- Submissions

Full Text
Environmental Analysis & Ecology Studies
Yield Potential and Aflatoxin Resistance in Subtropical Maize Hybrids
Noé D Sánchez Isordia1, Ricardo E Preciado Ortiz1*, Jorge Covarrubias Prieto2, Arturo D Terrón Ibarra1, César A Reyes Méndez3, Noel O Gomes Montiel4, Marco A García Perea2, Juan G Ramírez Pimentel2 and Ernesto Moreno Martínez5
1Maize Breeding Program of the National Forestry, Agriculture and Husbandry Research Institute, Bajío Experimental Station, México
2Graduate Program in Production and Seed Technology at Tecnológico Nacional de México, Instituto Tecnológico de Roque, México
3Rio Bravo Experimental Station, México
4Iguala Experimental Station, México
5Instituto de Ecología UNAM, México
*Corresponding author: Ricardo E Preciado-Ortiz, Maize Breeding Program of the National Forestry, Agriculture and Husbandry Research Institute, Bajío Experimental Station, México
Submission: September 25, 2019Published: July 17, 2020

ISSN 2578-0336 Volume7 Issue1
Abstract
Environmental variations caused by climate change have been increasing the impact and risks on maize (Zea mays L.) production, due to biotic factors like crop pests and diseases attack, which presence are altered by changes in abiotic climate conditions such as temperature and precipitation between others; the fungus Aspergillus flavus that in maize kernel develops aflatoxins (AF), a potent carcinogenic substance produced by this fungus which affects considerably animal and human health. For that reason, the development of AF resistance maize genotypes with good yield performance and agronomical quality become very important. Recent research conducted by National Institute of Forestry, Agricultural and Livestock Research (INIFAP) and the National Autonomous University of Mexico (UNAM) identified AF genetic resistant materials, nevertheless these materials had limited yield performance. INIFAP Maize Breeding Program initiated the development of superior inbred lines by pedigree selection, which might allow to obtain experimental hybrids with AF resistance and good grain yield potential. The objective of this study was to identify outstanding hybrids with AF contamination resistance. A new group of experimental hybrids formed by selected inbred lines were evaluated for grain yield during summer 2015 at Celaya, Gto. and Morelia, Mich., Mexico; under a 9 X 9 lattice design with two replications, another field experiment was conducted at Rio Bravo, Tam., Mexico were the presence of aflatoxins is endemic. At laboratory were analyzed two types of samples, the first from the inoculation with A. flavus of a set of seed of the hybrids used for field evaluation; and second from samples obtained from Rio Bravo field experiment. Results from field and laboratory allow identifying 19 statistically superior hybrids in yield and agronomic performance with a range of 8.6 to 11.6Mg ha-1 with good resistance to AF from the field, with values below 20ppb.
Keywords: Zea Mays L.; Aflatoxins resistance; Pedigree selection; Hybridization
Introduction
With the aim to reduce maize (Zea mays L.) production risks caused by several climate change (CC) factors like streamed temperatures, erratic precipitation, relative humidity, solar radiation, wind speed and so on [1]. These factors altered quantity, reproduction, dissemination, and survival of pest populations which higher incidence affected severely crop production. Pathological problems caused by fungal diseases have been also increased affecting ear and stalk rot. Aspergillus, Fusarium and Penicillium, are important fungus which affects maize grain in the field and in storage. These diseases produce substances known as mycotoxins. Specifically, Aspergillus flavus besides the presence of rotten ears, this fungus releases a highly carcinogenic compounds, named Aflatoxins (AF) which constitutes a serious human health and husbandry problems, and also causes important harvest loses, because contaminated grain has to be destroyed [2,3].
Highly carcinogenic AF represents a food contamination risk for human been, they might induce highly and chronic toxicity and caused terminal diseases. Animals also can be affected by AF contamination, provoking hepatic necrosis, nephritis, lung congestion and carcinogenic effects [4]. Nevertheless, actually the presence of AF contamination is focalized in specific regions, where climatic conditions are adequate for the fungus development (principally Southeast USA and Northeast Mexico regions), but with CC effects, this problem is latent in other maize production regions, as have happened in 2015 at Sinaloa, Mexico, were unusual frost forced farmers to replant, and in the replanted fields serious AF contamination problems were present.
Survive and development of Aspergillus flavus and Aflatoxinas pathological fungus depends on temperature and humidity conditions, affecting maize crop from the seed, field, post-harvest, transport and storage, affecting viability, nutritional quality and sanitary characteristics of grains and seeds [5,6]. Aspergillus flavus is an opportunist fungus with the particularity to produce AF [3,6], which are formed as a final product of a fungus secondary metabolism, and also AF is a defense mechanism from another microorganism [7]. The more important types of AF are: B1, B2, G1, M1 y M2, Aspergillus flavus is one of the principal producers of AF, but it only produces B1 y B2 types, where B1 is the more dangerous [4,8]. The economic impact of AF in food contamination, resulting in the increase of human and veterinary health costs, reducing animal production, among other economic and trading problems, some developed countries have clear legislations on the amount of AF allowed in their feed products, which cannot exceed 20 parts per billion (ppb) [8].
Factors which influence AF infestation
AF production principally depends on fungus development, so climatic conditions such as temperature and relative humidity are the main factors. The optimum temperature for AF production is 25 to 35°C, they do not occur below 10°C and above 45°C. The production range is between 12 to 40°C, in addition, the minimum relative humidity for the fungus development is 85% [2,9]. Aspergillus flavus achieves its greatest infestation mainly under plant water stress, due to high temperatures and poor agronomic management, in addition, some studies have found that plants stressed by drought, induces a large increase in the production of the amino acid proline, this amino acid like others such as glycine, glutamate, aspartate and glutamine stimulates AF production [2,10]. The presence of the fungus does not imply the production of AF, however when fungus is developed, the contamination risk can be higher in the first 24 hours, reaching the maximum contamination risk between 7-10 days [10].
Several biotic and abiotic factors enhance the development of the fungus and therefore AF contamination, some of these factors are; poor agronomic management, like inadequate soil preparation, inadequate planting dates, bad weed control, drought presence, high temperatures and relative humidity; the presence of pests and diseases are also of great importance for fungus development and subsequent AF contamination, ear and grain damage caused by several pests facilitate the entry and development of the fungus, mechanical damage as well, caused by deficient transporting and handling are another factors that caused AF contamination [7,11]. Biotic and abiotic factors caused by CC might favor AF contamination in maize production regions where the problem was not previously present. With the aim to increase maize production, and reduce CC risks, due to the eminent presence of adverse factors which could favor AF contamination in maize, it is necessary to generate AF resistance resistant and good productive potential maize germplasm by genetic improvement [11-13].
Recent research conducted by the Bajio Maize Breeding Program of the National Institute of Forestry, Agricultural and Livestock Research (INIFAP) and the Grains and Seeds Research Unit (UNIGRAS) of the National Autonomous University of Mexico (UNAM) have focused in the identification of the inheritance mechanism involved in the resistance of AF contamination by quantifying AF produced in grain by Aspergillus flavus on several maize genotypes. From this work, some genetic resistant materials were identified, unfortunately, the productive and agronomic performance of the resistant materials identified were limited; to solve this problem, at the INIFAP-Bajio Maize Breeding Program was started the process to generate superior inbred lines by pedigree selection to be able to develop hybrids with AF resistance and good productive potential. The objective of this research was to identify outstanding high yield potential hybrids with AF contamination resistance
Materials and Methods
The field activities of this research were conducted by the INIFAP-Maize Breeding Program at Bajio and Rio Bravo Experimental Stations, in collaboration with the Department of Graduate Studies of the National Technological of Mexico / Roque Technological Institute; and work was carried out in the UNAM-UNIGRAS- Laboratory (Table 1).
Genetic material. The germplasm involved in this research comes from INIFAP’s Germplasm Bank accessions, and INIFAP’s Corn Breeding Program populations and elite inbred lines (Table 2). These materials were classified as resistant and susceptible to AF contamination under several years of collaboration between INIFAP and UNAM. Unfortunately, resistant genotypes identified, presented poor agronomic and productive performance, for that reason, in order to fix and take advantage of the resistance, a pedigree selection process of was initiated by crossing resistant materials with elite tropical and subtropical inbred lines, with the aim to develop new inbred lines with AF resistance, more productive and superior agronomic characteristics; this process of advancing and selecting lines was carried out through several seasons at the INIFAP’s Bajio and Iguala Experimental Stations. With the new derived inbred lines, in 2014 a group of experimental hybrids were formed, with heterotically contrasting germplasm, which were evaluated in the field and in laboratory for 2015 summer season.
Table 1: Environment description of the locations where the field work was carried out for 2015 summer season.
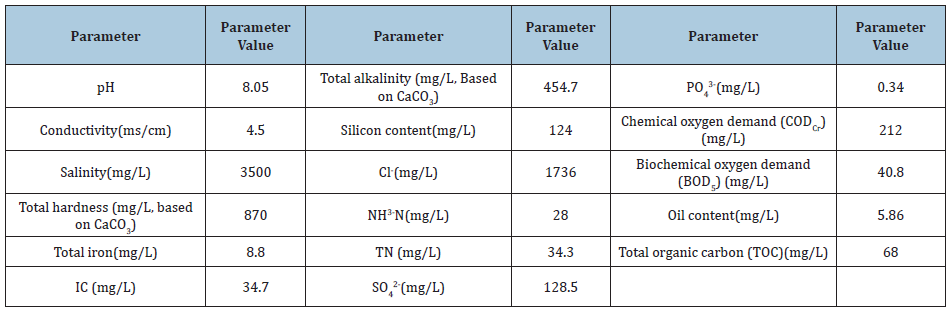
Table 2: Origin and Germplasm used in the genetic material involved in the hybrid combinations evaluated in the field at Celaya, Gto and Morelia, Mich, and in laboratory for AF contamination resistance.

Inoculation with Aspergillus flavus
Maize samples were washed, sterilized and inoculated by a potato-dextrose-agar medium, where the fungus A. flavus was incubated at 25°C (UNIGRAS strain 28); after eight days a portion of the medium was taken and was added 10 ml of sterile distilled water with a Tween solution 80 to 0.05%, stirred and spore counting was performed through Neubauer's chamber; moisture content (MH) of the seed was measured by the stove method [14] and inoculation was performed with 15,000 spores per experimental unit (EU).
Extraction and quantification of aflatoxins
In each EU, 10 grams of ground maize were weighed, and 40 ml methanol at 70% was added, was dissolved to obtain one ml of supernatant liquid, which was centrifuged for one minute, to dilute the extract and quantify the amount of AF using the Afla-V method (Kit Vicam Micotoxin Test), which allowed to quantify up to 150ppb of AF as a limit.
Experimental design and field variables
Field experiments for grain yield and agronomic characteristics were carried out at locations described in Table 1. The variables measured were: days to anthesis (pollen shed (AD) and silking (SD), plant (PH) and ear (EH) height, total root and stem lodging (TL), percentage of rotten ears (RE), poor ear coverage percentage (EC) and grain yield (GY). A 9x9 experimental lattice design was used per location, with 78 hybrids, 3 local commercial checks and 2 replicates, each EU consisting of two 5 m row with separation of 0.76 m, and 70 seeds per plot. Also, an experiment was established at Rio Bravo, Tamps. (region with endemic presence of AF), and because experiment was sown outside the recommended planting date, in order to ensure optimal environmental conditions for AF natural greater contamination in the grain, this situation caused low GY at harvest, so in this location genotypes only were sampled to measure AF incidence; however, agronomic evaluation was not considered.
Aflatoxins Evaluation
A sample of seed used to establish field experiments were inoculated in laboratory with A. flavus, in order to obtain information of AF contamination resistance, by extracting and quantifying, the amount of AF in the seed. Resistant hybrids were considered when they had less than 20ppb of AF in each sample. A completely random experimental design (CRED) was used; a total of 162EU (78 hybrids, three controls, and two repetitions), each sample consisting in 250 grams of inoculated and ground maize preserved in glass jars. Subsequently, in grain samples harvested at Rio Bravo, Tamps. the extraction and quantification of AF was carried out. Also, a CRED was used; with a total of 243EU (78 hybrids, three control checks and three replications), also each sample consisting in 250 grams of ground maize preserved in glass jars.
Statistical analysis
Analysis of variance of agronomic variables for each location and the combined for both locations were carried out, to determine the performance of the hybrids in both locations by the genotype x location interaction effect, with the aim to identify the outstanding hybrids for the region as well as the most stable ones. Tukey test comparison of means was performed in statistical significance agronomic variables and laboratory results; these analyses were performed using the SAS (Statistical Analysis System) statistical package, version 9.0.
Result and Discussion
Table 3 presents the combined analysis of variance (ANOVA) of the agronomic variables evaluated in the field experiments established at Celaya, Gto. and Morelia, Mich. Significant differences were observed for genotype and location in all variables, except for EH in genotype, which was only significant at (0.05). In genotype x location interaction, there were significant differences for GY, SD, and ER. From the three sources of variation, the variance due to location was greater, in all variables. Therefore, the significant differences observed in the agronomic variables of this research demonstrate the genetic variability of the genotypes evaluated, as well as the different environmental conditions like climate and soil which was present between both locations. Table 4 shows the means comparison of significant variables of the hybrids evaluated in both locations, since GY is the final expression of many agronomic characteristics that have manifested simultaneously [15], only superior GY hybrids, that were statistically similar are shown, as well as the laboratory results from them.
Table 3: Mean squares, degrees of freedom and statistical significance of the combined analysis of variance for grain yield and agronomic characteristics of the AF-resistant maize hybrids evaluated across Celaya, Gto and Morelia, Mich locations for 2035 Summer season.
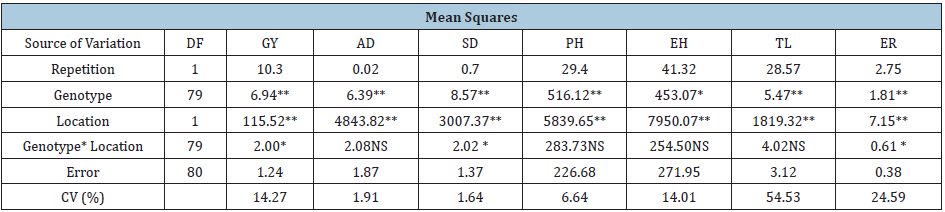
*, **, Significant at 0.05 and 0.01 of probability, respectively; NS: Not significant; CV: Coefficient of variation; DF: Degrees of freedom; GY: Grain yield; AD: days to Anthesis; SD: days to Silking; PH: Plant Height; EH: Ear Height; TL: Total Lodging; ER: Ear rot
Grain Yield (GY)
The GY highest statistical group formed by19 hybrids of were identified, with a range from 11.59 to 8.60Mg ha-1 (Table 4). There are some reports with equivalent yields in commercial and experimental hybrids adapted to similar regions [16-18]. So it can be assumed that the 19 outstanding hybrids of this research, might be considered competitive, in the locations that were evaluated, with greater potential for Morelia, Mich., which presented greater GY than some commercial hybrids evaluated in previous years, however, in the individual analyses of each location, the hybrids had higher GY at Celaya, Gto. Table 5. shows that hybrids 6 and 32 had similar GY in both locations. At Celaya, Gto., the hybrids of the superior statistical group for GY exceed 10Mg ha-1 and the hybrids (45, 28, 43, 44, 30 and 17)) surpassed the commercial check control (SULTAN). At Morelia, Mich., the hybrids 45, 6 and 28 with GY of 10.92, 10.22 and 9.84Mg ha-1 respectively, were statistically similar to the commercial check controls DK2027 and H-377 with 9.98 and 9.82Mg ha-1 respectively.
Table 4: Tukey´s Comparison means test of the evaluated variables from the combined analysis across Celaya Gto and Morelia, Mich 2015 summer season and laboratory results.
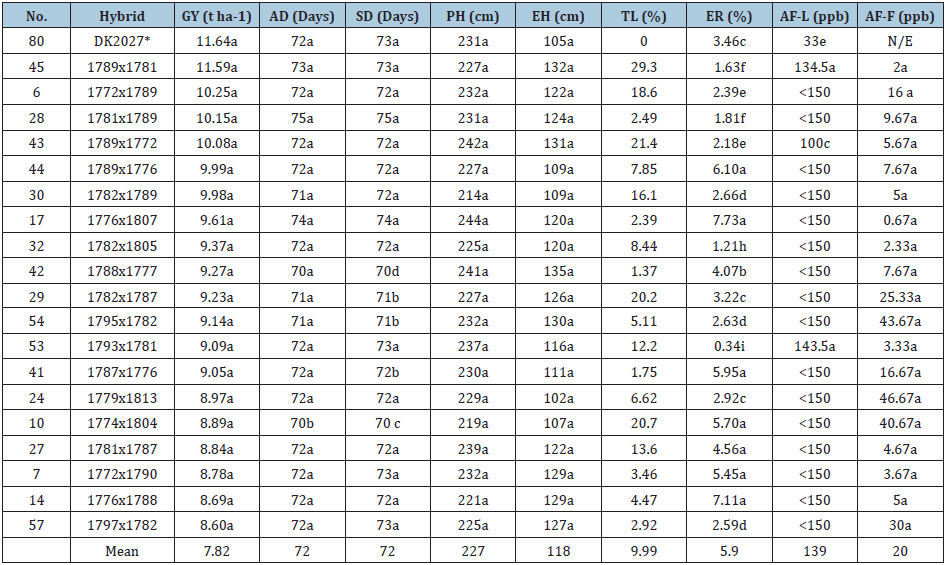
Means with the same letter are similar statistically, according to Tukey (p<0.05). GY: Grain Yield, AD: Days to Anthesis; SD: Days to Silking; PH: Plant Height; EH: Ear Height; TL: Total Lodging; ER: Ear rot; AF-L: Laboratory AF Quantity (Under Inoculation), AF-F: Amount in Non-Inoculated Field Samples, *-Commercial control , N/E-Not Evaluated.
Anthesis (AD) and Silking (SD) Days
Table 5: Comparison of the agronomic variables evaluated that showed significance in the individual analyses of Celaya, Gto. and Morelia Mich. 2015 Summer season.
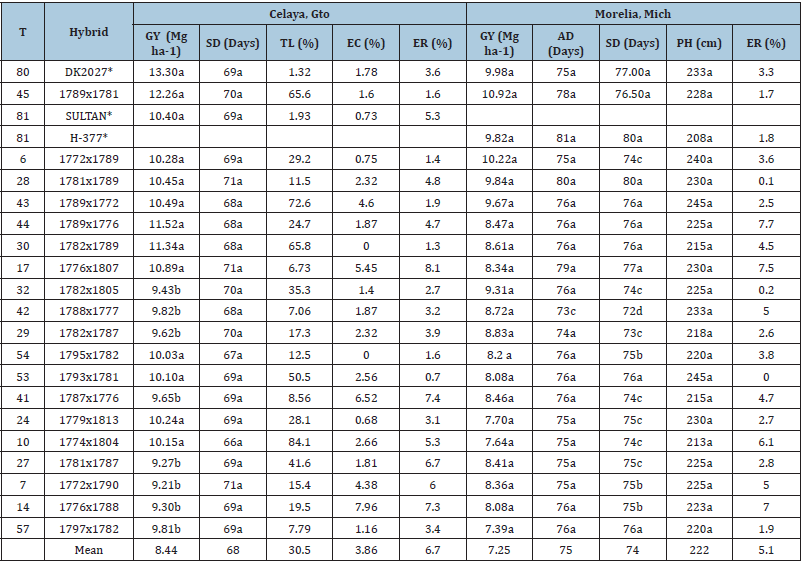
Means with the same letter are similar statistically, according to Tukey (p<0.05). GY: Grain Yield, AD: Days to Anthesis; SD: Days to Silking; PH: Plant Height; EH: Ear Height; TL: Total Lodging; ER: Ear rot; AF-L: AF Laboratory Quantity (Under Inoculation), AF-F: AF Amount in Field Samples (Non-Inoculated), *-Commercial control, N/E-Not Evaluated.
The combined analysis show that the mean for these variables were 72 days (Table 4), with a range from 67 to 75 days, so hybrids are considered intermediate maturity, as mentioned by Ledesma et al. [19], when evaluating testcrosses in Celaya, Morelia and other locations, with results ranges similar to those of this research. For subtropical conditions, early intermediate maturity, is defined from 62 to 66 days, and up to 78 days as intermediate maturity. Intermediate maturity materials for this region, represents an advantage for the use of rotating cultivation crops during the year [20]. The shorter vegetative materials are an alternative for delayed plant ing dates in the Bajío, in order to avoid the most intense periods of heat and higher water demands [17,20], so intermediate maturity hybrids evaluated, could be very useful by farmers in this region. The highly significant differences in both variables were of greater importance in the location factor (Table 3), and through individual analyses by locality it was obtained that these variables were later at Morelia, Mich. Due to its higher-altitude, lower mean temperature and different latitude (Table 1), as can be seen in days to SD (Table 5).
Plant (PH) and Ear (EH) Height
In several maize studies, lower PH genotypes are considering with some advantages because of the high correlation with EH [21], which allows to have shorter materials with accessible EH for manual harvest (thinking in tropical very tall maize cultivars), as well as positive correlations between PH an EH with lodging percentage, however some of the hybrids evaluated in this paper do not present these trends, as can be seen in the hybrids 28, 17, 54, 41 and 7, which had the higher PH, and are not necessarily higher EH and have low percentages of TL (Table 4).
Total Root and Stalk Lodging (TL)
Hybrids 28, 17, 42, 54, 41, 7, 14, and 57 were identified with low TL percentage in the combined analysis, as well as in the individual analysis at Celaya, Gto., where strong winds were present in the growing season (Table 4), besides those conditions it allowed to confirm that hybrids 28, 17 and 54 presented some resistance for this variable and their GY were above 10Mg ha-1 in this location (Table 5).
Ear Rot (ER)
High values of this variable can significantly reduce GY up to 40% [2,21], so most of the higher GY hybrids evaluated in this work did not have high values of ER, which had lower percentages than this variable average, except genotypes 44, 17, 41 and 14 (Table 5), which have the parent 1776 in common, which can be assumed to present some susceptibility to this character. Because the variation for location factor was greater (Table 3), the presence of diseases causing ER of some hybrids through environments is considered different, reflecting climate differences, relative humidity, cloudiness and presence of the fungus in the two evaluated locations, results similar to those of Sierra et al. [21].
Poor Ear Coverage (EC)
This variable was only evaluated at Celaya, Gto. EC is directly related to ER, since husk does not protect the maize ear from pests and other vectors which causing diseases. Hybrids 30, 6, 45, 54, 32, 24 and 57 had lower EC and ER percentages (Table 5), coinciding this positive trend with the results of Sierra et al. [21]. There are reports which mention that possibly the AF contamination resistance of the hybrid H-443 A is due to the good EC presented in this genotype [22]; in our research even hybrids 54, 24 and 57, mentioned above, presented lower percentages of EC and ER they had greater values than 20 ppb in AF-C (Table 4), so this variable might not be the only mechanism of AF contamination resistance.
Laboratory Aflatoxins Quantification (AF-L)
The values obtained for AF-L (under inoculation) are considered higher compared to the AF-F variable, because: a) the controlled conditions of the laboratory ensure the effectiveness of the inoculation; b) the strain of the fungus Aspergillus flavus used in inoculation was obtained and characterized by UNIGRAS, as highly toxigenic, so that even resistant materials, can have relatively high values AF values; c) in the inoculation was necessary an adjustment of the grain humidity content to provide the appropriate conditions for the development of the fungus, which involved physical deterioration of the grain, that possibly favored and increased AF contamination under inoculation [7,22]. On the other hand, the method used to quantify AF, Kit Afla-V (Vicam Mocotoxin Test Kit), has as a reading limit values of 150ppb so it did not allow readings above this value, limiting the comparison between a sample with 151ppb and a 3,000ppb (where the first could be considered with greater resistance than the second). From the group of 19 statistically superior hybrids in GY, three hybrids (45,43 and 53) with less than 150ppb of AF were observed in this variable (Table 4); with respect to check control DK2027 presented contamination with only 33ppb, this response was attributed that the seed of that check had being treated with fungicide. The results of this variable, of the three outstanding hybrids with less than 150ppb, values were lower compared to those reported by Alezones et al. [23], which although they used another technique and other strain, their results were very high, from 20,000 to 11,500,000ppb of AF when evaluating tropical maize germplasm for resistance to the same fungus and to the production of this toxin, as well as other similar work of inoculations in the grain mentioned in this same research, where they report AF values between 3,000 and 68,000ppb. There are some other research reports in which they worked with materials considered AF resistant and with the inoculation of the same strain from UNIGRAS, under greenhouse conditions [24], in which they found values of 0.5ppb in H-443A genotype reported as resistant to this toxin [22,24] and values of 349 and 510ppb in landrace maize accessions ZMT1 and ZMT2, considered tolerant to storage fungus development and physiological deterioration.
Field Aflatoxins quantification (AF-F)
The grain samples used to quantify AF-F, came from INIFAP's Rio Bravo Experimental Station in Tamaulipas State, as mentioned above, in that region, during the growing season it is common the presence and development of AF in the grain because the environmental conditions. Samples analyzed showed that more than 60% of the total hybrids evaluated presented AF levels below 20ppb. Mostly the higher statistical group of GY hybrids, had the same values (Table 4), so these materials have the potential to be exploited in improvement programs, by not exceeding the permissible limits of AF in grain [8,24]. Samples realized during 2007 in commercial farmer fields in this region, reported that the H-443A maize hybrid was the least contaminated with AF with 21ppb considered to be AF resistant, compared with other hybrids which had values of 36 to 323ppb [22,25]. In other studies, several authors report AF levels in samples of maize under natural infestation up to 990ppb [23,25].
Table 4 shows that most of the higher GY hybrids obtained in this research had fewer levels of AF contamination than values reported previously. Based on the first laboratory analysis, which allowed us to identify hybrids (45 (1789x1781), 43 (1789x1772) and 53 (1793x1781) with lower AF-L values under inoculation, and AF-F values of 2, 5.67 and 3.33ppb respectively, these three hybrids could be considered to be the most AF contamination resistant in this research, and with GY and agronomic characteristics statistically similar to control checks.
Table 6 summarizes the germplasm and pedigree involved in the superior in GY and AF resistance hybrids, in this Table can be appreciate the germplasm involved, which are AF resistance populations, Germplasm Bank AF resistance accessions, and elite inbred lines (Table 1). The parental inbred lines, derived from pedigree selection, 1789 and 1781 involved in superior GY and AF resistance hybrids could be used as sources of AF resistance hybrids for subtropical regions. The information presented in this research can be useful to develop maize hybrids which can face the potential problem of AF contamination in different maize production areas under climate change effects.
Table 6: Pedigree and Germplasm involved in the three superior AF resistance hybrids of this research.

AF-L Laboratory inoculation; AF-F Field samples.
Conclusion
Hybrids 45, 43 and 53 presented good performance in GY, agronomic characteristics and less presence of AF contamination in the two laboratory analyses, ear rot resistant, intermediate maturity and competitive performance for the Bajío region, Hybrid 45 (1789x1781) is considered with very good ear coverage. Most of the hybrids evaluated in this research contain values below 20ppb in contaminated grain from the field. Also, parental lines of the hybrid 45 can be used as source of AF resistance in breeding programs.
References
- Rosenzweig C, Iglesius A, Yang XB, Epstein PR, Chivian E et al., (2001) Climate change and extreme weather event-implications for food production, plant diseases, and pests. Global Change and Human Health 2: 90-104.
- Arrúa A, Moreno E, Quezada MY, Moreno J, Vázquez AM, et al. (2012) Aflatoxygenic aspergillus: Current taxonomic approach. Mexican Journal of Agricultural Sciences 3: 1047-1052.
- Martínez HY, Hernández S, Reyes CA, Vázquez G (2013) The genus Aspergillus and its mycotoxins in corn in Mexico: Problems and perspectives. Mexican Journal of Plant Pathology 31(2): 126-146.
- Bogantes P, Bogantesy SBD (2004) Aflatoxins. Costa Rican Medical Act 46: 174-178.
- Agrios GN (2005) Phytopathology. In: Agrios GN (Ed.), (2nd edn), Limusa, Mexico, p. 952.
- Carvajal M (2013) Transformation of aflatoxin B1 from food into the human carcinogen, AFB1-DNA adduct. TIP Specialized Journal of Chemical and Biological Sciences of the UNAM 16: 109-120.
- Klich MA (2007) Aspergillus flavus: The major producer of aflatoxin. Mol Plant Patho 8(6): 713-722.
- Serrano H, Cardona N (2015) Micotoxicosis y micotoxinas: Generalidades y aspectos bá Revista CES Medicina 29(1): 143-152.
- Moreno E, Gil M (1991) The Biology of Aspergillus flavus and the Production of Aflatoxins. Coordination of Scientific Research. University Food Program, National Autonomous University of Mexico. Mexico, p. 42.
- Moreno E (1988) Manual for the identification of fungi of grains and their derivatives. National Autonomous University of Mexico. Mexico, p. 109.
- Ureta C, Martínez E, Perales HR, Álvarez ER (2012) Projecting the effects of climate change on the distribution of maize races and their wild relatives in Mexico. Global Change Biology 18: 1073-1082.
- Castro S, Lopez JA, Pecina JA, Mendozay MC, Reyes (2013) Exploration of native corn germplasm in central and southern Tamaulipas. Mexican Magazine of Agricultural Sciences, Mexico 4(4): 645-653.
- Ahumada R, Velázquez G, Flores JR (2014) Potential impacts of climate change on corn production. Research and Science of the Autonomous University of Aguascalientes 22: 48-53.
- Moreno E (1996) Physical and biological analysis of agricultural seeds. National Autonomous University of Mexico, Mexico, p. 393.
- Hallauer AR, Miranda JB (1988) Quantitative genetics in maize breeding. In: Hallauer AR & Miranda JB (Eds.), University Press (2nd edn), Iowa State, Iowa, USA, p. 468.
- Torres JJ, Chapa JA, Martínez OA, Bucio CM (2011) Formation of multiple cross maize hybrids from commercial hybrids. University Act of the University of Guanajuato 21: 24-30.
- Peña A, González F, Santana OI, Robles FJ, Ramírez JL, et al. (2013) H-326: Early white corn hybrid for El bajío and north central mexico region. Technical Brochure No. 49. INIFAP. Mexico, p. 32.
- Ramírez CA, González JC, Hernández J (2015) Evaluation of the Zapalote Chico maize breed as the donor with the lowest ear height. Agricultural Science and Technology 3:10-19.
- Ledesma A, Ramírez JL, Vidal VA, Peña A, Ruíz JA, et al. (2015) Proposal to integrate a heterotic pattern of yellow grain corn for the transition zone of Mexico. Evaluation of crossbreeds and crosses. Mexican Plant Technology Magazine 38: 133-143.
- Preciado RE, Terrón AD (2001) Behavior and adaptation of two new trilinear corn hybrids, H-316 and H-317, for Bají Mexican Plant Technology Magazine 24: 235-239.
- Sierra M, Becerra EN, Palafox A, S. Barrón, Cano O, et al. (2004) Characterization of corn hybrids (Zea mays L.) with high protein quality for their performance and tolerance to ear rot in southeastern Mexico. Mexican Journal of Plant Pathology 22: 268-276.
- Cantú MA, Reyes CA, Caballero y GMG, Vázquez (2009) H-443A, yellow corn hybrid for Northeast Mexico. Mexico, p. 35.
- Alezones J, Asdrúbaly AGM (2014) Evaluation of tropical corn germplasm for resistance to Aspergillus flavus and the production of grain aflatoxins. Interciencia of the Central University of Venezuela 39: 803-808.
- Quezada MY, Flores A, Arrúa AA, Vázquez ME, Moreno E (2011) Resistance of corn plants to Aspergillus flavus Link infection in the greenhouse. New Era Agrarian Magazine 8: 15-20.
- Mendoza M, López A, Oyervides A, Martínez G, de León C, et al. (2003) Genetic and cytoplasmic inheritance of resistance to corn (Zea mays L.) ear rot caused by Fusarium moniliforme Mexican Journal of Plant Pathology 21: 267-271.
© 2020 Ricardo E Preciado Ortiz. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






