/L citric acid solution was added after which the pH was adjusted to 2 and the sample extracted three times with ethyl acetate. The ethyl acetate layer was dried with nitrogen at 40 °C, 0.5ml of the mobile phase was added, and the sample shaken on a vortex shaker to dissolve. The sample was then filtered through a 0.22μm syringe filter. Each sample preparation was repeated five times.
Determination of Endogenous Hormones by HPLC
Instruments and medicines: We used an Agilent 1100 high performance liquid chromatography system (Agilent Technologies; Palo Alto, CA, USA), including a chromatograph, high pressure infusion pump, and UV monitor, with a Zorbax C18 (Interchim; Montluçon, France) reversed phase chromatography column (250mm × 4.6mm, 5μm), ultrapure water preparation device, and hormone standards (GA, ABA, IAA, and ZT; Sigma). The acetic acid and methanol used in the mobile phase were of chromatographically pure grade and the remainder of the pharmaceutical reagents were analytical grade. The experimental water was ultrapure. Chromatographic conditions: Chromatographic conditions: 0.1% acetic acid aqueous solution: methanol (60% : 40%) as the mobile phase, a C18 reversed phase chromatography column, column temperature 30 ℃, detection wavelength 254nm, flow rate 0.8ml/ min gradient aliasing, injection volume 10μl, and aliasing time 45min.
Data analysis: All the data calculations were carried out in Excel 2016 (Microsoft corporation; Redmond, Washington, USA) and analyzed using SPSS 22.0 (IBM; Armonk, New York, USA) statistical analysis software. A one-way analysis of variance was used to test the differences between contents of the same hormone at different developmental stages, and multiple comparisons were used to test differences in the contents of the four endogenous hormones at different developmental stages.
Result
Reproductive characteristics of different developmental stages
In the relatively consistent and stable environment of the plantation, P. heterophylla began to germinate during late March, CH flowers began to appear in mid-April and reached full bloom in early May, while the blooming period ended in late May; the CH flower blooming period lasted about 40 days. CL flowers appeared in early May and reached their peak in mid-to-late June, then the number of CL flowers began to decrease and flowering ended in late July; the CL flower blooming period lasted about 80 days. During the entire flowering period, there were ~2.43 (±0.09) CH flowers and ~9.93 (±0.58) CL flowers on each plant. The number of CL flowers was four times that of CH flowers, and the difference was significant (F = 71.89, p < 0.001, n = 100). Since the fruit set from the two flower types on each plant was also significantly different (F = 11.89, p < 0.01), the seed yield from the CH flowers only accounted for 11.97% of the seed yield of the entire plant (Table 1). In addition, we observed that the original fleshy roots of this species began to shrivel in mid-to-late July, when the CL flowering period was about to end, forming multiple new fleshy roots from the top of the rhizome. The new fleshy roots matured during mid- September
Variation in Endogenous Hormone Contents at Different Developmental Stages
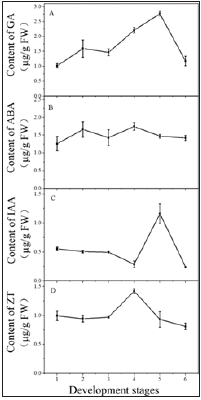
GA contents: The GA contents in the fleshy roots of P. heterophylla showed an overall upward trend during the first five stages of development; the contents rose from its lowest contents of 1.020μg/g at the germination stage to 2.771μg/g, the highest peak, at the CL seed stage, and then dropped sharply to 1.179μg/g at the plant withering and new fleshy roots stage, roughly recovering to the content found at the germination stage. Multiple comparisons showed that the GA contents at different developmental stages varied significantly (F = 183.19, p < 0.05) (Figure 2A).
ABA contents: Throughout development, the ABA contents did not change as dramatically as those of GA. The ABA contents were higher during the CH and CL flowering periods; the contents increased 31.72% from the germination stage (1.26μg/g) to the CH flower stage (1.66μg/g) and 22.38% from the CH fruit stage (1.43μg/g) to the CL flower stage (1.75μg/g), respectively, and the difference was significant (F = 121.37, p < 0.05). During the other four developmental periods, the ABA contents were relatively low and the differences in the contents between stages were insignificant (F = 12.06, p > 0.05) (Figure 2B).
IAA contents: The IAA contents showed a slow decline during the first four developmental stages, from 0.56μg/g during the germination stage to 0.29μg/g during the CL flower stage, which represented a decrease of 43.51%. However, with the end of the CL flower stage, the IAA contents increased rapidly, reaching a peak value of 1.16μg/g at the CL seed stage, which was twice that at the germination stage. Subsequently, the IAA contents dropped rapidly and reached the lowest content of 0.24μg/g at the new fleshy roots stage. Multiple comparisons showed that the IAA contents changed significantly throughout the developmental stages (F = 196.98, p < 0.001) (Figure 2C).
ZT contents: From the germination stage to the CH fruit stage, the ZT contents were relatively stable at a content close to 1. With the appearance of CL flowers, the ZT contents began to rise rapidly, until it reached a maximum value of 1.42μg/g, an increase of about 50%. Then, the concentration began to decline rapidly. At the plant withering and new fleshy roots stage, the ZT contents dropped to its lowest value, 0.81μg/g, which was lower than that seen at the germination stage, and the difference was significant (F = 80.67, p < 0.05) (Figure 2D).
Figure 2: Variation in four endogenous hormones in fleshy root tissue at different development stages in P. heterophylla 1. The germination stage; 2. The CH flowers stage; 3. The CH fruit stage; 4. The CL flower stage; 5. The CL seed stage; 6. The new fleshy roots stage. A, B, C, and D represent the four endogenous hormones GA, ABA, IAA, and ZT, respectively
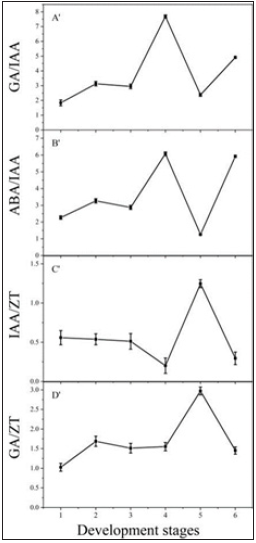
Variation in the ratio of endogenous hormones
We calculated the concentration ratios of four pairs of hormones. The results showed that the characteristic ratios of GA/IAA and ABA/IAA were similar; their ratios during first three developmental stages were relatively low, but at the CL flower stage, the ratios reached a peak, then decreased, and reached a low value at the CL seed stage. However, these ratios reached their second highest values again at the new fleshy roots stage (Figure 3A′ & Figure 3B′). Meanwhile, the ratios of IAA/ZT and GA/ZT also showed similarities; these ratios were relatively low during the first four developmental stages, and the highest values were seen in the CL seed stage. However, these ratios also returned to their lower values during the new fleshy roots stage (Figure 3C′ & Figure 3D′).Figure 3: Ratios of endogenous hormones in fleshy roots at different developmental stages in P. heterophylla
Discussion
Many studies have demonstrated that no matter how complex
the physiological processes of plants are, the initiation of each
developmental stage is based on the regulation of certain hormone
contents; that is, hormone contents are key to specific gene
expression patterns [18-23].
As the results of this study show, although plants with a
dimorphic mixed-mating system undergo a more complex physiological development process, with dimorphic flowers for
sexual reproduction at different periods and fleshy roots for
vegetative propagation [4], each developmental stage is closely
related to the contents of several endogenous hormones. For
example, the GA contents continued to increase up to 1.41-fold
during the process of sexual reproduction until the seeds reached
peak maturity but remained at a very low contents during the
subsequent vegetative propagation stage. Therefore, the contents of
GA must have greater relevance to sexual reproduction, especially
at the CL flower stage. Previous studies found that a low contents
of GA promotes flower bud differentiation, whereas a high contents
inhibits it [24], and that GA has the effect of promoting female
flower sex differentiation [7].
Our results are significantly different to those of previous
studies, and this may be related to the complex breeding mode of
this plant. Table 1 shows that the seed yield from CH flowers in
this species only accounts for 11.98% of the total seed yield, and
its fecundity mainly comes from the CL flowers. The significance
of CH flower-based reproduction is reflected in the opportunity
to outcross, thereby maintaining the genetic diversity of the
population [25]. The peculiarity of the CL flower is that its petals
are reduced, and the number of stamens is reduced from 10 to 2,
thereby saving resource investment in female organs and female
gametes. Therefore, the CL reproduction mode is more reflected in
the enhancement of female functions, which may be related to the
high contents of GA.
Table 1: The flower number and fecundity of P. heterophylla.

Different letters (a, b) indicate a significant difference between groups.
ABA is an important hormone that promotes the formation of
flowers. The ABA contents in the rhizomes of this species exhibited
a peak during the flowering stage of the dimorphic flowers,
indicating that high contents of ABA in the plant may promote the
development of the dimorphic flowers. Our results are similar to
those of previous studies. For example, Cao et al. [26] found that
the ABA contents increased significantly during the gestation of
apple flower buds, and that this hormone was also found at higher
contents during the induction and differentiation stages of olive
flower buds [27]. In addition, the ABA contents of flowering Moso
bamboo was observed to be significantly higher than that of nonflowering
M. bamboo. It can be seen that high contents of ABA are
necessary for plant flower formation and flower bud development.
The relationship between the contents of IAA and flower
formation or flower bud development has been controversial. Some
results have indicated that this hormone promotes the growth and
development of plant flowers. For example, during the flowering
period in Palmoxylon [28], IAA is at a high content. However, other
studies have suggested that IAA is an inhibitor of plant flower
formation and development. For example, Coptis deltoidei [29]
and July fresh jujube [30] contain low contents of IAA during the
flower bud development stage, indicating that high contents might
be necessary for promoting bud development; hence, the hormone
may have a dual effect of promoting and suppressing flowering,
depending on the species [27]. The results of this study show that
IAA in this species is always at a low content, whether the plant is
at the CH or subsequent CL flower stage, but the content rises to
a peak during the CL seed stage. IAA is at its highest during this
period, apart from when the seeds reach maturity, and also during
the rapid growth of new rhizomes. Our results appear to support
the latter of the reports mentioned above and may also indicate that
high contents of IAA play a key role in the development of atresia
fruits and tuber enlargement.
ZT is a type of cytokinin. Studies have shown that cytokinins
can regulate plant cell division, induce flower bud differentiation,
and promote the transformation of plants from vegetative to
reproductive growth [31]. For example, Peng et al. [32] studied Salix
viminalis flower formation and pointed out that ZT can promote
flower bud induction during the first step of flower formation.
Zhang et al. [33] studied the endogenous hormone changes of
Xanthoceras sorbifolium and found that high contents of ZT are
beneficial to the development of female flowers. In this study, we
found a very interesting and puzzling phenomenon; that is, the
effects of ZT contents on the development of CH and CL flowers are
opposing. Lower contents of ZT were beneficial to the development
of CH flowers, while high contents significantly promoted CL flower
development. We speculated that the effects of ZT contents on the
development of dimorphic flowers is likely to be affected by GA
regulation because previous studies have reported that GA may
have positive and negative regulatory effects during plant growth
and development. Moreover, there may be a crossover phenomenon
between the metabolic pathways of GA and cytokinins [26], but this
molecular regulation mechanism needs to be further studied.
Plant flowering or growth and development are often the result
of a variety of endogenous hormones interacting, antagonizing
each other, and promoting one another [16]. The dynamic balance
between endogenous hormones regulates the metabolism of nucleic
acids, proteins, and other substances and, in general, dominates
a plant’s growth and development process. For example, during
the growth and development of Phyllostachys edulis, the values
of ABA/IAA and GA/IAA at the flowering stage are significantly
increased. It is speculated that higher concentrations of ABA and
GA are beneficial to the formation of its flowers [34]. In the study
of two kinds of jujube, it was found that an increase in the ABA/
IAA ratio and decrease in the IAA/GA ratio is beneficial to flower
development [30].
Induction of bud or root differentiation by regulating the
ratio of hormones is a common method in tissue culture, the
most commonly hormones are cytokinin and auxin, and the ratio
of the two plays a key role in induction of plant shoots and roots
[35,36]. For example, in tissue culture of Pfaffia paniculate, higher
ratio cytokinin/ auxin promoted buds differentiation, while lower
ratio of Cytokinin/ Auxin promoted roots differentiation [37]. The
results of that study are basically consistent with our results. As
shown in Figure 3, the GA/IAA and ABA/IAA ratios changed in
similar patterns throughout the development process. The increase in these ratios was conducive to the differentiation and formation of
CL flowers. Conversely, the decrease in these ratios was conducive
to fruit setting and growth of the dimorphic flowers.
Related studies have also shown that hormone ratios and
contents play a certain role in the sex differentiation process
of Actinidia arguta female and male flowers and show certain
regularities; that is, an increase in the values of IAA/ABA and GA/
ABA is beneficial to flower bud formation in female plants, whereas
an increase in GA/ZT and IAA/ZT values has a greater impact in the
later stage of male flower bud development [7]. In this study, the
value of IAA/ZT was significantly higher during the CL seed stage
than at other developmental stages, which is somewhat different
from results of the previous studies described above. It is possible
that the change in the ratio of the two hormones has no obvious
effect on the growth and development of CH flowers. To summarize,
as the values of GA/IAA and ABA/IAA increase, and the value of
IAA/ZT decreases, both of which promote the formation of CL
flowers, the value of GA/ZT increases to promote CL seed ripening.
In terms of regulating gene expression, the SPY gene is not only
a negative regulator of GA signal transduction but also a positive
regulator of ABA signal transduction. SPY can interact with GI to
participate in the flower formation process, as regulated by light
signals [38]. We speculate that the biological clock-dependent ABA
signal transduction pathway may participate in and regulate flower
bud induction in the two types of flower, but there is no significant
difference in the ABA contents in the rhizome during the CH and
CL flowering periods. Therefore, the effect of ABA on the flowering
pattern of P. heterophylla cannot be determined.
The different contents of endogenous hormones in the
rhizomes of P. heterophylla at different developmental stages are
the result of regulated gene expression and may also be related
to environmental factors. Changes in environmental conditions
directly affect the flowering frequency and flowering time of CH and
CL flowers and in turn affect the flowering patterns of two types of
flower [38-42]. The dimorphic mixed mating system is regulated
by complex endogenous hormone contents and ratios, and this
provides us with strong evidence on which to base further studies
of the mechanism that underlies the maintenance of a dimorphic mixed-mating system in heterogeneous habitats [42-45].
Acknowledgement
We thank W. Si and D.-B. Zhuang for help in the field and lab. We thank Prof. L.-H. Zhang for the valuable comments. We thank International Science Editing (http://www. internationalscienceediting.com) for editing this manuscript. This work was supported by grants from the National Science Foundation of China to Zhang Yan-Wen (31370400, 31670426).
References
- Barnett LL, Troth A, Willis JH (2018) Plastic breeding system response to day length in the California wildflower Mimulus douglasii. American Journal of Botany 105(4): 779-787.
- Zhai XY, Ma DT, Wang CZ, Yan XB (2012) Effects of altitude on the mating system of alpine plant. Pratacultural Science 29(4): 640-645.
- Wang YL (2017) Research on reproductive ecology of Viola monbeigii with dimorphic flowers. Northwest Normal University Lanzhou, China.
- Zhang LH, Sun Q, Zhao JM, Zhang YW (2018) Plasticity in the reproductive strategy of a clonal cleistogamous species, Pseudostellaria heterophylla. Plant Ecology 219: 1493-1502.
- Lord EM (1981) Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. The Botany Review 47: 421-449.
- Culley TM, Klooster MR (2007) The cleistogamous breeding system: A Review of its frequency, evolution, and ecology in angiosperms. The Botanical Review 73(1): 1-30.
- Li QX, Huo QD, Wang J, Zhao J, Sun K, et al. (2016) Expression of B-class MADS-box genes in response to variations in photoperiod is associated with chasmogamous and cleistogamous flower development in Viola philippica. BMC Plant Biology 16: 151.
- Connor HE (1998) Breeding systems in New Zealand grasses XII, Cleistogamy in Festuca. New Zealand Journal of Botany 36: 471-476.
- Bell TJ, Quinn JA (1987) Effects of soil moisture and light intensity on the chasmogamous and cleistogamous components of reproductive effort of Dichanthelium clandestinum Canada Journal of Botany 65(11): 2243-2249.
- Li XY, Wang ZX, Qin HY, Fan ST, Ai J (2016) Dynamic variation of endogenous hormone during male and female flower Buds development of Actinidia arguta. Journal of Jilin Agricultural University 38(3): 281-286.
- Frankowski K, Kesy J, Wojciechowski W, Jan Kopcewicz (2009) Light-and IAA-regulated ACC synthase gene (Pn ACS) from Pharbitis nil and its possible role in IAA-mediated flower inhibition. Plant Physiol 166(2): 192-202.
- Rudich J, Halevy AH (1974) Involvenent of abscisic acid in the regulation of sex expression in the cucumber. Plant & Cell Physiol 15(4): 635-642.
- Shu K, Luo X, Meng Y, Yang W (2018) Toward a molecular understanding of abscisic acid actions in loral transition. Plant and Cell Physiology 59(2): 215-221.
- Minter TC, Lord EM (1983) Effects of water stress, abscisic acid, and gibberellic acid on flower production and differentianion in the cleistogamous species Collomia grandiflora Dougl. Ex Lindl. (Polemoniaceae). American Journal of Botany 70(4): 618-624.
- Campos RG, Osorio MP, Sánchez BR, Us Camas R, Duarte AF (2017) Plant hormone signaling in flowering: An epigenetic point of view. J Plant Physiol 214: 16-27.
- Conti L (2017) Hormonal control of the floral transition: Can one catch them all? Developmental Biology 430(2): 288-301.
- Liu YA (2012) The reproductive ecology of Pseudostellaria heterophylla with dimorphism reproductive system. Northeast Normal University, Changchun, China.
- Huang XZ, Hou LY, Meng JJ, You HW, Li Z, et al. (2018) The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in arabidopsis. Mol Plant 11(7): 970-982.
- Nguyen TN, Tuan PA, Mukherjee S, Son SH, Ayele BT (2018) Hormonal regulation in adventitious roots and during their emergence under waterlogged conditions in wheat. J Exp Bot 69(16): 4065-4082.
- Sakuraba Y, Kim D, Han SH, Kim SH, Piao WL, et al. (2020) Multilayered regulation of membrane-bound ONAC054 is essential for abscisic acid-induced leaf senescence in rice. Plant Cell 32(3): 630-649.
- Chen K, Li GJ, Bressan RA, Song CHP, Zhu JK, et al. (2020) Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62(1): 25-54.
- Liao K, Peng YJ, Yuan LB, Dai YS, Chen QF, et al. (2020) Brassinosteroids antagonize jasmonate-activated plant defense responses through BRI1-EMS-SUPPRESSOR1 (BES1). Plant Physiol 182(2): 1066-1082.
- Li W, Liu SW, Ma JJ, Liu HM, Han FX, et al. (2019) Pinus tabuliformis gibberellin signaling is required for far-red light-induced shoot elongation in seedlings. Plant Physiol 182(1): 658-668.
- Su JL, Jiang MY, Wang X, Yang YY, Fu HJ, et al. (2019) Changes of endogenous hormones and physiological and biochemical substances content during the flowering period of Sasaella kongosanensis 'Aureostriatus'. Journal of Anhui Agricultural University 46(1): 18-24.
- Wang N (2014) The population genetic structure of Pseudostellaria heterophylla with dimorphism breeding system. Northeast Normal University, Chagchun, China.
- Cao SY, Zhang JC, Wei LH (2000) Studies on the changes of endogenous hormones in the differentiation period of flower bud in apple trees. Journal of Fruit Science 4: 244-248.
- Ulger S, Sonmez S, Karkacier M, Nisa E, Ozgur A, et al. (2004) Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant Growth Regulation 42(1): 89-95.
- Wang Y, Zhao Y, Ran J (2018) Variation of endogenous hormone in the development of female and male. Seed 37(3): 7-11.
- Dai CC, Song LK, Li XF, Dong GT, He HY, et al. (2010) Effects of plant hormones on flower development in Coptis deltoidea and C omeiensis. Special Wild Economic Animal and Plant Research 32(1): 19-21.
- Niu HL (2015) Flower formation and endogenous hormones dynamic in Chinese Jujube. Northwest A&F University, Xi an, China.
- Lu ZG (2010) The study on the regulation on flower by endogenous hormone in leaves of Crassula argenten Thumb. Chinese Horticulture Abstracts 26(6): 27-28.
- Peng XY, Cheng YH, Li ZJ, Yu YC, Zou JZ, et al. (2018) Variations of endogenous hormones and polymines during flowering process in male and female Salix viminalis. Scientia SylvaeSinicae 54(8): 39-47.
- Zhang N, Huang YY, Ao Y, Su SC, Liu JF, et al. (2019) Flower bud differentiation and dynamic changes of endogenous hormone in Xanthoceras sorbifolium Bung. Journal of Nanjing Forestry University 43(4): 33-42.
- Qi FY, Peng ZH, Hu T, Gao J (2013) Changes of endogenous hormones in different organs during the flowering phase of Moso bamboo. Forest Research 26(3): 332-336.
- Poniewozik MK, Parzymies M, Szot P, Rubinowska K (2021) Paphiopedilum insigne morphological and physiological features during in Vitro rooting and ex Vitro acclimatization depending on the types of auxin and substrate. Plants (Basel) 10(3): 582.
- Devi TR, Dasgupta M, Sahoo MR, Kole PC, Prakash N (2021) High efficient de novo root-to-shoot organogenesis in Citrus jambhiri Lush: Gene expression, genetic stability and virus indexing. PLoS One 16(2): e0246971.
- Jiang H (2021) Rapid propagation system of Pfaffia paniculate tissue culture. Journal of West China Forestry Science 50(01): 138-144.
- Tseng TS, Salomé PA, McClung CR, Neil E Olszewski (2004) SPINDLY and GIGANTEA interact and act in Arabidopsis thaliana pathways involved in light responses, flowering, and rhythms in cotyledon movements. Plant Cell 16(6): 1550-1563.
- Waller DM (1984) Differences in fitness between seedlings derived from cleistogamous and chasmogamous flowers in impatiens capensis. Evolution 38(2): 427-440.
- Corff JL (1993) Effects of light and nutrient availability on chasmogamy and cleistogamy in anunderstory tropical herb, Calathea micans (Marantaceae). American Journal of Botany 80(12): 1392-1399.
- Koul M, Sharma N (2012) Rates and pattern of ovule abortion vis-à-vis in situ pollen germination in some populations of Trifolium fragiferum L. Journal of Biosciences 37(6): 1067-1077.
- Culley TM (2000) Inbreeding depression and floral type differences in Viola canadensis (Violaceae), a perennial herb with chasmogamous and cleistogamous flowers. Canada Journal of Botany 78: 1420-1429.
- Culley TM (2002) Reproductive biology and delayed selfing in Viola pubescens (Violaceae), an understory herb with chasmogamous and cleistogamous flowers. International Journal of Plant Science 163(1): 113-122.
- Iwona Ż, Ewa D, Monika K, Piotr W, Michal D, et al. (2015) Hormonal requirements for effective induction of microspore embryogenesis in triticale (×Triticosecale) anther cultures. Plant Cell Reports 34(1): 47-62.
- Wang JL, Wang Q, Wang J, Lu Y, Xiao X, et al. (2009) Effect of different plant growth regulators on micro-tuber induction and plant regeneration of Pinellia ternate (Thunb) Briet. Physiol Mol Biol Plants 15(4): 359-365.
© 2021 © Zhang Yan-Wen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






