- Submissions

Full Text
Environmental Analysis & Ecology Studies
Fluorescence and Molecular Weight of NOM Released from Forest Soil Under Different pH Conditions
Hongjie Gui 1* and Fusheng Li2
1School of Environmental Science and Engineering, China
2River Basin Research Center, Japan
*Corresponding author: Hongjie Gui, School of Environmental Science and Engineering, China
Submission: February 18, 2019Published: March 20, 2020

ISSN 2578-0336 Volume7 Issue1
Introduction
The concentration and composition of natural organic matter (NOM) dissolved in river water are greatly affected by the soil properties of different land types in the adjacent catchments. As an important source, forest soils supply a considerable amount of NOM to rivers through runoff during rainfall. With its properties of reacting with chlorine during drinking water disinfection process to form carcinogenic byproducts and competing with synthetic organic chemicals for adsorption in drinking water treatment, better understanding of the characteristics of NOM is important [1,2]. NOM is a mixture of a wide range of complicated constituents. Consisted mainly of humid and fulvic acids, NOM possesses different physicochemical properties in many aspects, such as molecular size, hydrophobicity and electric charge density. The content and composition of NOM in a river is not only associated with the soil types in its catchment but also affected by the pH of rainwater that contacts with the soils and carries NOM constituents into aquatic environment [3]. This study was performed to investigate the physicochemical characteristics of NOM released to Kani River from one of the major soil types distributed in its catchment. The investigation was based on specific ultraviolet absorbance (SUVA), fluorescence excitation emission matrix (EEM) and molecular weight distribution (MW).
Materials and Methods
Soil source and NOM releasing-A representative forest soil type in the Kani River catchment (Kani-city, Gifu, Japan) was chosen for this study. NOM in the soil was released under three different pH conditions (2.0, 7.0 and 11.0) following the procedures:
- adding 1kg of the forest soil in 6L of the pretreated tap water of Gifu University by activated carbon adsorption for removing dissolved organic matter contained therein
- agitating the soil solution with a mixer at 500rpm for 7 days and then allowing settlement for one day, and
- centrifuging the supernatant at 12000rpm for 5min, then filtering through 0.2μm membrane filter (Toyo Roshi, Japan).
The filtered water samples were used as the stock solutions containing released NOM for analysis. Specific ultraviolet absorbance (SUVA)- A parameter defined as the ratio of the ultraviolet absorbance at the wavelength of 260nm (UV260) to total dissolved organic carbon (DOC). UV260 and DOC were measured according to standard methods. Fluorescence EEM-Fluorescence EEM was measured with a spectrofluorometer (RF-5300, Shimadzu Co., Japan). The excitation and emission scans were performed in the range of 220-550nm with 5nm increments. Molecular weight (MW) distribution-MW distribution was analyzed using a high-performance size exclusion chromatography system (HPSEC) that consisted of a silica chromatographic column (GL-W520-X 10.7×450mm, Hitachi) and a UV-visible detector (Model LC-10AV, Shimadzu) with the wavelength of 260nm. Milli-Q that contained 0.02M Na2HPO4 and 0.02M KH2PO4, was used as elute with a constant rate of 0.5mL/min. The calibration of MW was made by three polystyrene sulfonate (PSS) with MW of 1430, 4950 and 6530g/mol. Based on the obtained MW chromatograms, the weight-averaged (Mw) and number-averaged (Mn) molecular weights were computed according to the following expressions [1]:
where, MWi (t) is the MW as a function of the elution time t, hi (t) is the detector response and Δt is the time interval. Polydispersity, a parameter calculated by the ratio of computed Mw to Mn (Polydispersity = Mw/Mn) was adopted for evaluating the heterogeneity of NOM in molecular weight.
Result and Discussion
SUVA-the differences in the UV-absorbing capability of NOM released from the forest soil under acidic, neutral and basic conditions were evaluated. As shown in Table 1, SUVA increased from 3.56 to 6.95[m-1/(mg/L)] as the initial pH used for releasing NOM increased from 2.0-11.0, indicating more species of NOM that possessed higher UV-adsorbing capability were released from the soil under higher pH. The values of DOC showed that, compared to neutral (6.8mg/L), more NOM species were released under acidic (18.5mg/L) and basic (278.2mg/L) conditions, with the released content under basic condition being tens of times higher. The significant differences in DOC reflected the differences in the easiness for soil humid molecules to get released into water under different pH environment and could be interpreted by their solubility differences as reported [3]. In a previous study, Li et al. [4] investigated the changes of SUVA for dissolved organic matter in Nagara River (Gifu, Japan) during a storm rain. The values of SUVA in the upstream, middle stream and downstream of this river varied in the range of 1.3-2.8[m-1/(mg/L)], showing a trend of increases as the river water level ascended and decreases as descended. Compared to these values, the values of SUVA of this study were comparatively larger, indicating the organic constituents released from the forest soil was mainly comprised of humid molecules.
Table 1: Specific ultraviolet absorbance of NOM released from forest soil under different pH conditions.
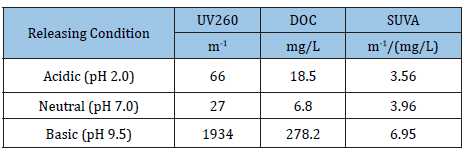
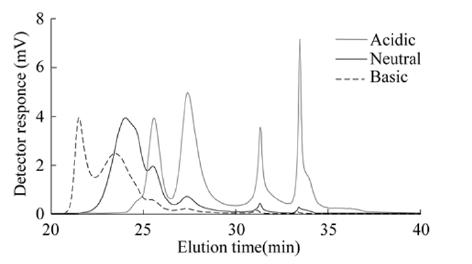
Florescence EEM-the characteristics of NOM released under different pH conditions based on fluorescence EEM are shown in Figure 1 using diluted samples having the same DOC concentration (1.6mg/L). The released NOM revealed two distinct peaks. The first and more intense peak (A') appeared in the excitation wavelengths of 220-240nm, and the second less intense one (A) in the range of 315-335nm. For both these peaks, the emission wavelengths are nearly identical, falling in the range of 420-450nm, a range well reported as the range for humic-like substances (humic acids and fulvic acids) in rivers and marines [5]. In addition, two weak peaks reflecting the tyrosine-like (B) and protein-like (C) molecules appeared only for the NOM released under the basic condition. MW distribution-the profiles of MW distribution of NOM are shown in Figure 2 with diluted samples. It is clear that, compared to the MW distribution for NOM released under the neutral pH condition; under the acidic condition, more organic molecules that possessed smaller molecular weight were released; while, under the basic condition, more molecules with larger molecular weight were released. The first peak appeared for NOM released under the acidic, neutral and basic condition corresponded to the apparent molecular weight of 6500, 7685 and 10122g/mol as PSS, respectively. Comparison of the MW distribution under the neutral pH condition with reported ones for dissolved organic matter in the less polluted Nagara River water in Gifu, and an underground peaty water and the Tokoro River water in Hokkaido made clear that their patterns were very close, thus suggesting NOM released from the forest soil is representative [2]. The computed values for Mw, Mn and polydispersity are shown in Table 2. For both Mw and Mn, from the acidic to basic condition, the averaged molecular weight for NOM molecules released from the soil increased by about one-fold, clearly indicating that more fulvic acids were released under the acidic condition and more humic acids were released under the basic condition.
Figure 1: Fluorescence EEM of NOM released from forest soil under different pH conditions (DOC=1.6mg/L)
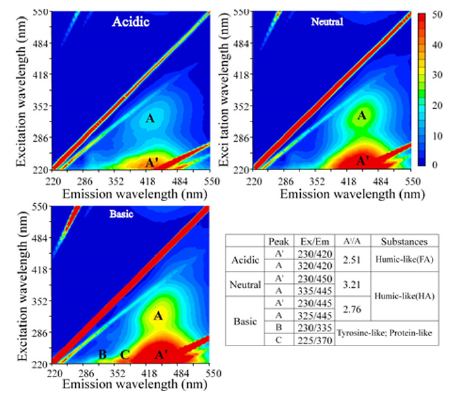
Figure 2: MW distribution of NOM released from forest soil under different pH conditions.
In regard of the heterogeneity in molecular weight, with a magnitude similar to Nagara River [2] before and after a storm rain (1.11-1.23), the computed polydispersity showed slight decreases (from 1.15 to 1.10) as the releasing pH changed from the acidic to basic condition, indicating the released NOM molecules were comparatively more heterogeneous under the acidic condition, judging from both their individual molecular weights and concentrations. The values of polydispersity are in close similarity with Nagara River but are obviously smaller than the market-available humic acids or humic substances isolated following extraction and purification [3].
References
- Kilduff JE, Karanfil T, Chin YP, Weber WJ (1996) Adsorption of natural organic polyelectrolytes by activated carbon: A size-exclusion chromatography study. Environ Sci Technol 30(4): 1336-1343.
- Li FS, Yuasa A, Chiharada H, Matsui Y (2003) Polydisperse absorbability composition of several natural and synthetic organic matrices. J Colloid Interface Sci 265(2): 265-275.
- Swift RS (1996) Organic matter characterization. In: Sparks DL, Page AL (Eds.) Methods of soil analysis (3rd part), Chemical methods, Soil Science Society of America, Inc, Wisconsin, USA.
- Li FS, Yuasa A, Muraki Y, Matsui Y (2005) Impacts of a heavy storm of rain upon dissolved and particulate organic C, N and P in the main river of a vegetation-rich basin area in Japan. Sci Total Environ 345(1-3): 99-113.
- Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Marine Chemistry 51(4): 325-346.
© 2020 Hongjie Gui. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






