- Submissions

Full Text
Environmental Analysis & Ecology Studies
Effect of a Pseudomonas Fluorescens-Based Biofertilizer on Sweet Potato Yield Components
Alexander Santana Fernández1, Yoel Beovides García2*, Jaime E Simó González2, María C Pérez Peñaranda3, Daniel Rodríguez Pérez2, Yenisey Gutiérrez Sánchez2, Jorge López Torres2 and Milagros Basail Pérez2
1Master of Science Student, University of Cienfuegos, Cienfuegos, Cuba
2Biotechnology Directorate, Research Institute of Tropical Roots and Tuber Crops (INIVIT), Cuba
3Unit Development and Innovation, Biological-Pharmaceutical Laboratories (LABIOFAM), Cuba
*Corresponding author: Yoel Beovides García, Research Institute of Tropical Roots and Tuber Crops (INIVIT), Cuba
Submission: June 23, 2020Published: June 17, 2021

ISSN 2578-0336 Volume8 Issue4
Abstract
Humanity needs an alternative agricultural development paradigm, sustainable and able to produce the food needed by the growing world population. The application of beneficial soil organisms offers a promising alternative for maintaining crop health and productivity. In this report, a field experiment was conducted to study the effect of a Pseudomonas fluorescens-based biofertilizer on sweet potato (Ipomoea batatas (L) Lam) yield. The cultivar INIVIT B 240-2006 was planted in an experiment with 12 treatmentcombinations; the method of application was by immersion of cuttings before sowing for 0, 5, 10 and 15 minutes and the studies included the bioproduct combination with doses of 0, 50 and 100% of NPK mineral fertilizer. All treatments were tested at a randomized block design with three replications. During the harvest after 130 days of growth, some measurements related to yield components were recorded on ten randomly selected plants from the central ridges of each plot. Field experiments showed a positive response in the agronomic variables in all the treatments where the immersion of cuttings in the solution of P. fluorescens was carried out. The combination with 100 % NPK and the immersion in P. fluorescens for 15min showed the highest yield (56.09 tha-1), followed by the other treatments with 100 % NPK and without statistical differences among them. The treatment with 50% NPK and the immersion in P. fluorescens for 15min (49.58 tha-1) had no statistical differences with the control variant (100 % NPK, 51.60 tha-1). Based on the results, it can be concluded that this biofertilizer could be an appropriate alternative to increase the sweet potato yield, saving the 50% of the current quantity of the recommended mineral fertilizer, through a more friendly environmental techniques to promote a sustainable, efficient and productive agriculture.
Keywords:Sweet potato; Biofertilizer; Fertilization; Phosphate solubilizer; Sustainable agriculture; Yield
Introduction
Modern agricultural systems promote the cultivation of high-input and high-yielding crop species and it implies to use a limited number of species, and it has caused a decline in crop diversity in agricultural systems across the world. Therefore, there are no doubts that humanity needs an alternative agricultural development paradigm, one that encourages more ecologically sound, biodiverse, resilient, sustainable and socially just forms of agriculture where genetic diversity is vital [1]. At the same time, the agricultural productivity and hence, food and nutrition security are being affected by climate changes while new thousands of people require food every day. Consequently, it is necessary to move towards a more sustainable and resilient production, in which the agroecological approach is the way forward to produce the food needed by the growing world population.
The ecological resilience of agroecosystems is closely linked to social resilience [2] and this in turn, requires the implementation of environmentally friendly crops and strategies, as well as timely training of producers for its use. Facing the great challenges that climate change implies for countries’ food sovereignty implies combining traditional crops and techniques with advances in science and technology. Sweet potato (Ipomoea batatas (L.) Lam.) (batata, boniato, camote) is a species of American origin [3] and constitutes the sixth most important food crop in the world [4]. Globally, 112 835 316 t of this tuberous root are produced in 9 202 777 ha with a yield of 12.26 t ha-1; in Cuba, sweet potato yields on smallholder farms stand at an average of 10.87 tha-1 [5]. It is a typical food for food safety since it can be harvested in just 4-6 months, an easily propagated crop able to provide carbohydrates, minerals and β-carotenes (pro-vitamin A). Its ability to face adverse climatic conditions, effective response to meteorological phenomena, its versatility and vegetative reproduction, places it above other crops of higher production [6].
As in other crops, fertilization is very important to get high yields. Sweet potato productivity is constrained by poor fertility, especially low potassium (K), phosphorus (P), nitrogen (N), sulfur (S), and some micronutrients [7,8]. Phosphorus requirements are quantitatively lower than K and N doses, but it affects increasing the average weight and the number of roots [9]. Therefore, P is one of the nutrients that, when deficient, most intensely affects crop yields, although plants need a smaller amount than other macronutrients for growth and development. Due to its slow diffusion and high degree of fixation, phosphorus is generally less available in the soil solution but, its uptake and utilization are essential on the final yield of agricultural crops [10].
Correct and timely fertilization are one of the requirements to take into account, however, the availability of chemical fertilizers, which are the most used in cultivation, is deficient many times. On the other hand, the application of chemicals in agriculture is often the cause of soil erosion and environmental deterioration, mainly when used indiscriminately. Fortunately, the use of natural sources, as biofertilizers, have been promoted in the nutrition of crops during the last decades; the relationships between plant and microorganism improve the assimilation of nutrients and, therefore, that allows to obtain better yields. Among the most commonly used biofertilizers in agricultural crops are mycorrhizae, azotobacter or phosphorin [11,12]. Other bioproducts, such as: Fitomas [13], VIUSID agro® [14] that stimulate vegetative development, flowering and fruiting, are also used successfully. Many studies have been carried out on sweet potato growth and productivity, including the effect of organic and inorganic fertilizers applications [15,16].
Among the most important beneficial microorganisms, different bacterial species of the genus Pseudomonas have been described; they act in a double way on crops: they promote plant growth and suppress pathogenic microorganisms. It has also been suggested that they stimulate the establishment of other beneficial microorganisms associated with roots, such as mycorrhizae [17,18]. Pseudomonas produces an increase in the availability of phosphorus and nitrogen in an assimilable way for the plant, due to the production of phytohormones that stimulate a vegetative activity, as well as the degradation of ethylene precursors [19]. Pseudomonas fluorescens Migula is a Gram-negative, aerobic bacilliform bacterium that has several polar flagella. They are known for their ability to stimulate the growth of plants that live in contact with them [20].
Recently, because of the need to promote the production of bioproducts with agricultural interest on a larger scale, researchers from the Cuban Business Group LABIOFAM developed a biofertilizer based on a P. fluorescens strain phosphate solubilizer [21]; the new product is in the process of technical validation for its future use in agriculture.
In the case of sweet potato, the effect of this biofertilizer or the most effective way to apply it to achieve sustainable productions is unknown. It is not also known if with the combined use of this bioproduct and doses of mineral fertilizer, the current doses of the chemical, expensive and environmental pollutants, could be reduced. Therefore, the objective of this work was to determine the effect of a P. fluorescens-based biofertilizer on the development and yield of sweet potato (Ipomoea batatas (L.) Lam) cv. INIVIT B 240- 2006.
Materials and Methods
The experiments were conducted on a neighboring experimental field site located at the Farm “La Dora” (Cienfuegos city, Cuba), between December/2018-April/2019 (low rainy period). The farm “La Dora” is on a slightly wavy relief (2.1-4.0%) to Wavy (4.1-8.0%); the field is situated 14m above sea level. This farm occupies a total area of 3.42 hectares of which 2.75ha represents the agricultural area. The last 20-years average annual rain fall is 1304.8mm (only 240.3mm during the growing season) with average temperatures from 20.9 °C (minimum) to 30.9 °C (maxim). The soil is brown without carbonates [22] with 3.2% of organic matter and a pH=6.8.
The commercial sweet potato cultivar ʹINIVIT B 240-2006ʹ was used. It is an early cultivar that has many appreciated characteristics by the producers: a good culinary quality of its elongated tuberous roots, the flesh or pulp is white, sweet and without fibers. The skin is light red and smooth with a very good presence for commercialization. It has a short production cycle (four months) with potential yields greater than 55t.ha-1.
The effect of a P. fluorescens-based biofertilizer was evaluated at the dose recommended by the manufacturer (Labiofam) of 20Lha-1. The application method was by immersion of cuttings before sowing for 5, 10 and 15 minutes and the studies included the combination of the bioproduct with doses of 0, 50 and 100 % of the NPK mineral fertilizer (complete formula: 9-13-17). In field, plants from all tested groups were exposed to the same agricultural conditions according to the sweet potato Technical Instructive [23]. On-farm field experiments were conducted with 12 treatments:
T1. Absolute Control (a control group without the application of any mineral fertilizer or Pseudomonas)
T2. Cuttings with immersion in P. fluorescens for 5min
T3. Cuttings with immersion in P. fluorescens for 10min
T4. Cuttings with immersion in P. fluorescens for 15min
T5. Cuttings with 100% of complete fertilization (NPK) (a control group technically recommended by [23])
T6. Cuttings with 100% NPK fertilization plus immersion in P. fluorescens for 5min
T7. Cuttings with 100% NPK fertilization plus immersion in P. fluorescens for 10min
T8. Cuttings with 100% NPK fertilization plus immersion in P. fluorescens for 15min.
T9. Cuttings with 50% NPK fertilization
T10. Cuttings with 50% NPK fertilization plus immersion in P. fluorescens for 5min
T11. Cuttings with 50% NPK fertilization plus immersion in P. fluorescens for 10min
T12. Cuttings with 50% NPK fertilization plus immersion in P. fluorescens for 15min.
100% NPK fertilizer was applied as 90kg N ha-1, 75kg P2O5 ha-1 and 150kg K2O ha-1, according to the technical recommendations for this edaphic condition [23].
The vine cutting, 20-30cm in length, was used as planting material. A unique planting distance of 0.90m X 0.30m was used according to [23]. Plants were grown in field conditions and the experiments were conducted in completely randomized blocks with 12 treatments in three replicates (plots of 10.80m2). The plantation was manually, on the ridge and with the soil properly humid, and in independent plots. Routine agronomic package of practices and plant protection measures recommended for this crop were applied to raise a good crop [23]. Each treatment was represented in three replicates with 50 plants. The measurements were recorded on ten randomly selected plants from the central ridges of each plot, and they consisted in different qualitative and quantitative characters, especially those related with the yield: Number of Total Tuberous Roots per plant (NTTR), Number of Commercial (NCTR) and Noncommercial (NNCTR) tuberous roots per plant, Fresh Weight of Commercial (FWCTR, g) and Noncommercial (FWNCTR, g) tuberous roots per plant, and commercial yield per hectare (CY, tha- 1). The collected data were averaged to get mean values of these characters that have been affected by the studied treatments.
After 130 days of growth, the plots were harvested and data for the mentioned characters, especially the number and weight of marketable tubers per plant were recorded. Root and vine characteristics were described previously after 90 days of growth, according to sweet potato classification system defined by [24,25].
The collected data were subjected to the analysis of variance (ANOVA) appropriate to the design of completely randomized blocks with factorial arrangement (3x4), where the factors were: A-four immersion times in a P. fluorescens-based biofertilizer (0, 5, 10 y 15min), and B-three doses of NPK mineral fertilizer (0, 50y 100%). All statistical procedure was according to the experimental design and using the tools from the SPSS/PC+ statistical package version 15.0 for Windows®. Whenever differences existed among means values, the comparison of them was carried out with the Tukey’s honest significant difference (HSD) for P≤ 0.05.
Result and Discussion
Observations of the sprouting percentage 20 days after planting always behaved above 95 % (absolute control treatment without any fertilizer) for a general average of 98.06%. In all cases, the result coincides with the one expected by this commercial cultivar and it was higher to the 90%, technically demanded as a permissible value on the sweet potato crop production [23]. The vigor and the positive response observed in all treatments is firstly related with the good quality of cuttings (tips) that came from areas of categorized seed (original seed) of the cultivar at the Research Institute of Tropical Roots and Tuber Crops (INIVIT). In addition, cuttings with 30 cm are the best option to get a better development of this crop; a recent study made by Dumbuya et al. [26] confirmed that 30cm-long sweet potato vine cuttings produced the greatest growth and yield. Therefore, the planting material used had enough moisture and nutrients to achieve an optimum sprouting, after the agrotechnical work to the soil was carried out properly before, during and after sowing, especially the irrigation.
The effect of different treatments on the growth parameters of sweet potato was observed. However, the morphological characteristics did not change, especially those related with the color of immature (lightly purple) and mature leaf (green), the tuberous root form (lengthened), and the predominant light red skin color and the white tuberous roots pulp. All plants, regardless of the treatment received, maintained the characteristics of the cultivar INIVIT B-240-2006, among them: vigorous foliage with green stems and green nodes. The leaves were heart-shaped, slightly dentate of medium size and green color, with green ribs slightly pigmented on the underside with the purple limbo-petiole insertion point.
On the other hand, a positive response was measured of the evaluated agronomic variables in all treatments where the immersion of cuttings in the solution of P. fluorescens (20 tha-1) was carried out (Table 1); when the biofertilizer was combined with 100 % of NPK fertilization, the results were significantly higher over the rest of treatments. No statistical differences were shown among the treatments for the Number of Total Tuberous Roots (NTTR), the Number of Noncommercial Tuberous Roots (NNCTR) and its Fresh Weight (FWNCTR). However, significant differences appeared for other variables, especially for those related with the yield. The worst treatment for all variables was the absolute control (without any fertilizer) with significant statistical differences with the rest of combinations. The control treatment with 100% of the mineral fertilizer (NPK) and its combinations with 5,10 and15min immersion in the biofertilizer expressed statistical differences regarding to the rest of treatments. The mixture of 50% NPK and the immersion during 15min in the biofertilizer don’t differ statistically with the control with 100% NPK. That’s a good finding because it shows the possibility to save mineral fertilizer to increase new areas when immersions for 15min in the P. fluorescens-based biofertilizer could be made to improve sweet potato production significantly.
Table 1:Effect of a P. fluorescens-based biofertilizer on the yield components on the sweet potato cultivar INIVIT B 240- 2006 at La Dora farm (Abreus, Cienfuegos).
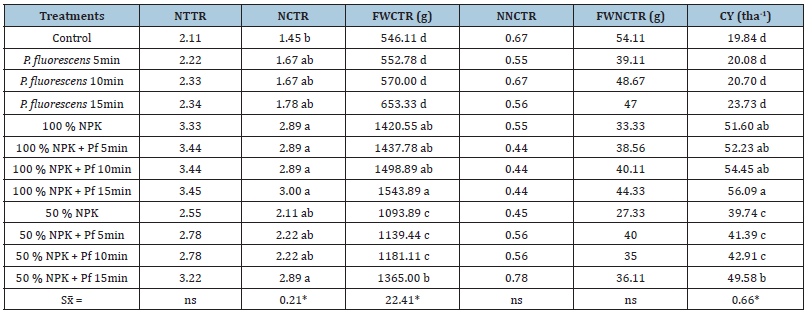
*Means followed by the same letter in a column are not significantly different according to Tukey’s HSD test for P≤0.05. Legend: NPK-mineral fertilizer (complete formula 9-13- 17); Pf: P. fluorescens; NTTR: Number of Total Tuberous Roots Per Plant; NCTR: Number of Commercial Tuberous Roots Per Plant; FWCTR(g): Fresh Weight of Commercial Tuberous Roots Per Plant; NNCTR: Number of Non- Commercial Tuberous Roots Per Plant; FWNCTR: Fresh Weight of Non-Commercial Tuberous Roots Per Plant(g); CY: Commercial Yield(tha-1).
When analyzing the effect of the bioproduct on the yield, the combination with 100% NPK and the immersion in P. fluorescens for 15min showed the highest yield (56.09 tha-1), followed by the other treatments with 100% NPK and without statistical differences among them. It was interesting that the treatment with 50% NPK and the immersion in P. fluorescens for 15min (49.58 tha-1) had no statistical differences with the control variant (100 % NPK, 51.60 tha-1). In general, the effect of this biofertilizer and its ability to improve plant growth and productivity confirms an option to minimize the agricultural chemical footprint on sweet potato.
Beneficial Pseudomonas strains are frequently found associated with plants where they act as Plant Growth Promoting Bacteria (PGPB) by suppressing growth of pathogens or by producing plant growth hormones [27]. Bio-fertilizer liberates growth promoting substances and vitamins and helps in maintaining the soil fertility. They acts as antagonists and suppress the incidence of soil borne plant pathogens [16]. They are one of the most important organic sources, containing beneficial viable organisms which have ability to mobilize nutritionally important elements from nonusable to usable form through biological processes [28]. On the other hand, there are many examples where the combination of inorganic fertilizer with biofertilizers produce better productive results than when they were applied individually; Abdel-Razzak et al. [29] reported an improved root quality and productivity in sweet potato when combining arbuscular mycorrhizal fungi (G. mosseae) inoculum with the recommended P level (100% P2O5) (superphosphate fertilizer).
Usually, chemical fertilizers make the difference necessary to guarantee the increase of yields [30], but the excessive mineral fertilization to one side have adverse financial effects and also represents an environmental burden [31]. The vision to reduce the dependency on synthetic fertilizers requires effective biologicalbased alternatives. In this sense, P. fluorescens is a new input for sweet potato crop that can reduce the application rates of chemical fertilizers, offering an alternative to traditional agricultural practices like in other crops [32,33].
Conclusion
Due to the worldwide current multifactorial crisis (ecological, economic and social), the increase of the world population and the necessity of foods for millions of people, where climate change represents a threat to agriculture and food security, agroecological methods offer comprehensive solutions for food systems. Farmers have to fight with all sorts of problems, including the high prices of fertilizers, the soil degradation, the reduction of crops productivity, the increment of plagues and droughts, among others. In this context, the application of environment-friendly farming practices is not an alternative, is the urgent response to guarantee the food sovereignty. In consequence, although it would be necessary to know the productive response during the rainy period (in execution), the results of this research are the first scientific report about the use of this new P. fluorescens-based biofertilizer to improve the sweet potato yield significantly. This is a very important alternative for more sustainable agricultural practices without affecting the growth and productivity of this important crop. The study concludes that the P. fluorescens-based biofertilizer stimulated the commercial yield of sweet potato (Ipomoea batatas (L.) Lam) cv. INIVIT B 240-2006; the combination of 50% NPK fertilization and the immersion of vine cuttings in P. fluorescens during 15 min after planting, can equal the productive results reached with 100% of the mineral fertilizer.
Acknowledgement
The authors would like to thank Ms. Geisy Díaz-Roche for her contribution to the revision and comments that greatly improved the original manuscript. Thanks to the National Fund of Science and Innovation from the Cuban Ministry of Science, Technology and Innovation for the financial support for the research. The authors would also wish to thank the Bioali-Cyted Network for its support in the development of this research.
References
- Altieri MA, Nicholls CI (2012) Agroecology scaling up for food sovereignty and resiliency. In: Lichtfouse E (Ed.), Sustainable Agriculture Reviews. ©Springer Science+Business Media Dordrecht, Germany, 11(1).
- Nicholls CI, Osorio LAR, Altieri MA (2013) Agroecology and socio-ecological resilience adapting to climate change. REDAGRES and SOCLA. CYTED, Medellin, Colombia.
- Roullier CA, Duputié A, Wennekes P, Benoit L, Bringas VMF, et al. (2013) Disentangling the origins of cultivated sweet potato (Ipomoea batatas (L.) Lam.). PLoS One 8(5): e62707.
- Rossel G, Alagon R, Tay D, Simon R, Grueneberg W, et al. (2014) Development of a globally representative SSR marker kit for sweetpotato (lpomoea batatas). Plant and Animal Genome XXll, p. 738.
- FAOSTAT (2019) Production, crops, sweet potato, 2017 data. ©FAO (Food and Agriculture Organization) Canada.
- Lebot V (2010) Root and tuber crops chapter 3 sweet potato. In: JE Bradshaw (Ed.), Handbook of Plant Breeding. Springer Science Business Media, Germany, pp. 97-125.
- Bailey JS, Ramakrishna A, Kirchhof G (2008) An evaluation of nutritional constraints on sweet potato (Ipomoea batatas) production in the central highlands of Papua New Guinea. Plant Soil 316: 97-106.
- Uwah DF, Undie UL, John NM, Ukoha GO (2013) Growth and yield response of improved sweet potato (Ipomoea batatas (L.) Lam) varieties to different rates of potassium fertilizer in Calabar, Nigeria. J Agric Sci 5(7): 61-69.
- Ruiz L, Simó J, Rodríguez S, Rivera R (2012) Las micorrizas en cultivos tropicales. Una contribución a la sostenibilidad agroalimentaria. Ed Académica Española, p. 239.
- Kareem I, Akinrinde EA (2018) Impact of phosphorus release dynamics on sweet potato production. Sci Agri 21(1): 26-34.
- Ruiz LA, Carvajal D, Espinosa E, Simó J, Rivera R, et al. (2015) Efecto de las micorrizas y bioplaguicidas sobre cultivares de raíces y tubérculos en un suelo pardo mullido carbonatado. Rev Agric Trop 1(1): 1-6.
- Pérez JV, Sánchez DB (2017) Characterization and effect of azotobacter, azospirillum and Pseudomonas associated with Ipomoea Batatas from the Colombian. Rev Colomb Biotecnol 19(2): 35-46.
- Fundora LR, Cabrera JA, González J, Ruiz LA (2009) Increases in the yields of Boniate cultivation due to the combined use of the phytostimulant fitomas-e and the ecomic® biofertilizer under production conditions. Cult Trop 30(3): 14-17.
- Peña K, Rodríguez JC, Olivera D, Meléndrez JF, Rodríguez L, et al. (2017) Effect of growth promoter on different vegetable crops. Int J Dev Res 7(2): 11737-11743.
- Adeyeye AS, Akanbi WB, Sobola OO, Lamidi WA, Olalekan KK (2016) Comparative effect of organic and in-organic fertilizer treatment on the growth and tuber yield of sweet potato (Ipomoea batatas L). Int J Sustain Agric Res 3(3): 54-57.
- Singh J, Sharma MK, Singh SP, Bano R, Mahawar AK (2018) Effect of organic and inorganic sources of NPK and bio-fertilizer on enhancement of growth attributes and chlorophyll content of sweet potato. Int J Curr Microbiol App Sci 7(9): 3659-3667.
- Behn O (2008) Influence of Pseudomonas fluorescens and arbuscular mycorrhiza on the growth, yield, quality and resistance of wheat infected with Gaeumannomyces graminis. J Pant Dis Protect 115(1): 4-8.
- Imperiali N, Chiriboga X, Schlaeppi K, Fesselet M, Villacrés D, et al. (2017) Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi, and entomopathogenic nematodes and their effects on wheat performance. Front Plant Sci 8: 1809.
- Nieto P (2016) Pseudomonas, microorganismos de biocontrol en agricultura. Control Bí
- Madigan M, Martinko J (2019) Brock biology of microorganisms. (15th edn), Pearson Educational Limited (Pearson Global Edition), Pearson, UK, p. 1056.
- Pérez MC, Oramas J, Sotolongo EA, Miranda A, Román Y, et al. (2019) Optimización del medio de cultivo y las condiciones de fermentación para la producción de un biofertilizante a base de Pseudomonas fluorescens. Biot Veg 19(2): 127-138.
- Hernández JA, Pérez JJM, Bosch ID, Castro SN (2015) Clasificación de los suelos de Cuba. Ediciones INCA, Cuba, p. 93.
- INIVIT (2012) Instructivo técnico para la producción de semillas de viandas. Martínez E (Ed.) Instituto de Investigaciones de Viandas Tropicales (INIVIT), Ministerio de la Agricultura, Cuba, p. 162.
- CIP, AVRDC, IBPGR (1991) Descriptores de la batata. Huamán Z (Ed.), International Board for Plant Genetic Resources (IBPGR), Rome, Italy, p. 132.
- Shiotani I, Yoshida S, Kawase T (1990) Numerical taxonomic analysis and cross ability of diploid Ipomoea species related to the sweet potato. J Breed 40(2): 159-174.
- Dumbuya G, Addo JS, Daramy MA, Jalloh M (2017) Effect of vine cutting length and potassium fertilizer rates on sweet potato growth and yield components. Int J Agric For 7(4): 88-94.
- Khan Z, Doty SL (2009) Characterization of bacterial endophytes of sweet potato plants. Plant Soil 322: 197-207.
- Oliveira AP, Santos JF, Cavalcante LF, Pereira WE, Santos MCCA, et al. (2010) Yield of sweet potato fertilized with cattle manure and biofertilizer. Hortic Bras 28(3): 277-281.
- Razzak HSA, Moussa AG, Fattah MA, Morabet GA (2013) Response of sweet potato to integrated effect of chemical and natural phosphorus fertilizer and their levels in combination with mycorrhizal inoculation. J Biol Sci 13(3): 112-122.
- Alane F, Karima BM, Chabaca R, Abdelguerfi A (2019) Characterization of two oasis luzerns (El Menea, Tamentit) at the floral bud and early flowering stages. Environ Anal Eco Stud 6(3): 639-646.
- Halpern M, Tal AB, Ofek M, Minz D, Muller T, et al. (2015) The use of biostimulants for enhancing nutrient uptake. In: DL Sparks (Ed.), Adv Agron, Elsevier, California, USA, pp. 141-174.
- Oosten MJ, Pepe O, Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric 4: 5.
- Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front. Plant Sci 7: 671.
© 2021 Yoel Beovides García. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






