- Submissions

Full Text
Environmental Analysis & Ecology Studies
Status of Surface Water from Selected Areas of Coastal Guyana and Selective Removal of Pb2+ and Fe2+ using Pulverized Coconut Fibres
Jagessar RC* and Lord B
Department of Chemistry, University of Guyana, South America
*Corresponding author: Jagessar RC, Senior Lecturer, Department of Chemistry, University of Guyana, Turkeyen Campus, Georgetown, Guyana, South America
Submission: September 1, 2020Published: December 14, 2020

ISSN 2578-0336 Volume7 Issue4
Abstract
Guyana’s surface and domestic water needs constant monitoring to assess the concentration of toxic anions and cations. The status of surface water at five selected areas: Blairmont, Bath, Bushlot, Belladrum and Mahaicony surface water was assessed in terms of the parameters discussed. In all cases, the concentration of cations and anions were below the WHO standard. Only at Mahaicony surface water, the concentration of Cl¯ was above the WHO standards. The adsorbents (coconut fibres) was selective in its removal of Pb2+ at Bushlot, Mahaicony and Belladrum surface water. Also, it showed selectivity for removal of Fe3+ at all cases, whilst the concentration of Mn2+ remained the same for treated and untreated water. For example, the concentration of Fe2+ in the surface water at Bath for treated and untreated water was (7.31 ± 0.44mg/L) and (6.88 ± 0.51mg/l) respectively. It was also shown to reduce the turbidity in all cases, whilst elevating the pH.
Keywords: Guyana’s surface water; WHO standards;Cations;Anions;Selective areas;Concentration
Introduction
Water is a universal solvent that sustains all life forms. Much of the current concern with regards to environmental quality is focused on water, because of its importance in maintaining the human health and health of the ecosystem. Surface water is water on the surface of the planet, such as in a stream, river, lake, wetland, or ocean. It can be contrasted with groundwater and atmospheric water [1-7]. Providing sufficient quantities of high quality water to satisfy our domestic, industrial and agricultural needs is an ongoing global problem. Increasing population size, climate change and pollution will only exacerbate the global status. There is no physical shortage of water on the planet earth as it covers 70% of the globe. However, 97% of the world water is saline and is thus non-drinkable, 2% is locked in glaciers and polar ice caps, resulting in 1% to meet humanity needs [7,8]. Guyana water need continual monitoring to assess the concentration of toxic elements8. Surface water plays a very vital role in economics and the functioning of ecosystems [9]. In Guyana, surface water is primarily used for agricultural, industrial and commercial purposes. Pollution of surface water, due to industrial effluents and municipal waste in water bodies is a major concern in Georgetown, Linden and many other regions in Guyana.
Surface water is usually rainwater that collects in surface water bodies, like oceans, lakes, or streams. Another source of surface water is groundwater that discharges to the surface from springs. Guyana has abundant surface and ground water supplies near all populated centers. Both surface and ground water resources are relied upon for water supply requirements. Heavy amounts of precipitation provide high amounts of surface runoff and ground water recharge. Most of the domestic water supply comes from ground water resources, while most of the water supply for agriculture (such as, sugarcane and rice) and industry comes from surface water.
Surface water pollution occurs when hazardous substances come into contact and either dissolve or physically mix with the water [10,11]. Contamination of surface water can occur when hazardous substances are discharged directly from an outfall pipe or channel or when they receive contaminated storm water runoff. On the other hand, direct discharges can come from industrial sources or from certain older sewer systems that overflow during wet weather. Storm water runoff becomes contaminated when rainwater comes into contact with contaminated soil and either dissolves the contamination or carries contaminated soil particles. Surface water can also be contaminated when contaminated groundwater reaches the surface through a spring, or when contaminants in the air are deposited on the surface water. Contaminated soil particles carried by storm water runoff or contaminants from the air can sink to the bottom of a surface water body, mix with the sediment, and remain [12].
Subsequently, all levels of an ecosystem will be negatively impacted due to contamination of surface water. This is due to the fact that there will be a great change in the water chemistry. It can impact the health of lower food chain organisms and, consequently, the availability of the food supply up through the food chain. It can also impact the health of wetlands and impair their ability to support healthy ecosystems, control flooding, and filter pollutants from storm water runoff. Contaminated surface water can also affect the health of animals and humans when they drink or bathe in contaminated water or, for aquatic organisms, when they ingest contaminated sediments. One of the major concerns associated with contaminated surface water is the ability of aquatic organisms, like fish, to accumulate and concentrate contaminants in their bodies. When other animals or humans ingest these organisms, they receive a much higher dose of contamination than they would have if they had been directly exposed to the original source of the contamination.
The most effective approach for cleaning up contaminated surface water is to prevent further discharges from contaminated sources and enable natural biological, chemical, and physical processes to break down the existing contamination. In some surface water bodies where natural processes are not enough to break down the contaminants, other cleanup approaches such as ion exchange, reverse osmosis, precipitation, solvent extraction, membrane technologies, electrochemical treatment, sorption, etc. The latter is by far the most versatile and widely used, and activated carbon is the most commonly used sorbent. However, the use of activated carbon is expensive, so there has been considerable interest in the use of other sorbent materials, particularly biosorbents [13]. So the technique used is this study was biosorption. A pulverized plant material (coconut fibre from the plant Cocos nucifera) was used to aid in the removal of toxic metal ions from the surface water of selected areas or Mahaica- Berbice administrative region. Mahaica- Berbice was chosen as the study site, since it is well known for its large scale agricultural and industrial activities, which are major sources of surface water, and also due to the fact that surface water to be evaluated have not been previously analyzed.
According to literature, coconut fiber is rich in lignin (35-45%) and cellulose (23-43%), providing greater application potential in the treatment of aqueous solutions for the removal of heavy metals. Coconut fibres contain cellulose, hemi-cellulose and lignin as major composition. These compositions affect the different properties of coconut fibres. The pre-treatment of fibres changes the composition and ultimately changes not only its properties, but also the properties of composites. Sometimes it improves the behaviour of fibres, but sometimes its effect is not favourable [14].
Literature review reports that aquatic macrophytes, potential for the simultaneous removal of heavy metals such as Fe, Cu, Zn, Mn, Cr and Pb have been reported [15]. High metal removal percentages were reported for all metals. The removal of toxic metals: zinc, copper, mercury, cadmium or lead, from aqueous solutions by fungal biomass of Agaricus macrosporus has been reported16. The highest percentage uptake being of cadmium (96%).
The evaluation of a low-cost adsorbent in sugar cane residue or bagasse for the removal of toxic metal ions such as Cu2+, Ni2+ and Zn2+ from wastewater of an electroplating factory has been reported. The removal percentage being 95.5%, 96.3% and 97.1% [16,17]. The removal of ten metals (Se, Zn, Fe, Ni, Co, Pb, Mn, Hg, Cr and Cu) and the metalloid Arsenic, As by plant biomass for the detoxification of industrial effluents for environmental protection has been noted.18
The Aim and Objectives of this research were to examine the water quality of selected areas of region 5, Mahaica-Berbice of coastal Guyana and to evaluate the concentration of toxic metal cation and anion before and after the addition of pulverized coconut fibre. Guyana is a sovereign state on the northern mainland of South America and is also part of the Caribbean region. Guyana (83,000 square miles) is bordered by the Atlantic Ocean to the north, Brazil to the south and southwest, Suriname to the east and Venezuela to the west [18,19], Figure 1 & 2 is a map of two of the selected areas of coastal Guyana.
Methodology
This project was conducted in Guyana’s fifth administrative region: Mahaica Berbice. Selected areas being: surface water under Mahicony Bridge, Bushlot, Beladrum, Blairmont and Bath Settlement. These sites were selected based on a random sampling method where the names of the villages were placed in a bag and the first five were selected as the sampling sites. It so happened that each site had his own uniqueness i.e Blairmont has a Sugar Estate, Bushlot has a Rice Mill, Bath Settlement and Belladrum has lots of agriculture, and Mahaicony Bridge has fishing and a mode of transportation for farmers. Two photographs of one of the sampling sites are placed below.
Methods
Sample collection
The water samples were collected between 18:30hrs-19:30hrs on April 07, 2016, from the five sites previously described. The samples were collected (in triplicates) in plastic containers and stored on ice in a cooler. The samples were then submitted the next day for analysis at the Institute of Applied Science and Technology (IAST) laboratory.
Analysis of water samples
The surface water from selected areas of coastal Guyana was analysed according to standard procedure [20,21].
Turbidity: A representative sample was collected in a clean container. A sample cell was filled to the line (about 15mL), taking care to handle the sample cell by the top. The cell was capped. and wiped with a soft, lint-free cloth to remove water spots and fingerprints. A thin film of silicone oil was applied and wiped with a soft cloth to obtain an even film over the entire surface. The instrument was placed on a flat, sturdy surface. The sample cell was inserted in the instrument cell compartment so the diamond or orientation mark aligns with the raised orientation mark in front of the cell compartment. The lid was closed. The Manual or Automatic RANGE key was selected. Signal averaging mode was selected by pressing the SIGNAL AVERAGE key. The READ was pressed. The display showed ---NTU, then the turbidity in NTU. The turbidity was recorded after the lamp symbol turned off [22].
Determination of pH: After a successful calibration, the electrode was rinsed in deionized water. The electrode was then placed in the sample. The READ button was pressed. The sample temperature and the pH reading appeared. These values fluctuated until the system became stable. The pH was recorded. The electrode from the sample was rinsed with deionized water and placed in the next sample. Steps 2-4 were repeated for each sample. When finished, the meter was turned off. The electrode was rinsed with deionized water and blot dry. The protective cap on the electrode was replaced in the electrode holder.
Digestion of the samples: 100mL of sample was transferred to a 250mL conical flask and the volume was reduced on heat to approximately 50mL. It was then cooled, 10mL of concentrated nitric acid was added and samples further digested to approximately 5mL. Dark samples were cleared up by adding 30% hydrogen peroxide dropwise. 100mL of a blank sample was prepared and digested in the same manner. 100mL of each of the reference solutions were prepared and digested using the same procedure. The samples, blank and reference solutions were cooled and transferred quantitatively to a 100mL volumetric flask using Whatman #541 (7cm) filter paper to remove any residual precipitate. It was then diluted to the mark using distilled water and mixed well. The US-Vis Spectrophotometer was switched on and allowed to warm up for at least 15 minutes. A 10mL aliquot of each of the samples, blank, reference solution and standards were transferred to separate 50mL volumetric flasks. 20mL of distilled water, 1mL of 6M hydrochloric acid, 1mL of gum acacia and 0.5g of barium chloride dihydride were added to each of the samples, blank, reference solutions and standards. It was ensured that all solids dissolved, then diluted to the 50mL mark using distilled water. It was then shaken vigorously to mix. The absorbance was measured at 420nm.
Total Kjeldhal Nitrogen: 100mL of sample was transferred to a 250mL Erlenmeyer flask. 50mL of digestive reagent was added and samples heated on a hotplate to digest to ~50mL. Dark samples were cleared up by adding 30% hydrogen peroxide dropwise. 100mL of the control (TKN reference solution or Nutrients wastewater standard) was digested and a blank, using the same procedure. Samples, blank and reference solution were further digested to 5mL, then cool and transfer quantitatively to 100mL volumetric flask using 7cm Whatman #541 filter paper. It was then diluted to the mark with distilled water and mixed well. When samples crystallized after cooling, a minimum amount of distilled water was added, it was then shaken to dissolve crystals and reheated for about 15 minutes. 5mL each of the sample, blank and reference solutions were transferrred to separate 50 mL volumetric flasks. A piece of litmus paper (litmus turns pink) was added to each and neutralized with 10% NaOH (litmus paper turns blue). The spectrophotometer was switched on and allowed to warm up for at least 15min. A 100mg/L nitrogen standard was prepared by transferring 10mL of 1000mg/L stock standard to a 100mL volumetric flask, then diluting to the mark with distilled water and mixing thoroughly [23,24].
10mL of this solution was transferred to 100mL volumetric flaks and diluted with distilled water to give a 10mg/L nitrogen standard. It was mixed thoroughly. 1, 2 and 3mL each of the 10mg/L nitrogen standard was pipetted into 50mL volumetric flasks, 20mL of distilled water and 1mL of 10% NaOH were added and diluted to the mark. These volumes gave standards of 0.2, 0.45, and 0.6mg/L nitrogen. 1mL of Na2OSiO2 was added, followed by 2mL Nessler’s reagent to the sample, blank and reference solutions as well as to the standards from the previous step. It was then diluted to the mark with distilled water, shaken vigorously and absorbances at 420nm was measured using the UV-Vis Spectrophotometer.
Chloride- mohr argentometric method using phenolphthalein: 50mL of sample was transferred to an Erlenmeyer flask. 3 drops of phenolphthalein indicator solution was added, followed by 3 drops of sodium hydroxide solution. The solution developed a deep pink color. Six drops or more of acetic acid were added for neutralization. The solution became clear and colourless. Six drops of potassium chromate indicator solution were added. The sample was titrated against 0.1M AgNO3, until a permanent reddishbrown color appeared. Recorded titer as A. Quality control - determined the chloride concentration of the reference solution (10mL) described using the same procedure. A blank titration was performed using 50mL of distilled water. The titre was recorded as B. The AgNO3 solution was periodically standardarised with 0.1M NaCl solution.
Phosphates: Stannous Chloride Molybdate Colorimetric method: 100mL of sample was transferred to a 250mL conical flask. 10mL of 10% H2SO4 were added and digested to 50mL. The solution was cooled, then 10 mL of conc. HNO3 were added and samples further digested to 5mL. Dark samples were cleared up by adding 30% H2O2 dropwise. A sample blank and reference were prepared by digesting 100mL of water and reference solution using the same procedure. The solutions were transferred quantitatively to 100mL volumetric flasks using the Whatman #541, 11.0cm filter paper. It was then diluted to mark with distilled water and mixed well. A small piece of blue litmus paper was added to each aliquot. The paper turned red. NH3 solution (1:3 v/v) was added, until the red litmus paper changed to blue, then 10% H2SO4 was added until the blue litmus paper changed back to red. A 100mg/L phosphorous standard was prepared by transferring 10mL of 1000mg/L stock standard to a 100mL volumetric flask, then diluted to the mark with distilled water and mixed thoroughly. 5mL of this solution was transferred to a 100mL volumetric flask and diluted with distilled water to give a 5mg/L phosphorous standard. It was then mixed thoroughly. 1, 2, 3, 4 and 5mL each of the 5mg/L phosphorous standard was pipetted into 50mL volumetric flasks. Approximately 20mL of distilled water, 2mL of molybdate solution and 4 drops of stannous chloride solution were added to the sample, blank and reference solutions. The solutions were diluted to the mark with distilled water, shaken vigorously and absorbances measured at 660nm, using the UV-VIS Spectrophotometer.
Total metals: The standards were prepared by diluting the necessary volume of stock solution with distilled water in volumetric flasks. The sample was gently agitated to homogenicity, then 100mL was measured into a 250ml glass beaker. 5mL of concentrated nitric acid were added and the beaker was placed on the hot plate to allow the sample to digest. The volume was evaporated to about 4-5mL and transferred to a 25mL measuring cylinder. The solution was made up to 20mL with distilled water and transferred to a 20mL test tube. It was then covered with a stopper or a piece of plastic wrap and shaken well. Three blanks with distilled water were prepared using the above procedure. The AAS readings of these were averaged to obtain a more accurate blank concentration. The AAS was calibrated, using the prepared standards and the correct hollow cathode lamp. The samples were read on a calibrated AAS. Absorbance was measured at 420nm. It was ensured that the concentration of the sample falls within the calibrated range of the standard. If it did not, the concentration (either up or down) of the sample or the standards was adjusted. After the first laboratory tests of the methods listed above, 5g of the absorbent (coconut fiber) were added to 800mL of the remaining surface water samples and bottles underwent, an intermittent swirling motion for two hours. The water was then filtered, and the methods explained above were repeated on the filtrate. Results were noted and recorded.
Result
Table 1: Table showing the mean with standard deviation, variance and confidence limit values of the parameter’s pH and turbidity before and after the treatment with absorbent.
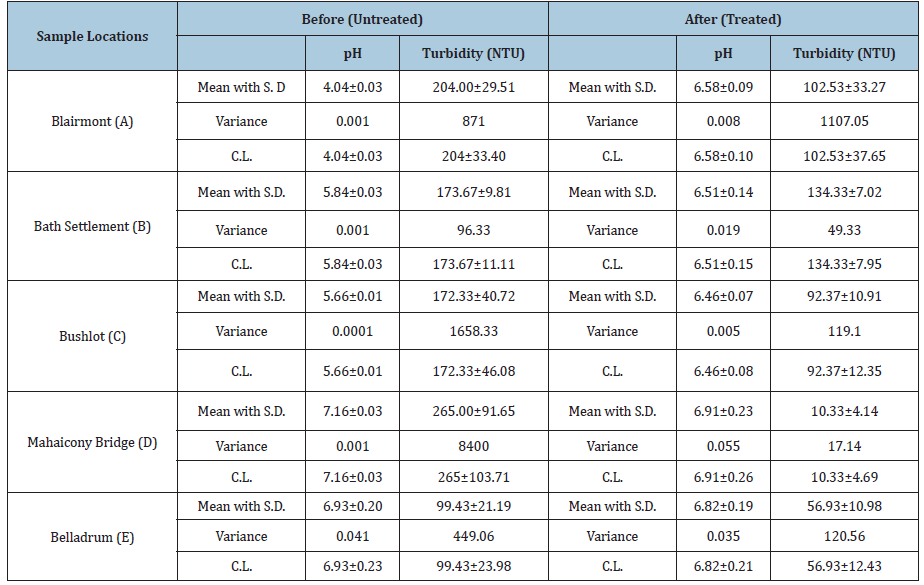
Table 2: Table showing the mean, standard deviation, variance and confidence limit values of the anions before and after the treatment with absorbent.

Table 3: Table showing the mean, standard deviation, variance and confidence limit values of the anions before and after the treatment with absorbent.

Table 4: Table showing the mean, standard deviation, variance and confidence limit values of the cations and total nitrogen before and after the treatment with absorbent.

Table 5: Table showing the mean, standard deviation, variance and confidence limit values of the cations and total nitrogen before and after the treatment with absorbent.
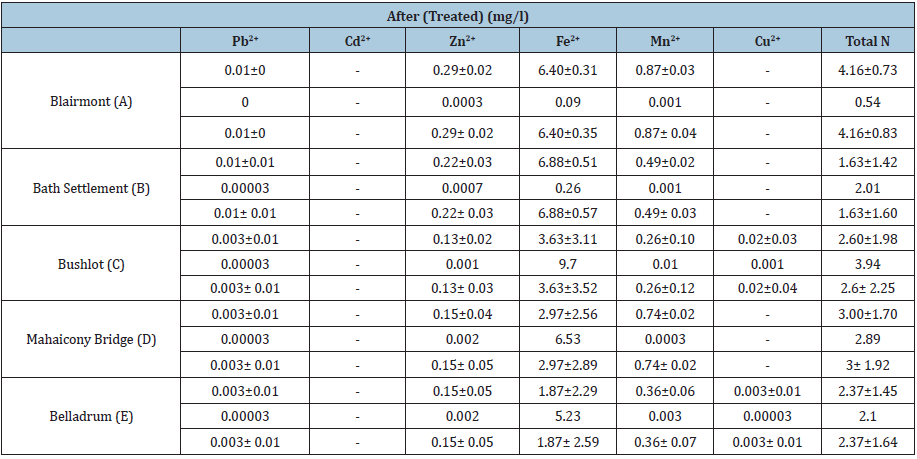
Table 6: Table showing the results for the Analysis of Variance (ANOVA) test of the parameters tested at the sample locations before treatment.
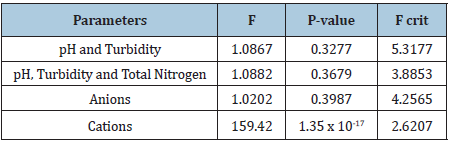
Table 7: Showing the results for the Analyses of Variance (ANOVA) test of the parameters tested at sample locations after treatment.
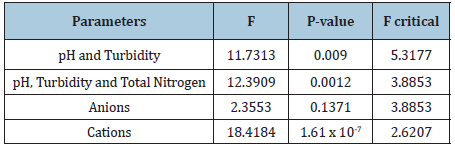
Table 8: Data for the determination of the cations: copper, zinc, cadmium, manganese, iron and lead content of the standard samples at varying concentration versus absorbance.
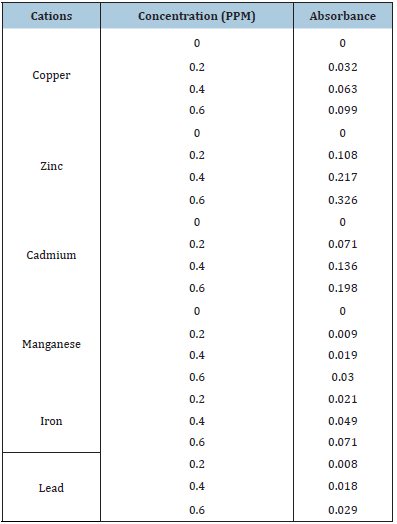
Table 9: Data for the determination of Total Nitrogen and the anions: sulphate and phosphate content of the standard samples at varying concentration versus absorbance.
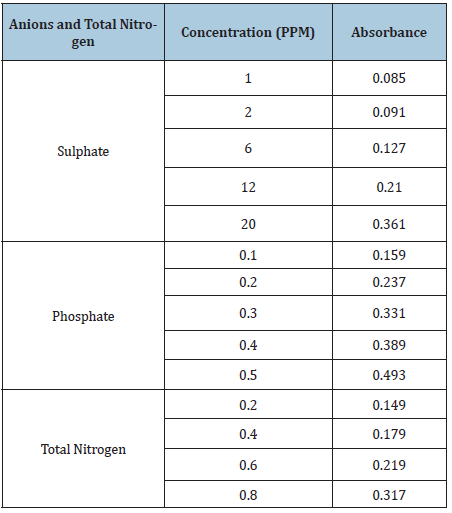
Figure 1: Map of Guyana.
Figure 2: Map of two of the selected areas of Coastal Guyana.
Figure 3: Surface water from one of the selected areas.

Figure 4: Collection of surface water from one of the selected areas.

Figure 5: Line Graph showing a plot of the absorbance vs concentration of the copper standard at concentration of 0, 0.2, 0.4 and 0.6 ppm copper; Graph. 5(b): Line Graph showing a plot of the absorbance vs concentration of the zinc standard at concentration of 0, 0.2, 0.4 and 0.6 ppm zinc.
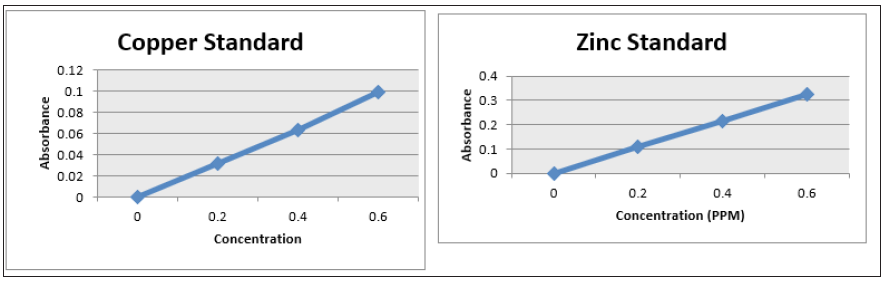
Figure 6: Line Graph showing a plot of the absorbance vs concentration of the cadmium standard at concentration of 0, 0.2, 0.4 and 0.6 ppm cadmium; Graph. 6(b): Line Graph showing a plot of the absorbance vs concentration of the manganese standard at concentration of 0, 0.2, 0.4 and 0.6 ppm manganese.
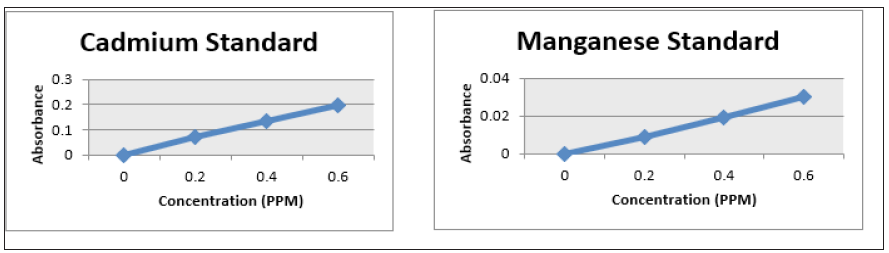
Figure 7: Line Graph showing a plot of the absorbance vs concentration of the iron standard at concentration of 0.2, 0.4 and 0.6 ppm iron. 7(b): Line Graph showing a plot of the absorbance vs concentration of the lead standard at concentration of 0.2, 0.4 and 0.6 ppm lead.
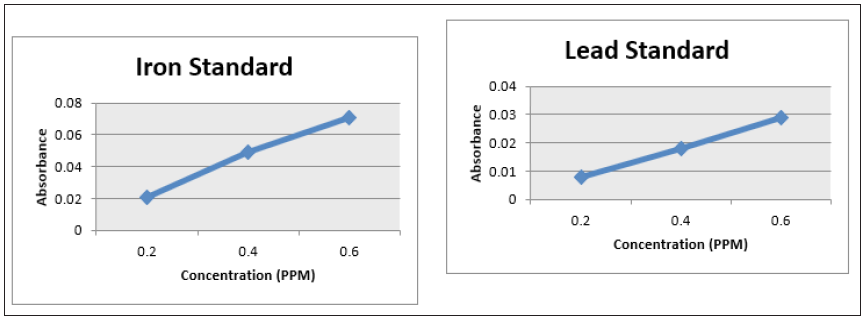
Figure 8: Line Graph showing a plot of the absorbance vs concentration of the sulphate standard at concentration of 1, 2, 6, 12 and 20ppm sulphate; Graph. 8(b): Line Graph showing a plot of the absorbance vs concentration of the phosphate standard at concentration of 0.1, 0.2, 0.3, 0.4 and 0.5ppm phosphate.
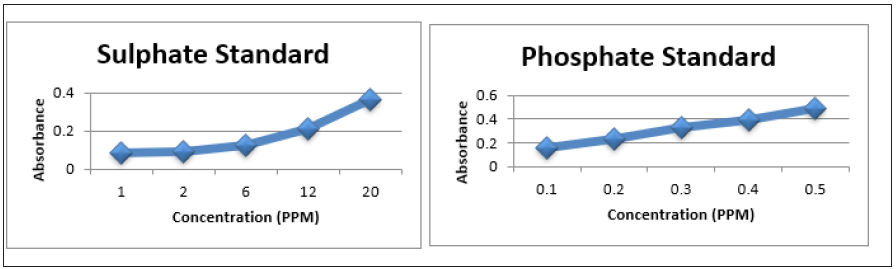
Figure 9: Line Graph showing a plot of the absorbance vs concentration of the total nitrogen standard at concentration of 0.2, 0.4, 0.6, 0.8 and 1.0ppm total nitrogen.
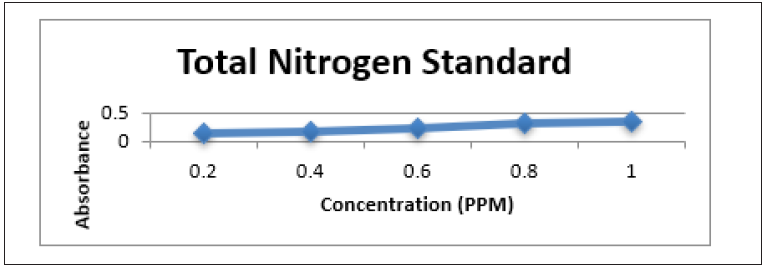
Figure 10: Bar Graph showing the mean pH levels at the various sample sites before and after the addition of the absorbent.
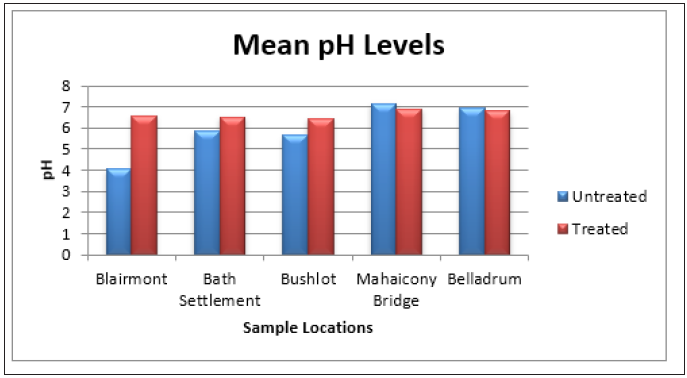
Figure 11: Bar Graph showing the mean turbidity levels at the various sample sites before and after the addition of the absorbent.
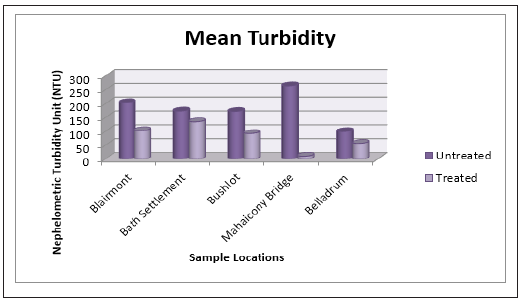
Figure 12: Bar Graph showing the mean concentration of iron at various sample sites before and after the addition of the absorbent.

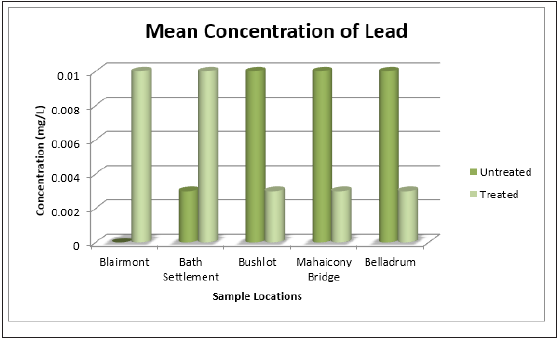
Figure 13: Bar Graph showing the mean concentration of lead at the various sample sites before and after the addition of the absorbent.
Conceptuality, validity, sensitivity, specificity, feasibility, reliability, sustainability, understandability, timeliness, and flexible have been kept in mind while selecting the indictor as well as data set. Data for PM10 is taken from UP Pollution Control Board from 2008 to 2017 from all seven stations across the city. The annual average from 2008 to 2017 have been averaged to get cumulative annual average in µg/m3. Interpolation techniques is used to assign readings to the surrounding wards in such manner as no ward is left unserved, more less in circular fashion. Data for population density is taken Census of India, 2011 for each ward of the city. Percentage of slum population for each ward is taken from Rajiv Aawas Yojana Slum Free city program, 2012. Data for literacy rates is taken from Census of India, 2011. Road density data for each ward is taken from Lucknow Municipal Corporation (LMC), 2015. Data of hospitals is generated by combining two different lists of hospitals one each for Private and government hospitals. Government hospitals number is given by NUHM Lucknow, Government of Uttar Pradesh, 2013-14. Private hospitals’ number is released by Lucknow Nursing Home Association, 2016. Both of numbers are combined and placed on google maps to get the exact location, based on which a ward wise dataset is prepared. This hospital data excludes private clinics and quacks and counts only the major hospitals with significant beds and health infrastructure. The source for park area is again LMC which provides the zone wise park areas in hectares and average park area for each ward is obtained. Lastly, ward wise primary garbage collection figures are taken again from LMC by averaging out share of each ward from the respective zone (Table 1).
Discussion
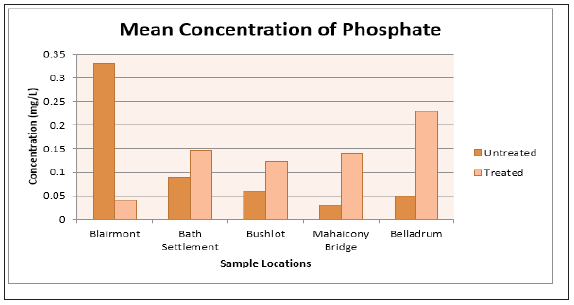
The status of surface water was measured at five selected areas of Blairmont, Bath, Bushlot, Belladrum and Mahaicony. The physical parameters tested for were pH and Turbidity. First the status of the surface water before treatments needs to be discussed: Consider the anions, Table 2, PO43¯ ion concentration range from (0.06 ± 0.02mg/L) to (0.33 ± 0.01mg/L). The highest concentration of PO43¯ (0.33 ± 0.1mg/L), was detected at Blairmont surface water. The concentration of PO43¯ was less than the WHO standard of 5.0mg/L for all the selected areas For Cl¯ anion, the highest concentration of (20, 070 ± 3.72mg/L) was detected at Mahaicony surface water and this was exceedingly above the WHO Standard of 250mg/L. The Cl¯ ion concentration for the other areas range from (4.46 ± 7.72mg/L to 75.79 ± 91.72mg/L) and these values are below the WHO Standard value of 250mg/L. SO4 2¯ was undetected for all the areas, with the exception of Mahaicony surface water, which registered a value of (0.05 ± 0.00mg/L) which was less than the WHO Standard of 200mg/L.
With respective to cations, Pb2+ concentration range from (0.003 ± 0.01mg/L to 0.01 ± 0.0mg/L). These values are below the WHO Standard of 0.1mg/L. There was no detection for Cd2+. Zn2+ concentration range from (0.003 ± 0.0mg/L to 0.01 ± 0.0mg/L) and these values are below the WHO standard of 3mg/L. Fe2+ concentration range from (5.07 ± 0.39mg/L) to (7.09 ± 0.03mg/L). These values are above the WHO standard of 0.3mg/L. Mn2+ concentration range from (0.26 ± 0.12mg/L to 0.87 ± 0.03mg/L). These values are below the WHO standard of -----. There wasn’t any detection for Cu2+.
The surface water was tested for pH and turbidity before and after treatment with the adsorbent, Table 1. It was noticeable that after treatment with the adsorbent, the turbidity decrease in all cases, whereas the pH was elevated for Blairmont, Bath settlement and Bushlot surface water but decrease for Mahaicony and Belladrum surface water. For example, for Blairmont and Bush Lot surface water, the turbidity decreases from (204.00 ± 29.5NTU), (172.33 ± 40.72NTU) to (102.53 ± 33.27NTU), (92.37 ± 10.91NTU) respectively after treatment. At Bath and Mahaicony, the pH was (5.84 ± 0.03mg/L), (7.16 ± 0.03mg/L) before treatment and after treatment, the pH was (6.51 ± 0.14), (6.91 ± 0.23). Graphs shows the variation of physical parameters such as pH and Turbidity verses selected areas. The pH was elevated for Blairmont, Bath settlement and Bushlot surface water, but decrease for Mahaicony and Belladrum surface water. For example, for Blairmont surface water, the pH was (4.04 ± 0.03mg/L) and (6.58 ± 0.09mg/dl) before and after treatment with coconut fibre. For Mahaicony and Belladrum surface water, there was a decrease in pH. For example, for Belladrum surface water, the pH decrease from (6.093 ± 0.20mg/L) to (6.82 ± 0.19mg/l). Figure 6 & 7 show the variation of the mean pH and Turbidity before and after treatments.
The surface water was tested for the following anions: Cl¯, PO43¯, SO4 2¯ before and after treatments. For Blairmont, Bath settlements and Bushlot surface water, there was a significant increase in the Cl¯ concentrations. For the Mahaicony surface and Belladrum surface water, there was a significant decrease in the Cl¯ concentrations. With regards to the PO43¯ concentrations, there was an increase after treatments. For example, the PO43¯ concentration at Blairmont and Bushlot surface water before treatment was (0.33 ± 0.01mg/L) and (0.06 ± 0.02mg/L) and after treatment was (0.40 ± 0.13mg/L) and (0.12 ± 0.06mg/L) respectively. The coconut fibres wasn’t effective in removing PO43¯ ions. Negligible SO4 2¯ was detected for Blairmont, Bath Settlement, Bushlot and Belladrum surface water, before treatment. However, Mahaicony surface water showed a concentration of (0.05 ± 0.00mg/L). After treatment, with adsorbent, the SO4 2¯ seems to be present for Blairmont (0.003 ± 0.006 mg/L) and Belladrum (0.01 ± 0.02 mg/L) surface water. Figure 12 & 13, graphs shows the variation of the mean concentration of anions before and after treatment with the adsorbent, coconut fibres.
The status of the surface water for the presence of Pb<2+, Cd2+, Zn2+, Fe2+, Mn2+ and Cu2+ before and after treatments was also determined. It was noticeable at Bushlot, Mahaicony and Belladrum surface water, there was a decrease in the concentration of Pb2+. The coconut adsorbent was effective in removing Pb2+ cations at these three locations. For example, at Bushlot and Belladrum surface water, the concentration of Pb2+ was (0.01 ± 0.01mg/L) and (0.01 ± 0.00mg/L) respectively before treatments and after treatments was (0.003 ± 0.01mg/L) and (0.003 ± 0.01mg/L) respectively. For Blairmont & Bath surface water, the opposite was noted. There was an increase in Pb2+ ion concentration. Before treatment, Pb2+ concentration was found to be (0.00 ± 0.0mg/L) and (0.003 ± 0.01mg/L) respectively. After treatment, the concentration of Pb2+ was found to be (0.01 ± 0.0mg/L) and (0.01 ± 0.01mg/L). For Cd2+, there was no detection for untreated & treated H2O. For Zn2+, there was an increase in concentration for treated water. The adsorbent wasn’t effective in removing Zn2+.
At Bath and Belladrum surface water, the concentration of Zn2+ before and after treatment was (0.003 ± 0.01 mg/L), (0.02 ± 0.0mg/L) and (0.22 ± 0.03mg/L), (0.15 ± 0.05mg/L) respectively. For Fe3+, there seems to be a decrease in concentration after treatment with the adsorbents for all regions. For example, the concentration, of Fe2+ at Blairmont and Bath Settlement was (7.09 ± 0.03mg/L), (7.31 ± 0.44mg/L) and (6.40 ± 0.31mg/L), (6.88 ± 0.51mg/L) before and after treatments respectively. This again shows that the adsorbent was effective in removing Fe2+ ions. With regards to Mn2+, the concentration of Mn2+ was the same for the surface water water at all five locations, before and after water treatments. There wasn’t any detection for Cu2+ in the untreated water. However, for the treated water, the concentration of Cu2+ was the same, with the exception for Bushlot (0.02 ± 0.03mg/L) and Belladrum (0.003 ± 0.01mg/L) surface water, indicating that Cu2+ has been leached out of the coconut fibres.
There was also an increase in the total nitrogen content of the treated water as compared to the untreated water for all selected areas. For example, the total nitrogen content for the Mahaicony surface water and Belladrum surface water before treatment was (0.03 ± 0.01mg/L), (0.02 ± 0.01mg/L) respectively for the same water subjected to treatment with adsorbents. Values registered were (3.00 ± 1.70mg/L) and (2.37 ± 1.45mg/L) respectively. Figure 8 & 11 shows the variation in the mean concentration of cations verses selected areas for untreated and treated water. Figure 1 to Figure 5a are line graphs, showing a plot of the absorption vs. concentration of various cations and anions tested for the standard samples. Anova factor with two replication was used to assess whether there is a significant difference in physical parameters such as pH, Turbidity, cation and anion concentration for the untreated and treated water. Table 6 indicates that for untreated water, there wasn’t any significance difference between pH & Turbidity, the p value, 0.3277 is greater than 0.05 and the F value (1.0867) < F critical (5.3177). There was also no significant difference between pH, Turbidity and the total nitrogen content, as the p-value (0.3679) is greater than 0.05 and F value (1.0869) < F critical (3.8853). There was also no significant difference between the concentration of the anions as P-value (0.3987) is greater than 0.05 and F value (1.0202) < F critical (4.2565). However, for cations, there was significance differences in the concentration as P (1.35 x 10-17) < 0.05 and F value (159. 42) > F critical (2.6207). Table 7 shows the Anova results for the physical parameters (pH and Turbidity) and cation and anion concentration after the water was treated. For (pH and Turbidity) and (pH, Turbidity and Total nitrogen), there seems to be a significant differences as the individual P-values are less than 0.05. For anions, no significant differences, P(0.1371) > 0.05 and F (2.3553) < F critical (3.8853). For cations, P (1.61 x 10-7) < 0.05, indicating significance differences.
Conclusion
The status of surface water at the five selected areas: Blairmont, Bath, Bushlot, Belladrum and Mahaicony of coastal Guyana was assessed in terms of the parameters discussed. In all cases, the concentration of cations and anions were below the WHO standard. Only at Mahaicony surface water, the concentration of Cl¯ was above the WHO standards. Also, the Fe2+ concentration of the untreated water was above the WHO standards in all cases. The adsorbents was selective in its removal of Pb2+ at Bushlot, Mahaicony and Belladrum surface water. Also, it showed selectivity for removal of Fe3+ at all cases. The concentration of Mn2+ remained the same for untreated and treated water in all cases. It was also shown to reduce the turbidity for all selected surface water, whilst elevating the pH. In some cases, the adsorbent was effective in removing a particular metal cation from some surface water after treatment, whereas the same metal ion concentration, increased in other areas. Thus, coconut fibres should be an excellent candidate for the removal of Pb2+ and Fe3+ ions from contaminated water and to induce a decrease in turbidity Figure 13-17.
Figure 14: Bar Graph showing the mean concentration of zinc at the various sample sites before and after the addition of the absorbent.
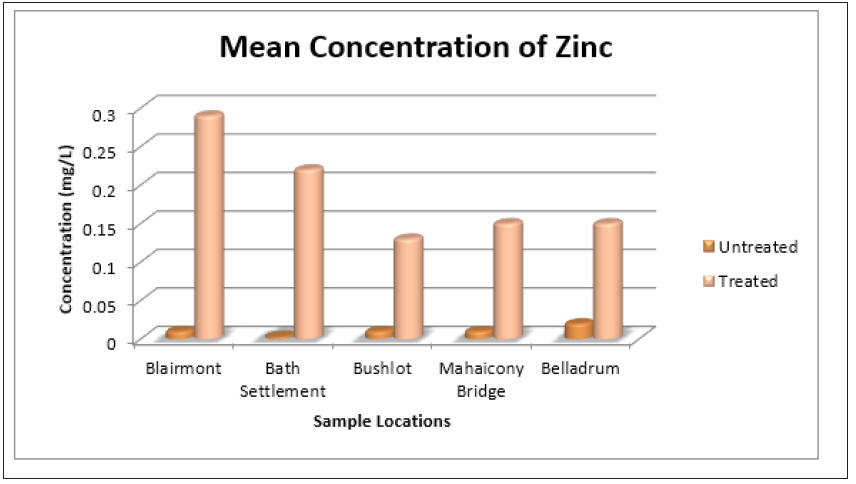
Figure 15: Bar Graph showing the mean concentration of manganese at the various sample sites before and after the addition of the absorbent.

Figure 16: Bar Graph showing the mean concentration of chloride at the various sample sites before and after the addition of the absorbent.
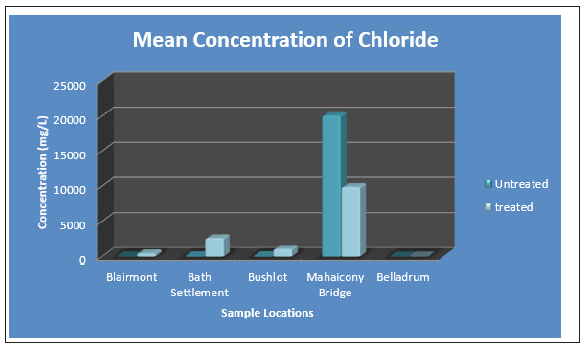
Figure 17: Bar Graph showing the mean concentration of phosphate at the various sample sites before and after the addition of the absorbent.
Acknowledgement
We thank the Institute of Applied Science and Technology (IAST) analytical lab for paid Analytical Services.
References
- Young RA, Bredehoeft JD (1972) Digital simulation for solving management problems with conjunctive groundwater and surface water systems. Water Resources Research 8(3): 533-556.
- Eaton AD, Clessicens SL, Greenberg EA (1995) Standard methods for the Examination of Water and Wastewater, (19th edn), United Book Press Inc, Baltimore, Maryland, USA, pp. 4-48.
- Eaton AD, AWWA, Chair, et al, (1995) Standard methods for the examination of Water and Wastewater, (19th edn), United Book Press Inc. Baltimore, Maryland USA, pp. 4-67.
- Booth RL (1983) Methods for the chemical analysis of water and wastes, (2nd edn), Environmental monitoring and support laboratory, Office of Research and Development, US Environmental Protection Agency, Ohio, USA, p. 352.
- Hach company, Water Analyses handbook, 3rd ed. Loveland Colorado, USA; 1997, 304-307.
- Radojevic M, Bashkin VN (1999) Practical environmental analysis, Royal Society of Chemistry, Cambridge, UK.
- Elliot S (2008) Testing the water, Royal Society of Chemistry, RSC, News Magazine 12(5): 12-13.
- Williams N (2010) Guyana Times, a News Magazine 11:
- Khublaryan MG (1994) Surface Waters: Rivers, streams, lakes and wetlands. Encylopaedia of Life Support Systems. Types and Properties of Water 1:
- EPA (2011) Drinking water contaminants.
- EPA (2013) Total Nitrogen. Environmental Protection Agency, USA.
- Carr GM, Neary JP (2008) Water quality for ecosystem and human health 2006. United Nations environment programme global environment monitoring system/water programme, Burlington, Canada, p. 132.
- Ng J, Cheung W, McKay G (2003) Equilibrium studies for the sorption of lead from effluents using chitosan. Elsevier Science, Chemosphere 52(6): 1021-1030.
- Hossain M, Nalsy M, Sobohan M (2013) Effect of industrial pollution on the spatial variation of surface water quality. American Journal of Environmental Sciences 9(2): 120-129.
- Miretzky P, Saralegui A, Cirelli AF (2004) Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere 57(8): 997-1005.
- Melgar MJ, Alonso J, Garcia MA (2007) Removal of toxic metals from aqueous solutions by fungal biomass of Agaricus macrosporus. Total Environ 385(1-3): 12-19.
- Sousa FW, Sousa MJ, Oliveira IRN, Oliveira AG, Cavalcante RM, et al. (2009) Evaluation of a low-cost adsorbent for removal of toxic metal ions from wastewater of an electroplating factory. Journal of Environmental Management 90(11): 3340-3344.
- Sekhar KC, Kamala CT, Chary NS, Anjaneyulu Y (2003) International Journal of Mineral Processing, 68(1-4): 37-45.
- worldatlas.com/webimage/countrys/samerica/gy.html
- Eaton AD (1995) Standard methods for the examination of water and waste water. United Book Press Inc, Baltimore, USA.
- Methods for Chemical Analysis of Water and Wastes. (n.d.). US Environmental Protection Agency, Ohio, USA.
- Jagessar R (2015) An evaluation of metal cations and anions concentration in surface water from six selected areas of coastal Guyana via Flame Atomic Spectroscopy. International Journal of Research in Chemistry and Environment 5(3): 10-17.
- Jagessar R, Alleyne O (2011) Determination of nitrate anions concentrations in wastewater from selected areas of coastal Guyana via spectrometric method. International Journal of Academic Research 3 (1): 443-453.
- Jagessar R, Sooknundun L (2010) Determination of Nitrate Anion in waste water from nine selected areas of coastal Guyana via a Spectrometric method. International Journal of Research and Reviews in Applied Sciences 7(2): 203-212.
© 2020 Jagessar RC. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






