- Submissions

Full Text
Environmental Analysis & Ecology Studies
Current Opinion in Artificial Photosynthesis with Molecular Catalysts
Carla Casadevall*
Department of Chemistry, University of Cambridge, UK
*Corresponding author: Carla Casadevall, Department of Chemistry, University of Cambridge, UK
Submission: October 21, 2019Published: February 20, 2020

ISSN 2578-0336 Volume7 Issue1
Abstract
The transition to a green and sustainable energy-based scheme is one of the most important challenges that faces our society. Natural photosynthesis is the process by which sunlight energy is stored into chemical bonds to sustain life, producing only O2 as a by-product. Therefore, an appealing approach is the application of Artificial Photosynthetic (AP) schemes to produce the so-called solar fuels and fine solar chemicals from CO2 and water using sunlight as driving force. However, both CO2 reduction and water oxidation (WO) are challenging processes and remain bottlenecks for the development of efficient AP. In addition, a viable artificial photosynthetic approach should also rely on inexpensive and long-lasting photocatalytic materials. In this regard, new sustainable, modular, robust and efficient catalytic platforms are needed. Moreover, it is important to notice that to design efficient and robust artificial photosynthetic systems, a fundamental understanding of the factors that control both the catalytic activity and selectivity is necessary.
Main Text
Natural photosynthesis elegantly transforms solar energy into chemical energy, obtaining the chemical products plants need to sustain their life. In this regard, one of the most important challenges is to mimic this process artificially to develop sustainable and greener synthetic organic methodologies to produce solar fuels and fine chemicals based on the application of artificial photosynthetic schemes [1,2]. However, both CO2 reduction and Water Oxidation (WO) are challenging processes and remain bottlenecks for the development of efficient artificial photosynthesis. A large number of accessible reaction pathways with similar thermodynamic reduction potential (Eq.I.3-5) [3,4] and the multi-proton-electron transformations involved in the CO2 reduction difficult the selectivity [5]. On the other hand, WO (Eq.I.1) is a highly endergonic process that requires the access of very high oxidation states at the metal center, which often leads to oxidative damage side reactions. In addition, a viable artificial photosynthetic approach should also rely on inexpensive, non-toxic and robust photocatalytic materials [6]. In this regard, new sustainable, modular, robust and efficient catalytic platforms are needed (Figure 1). Moreover, it is also important to notice that to design efficient and robust artificial photosynthetic systems, a fundamental understanding of the factors that control both the catalytic activity and selectivity is necessary [7].
Figure 1:

Despite all the studies related to understanding all the processes in natural photosynthesis in detail, still, there are some mechanisms that remain not fully understood, such as the O-O bond formation in water oxidation (WO) by the OEC. The study of photosynthesis despite being challenging due to the high amount of simultaneous multi-proton and electron transfer processes is needed to understand how this natural process works so that it can be mimicked and even improved. A better understanding of the essential components is still required to construct efficient artificial photosynthetic systems (Figure 2):
Figure 1: Simplified extended artificial photosynthetic scheme representing the use of electron from the oxidation of water to perform reductive transformations. WOC stands for Water Oxidation Catalysts and RC stands for Reduction Catalysts.
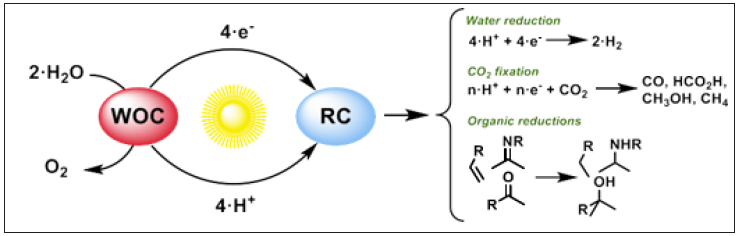
- light harvesting,
- charge separation,
- water oxidation (Eq.I.2),
- proton and electron transport,
- proton reduction (Eq.I.2) and
- CO2 fixation (Eq.I.3-5). In this line, the study of the mechanisms involved in “natural” photosynthesis very much benefit from the development of synthetic models mimicking the natural machinery that allows for the investigation of the different processes involved in such complex transformation individually, such as light harvesting, charge transfer and separation, water oxidation and reductive transformations [8].
In the case of WO, the key aspects are the generation and stabilization of high oxidation states at the metal centers needed to form the critical O-O bond formation with concomitant O2 release. A detailed mechanistic understanding of these key aspects can lead to the development of robust and efficient systems. In this regard, catalysts based on biomimetic systems of the OEC, coordination complexes and organometallic complexes are the most suitable ones for the mechanistic study of water oxidation. Those systems have allowed to shed some light in the mechanisms [9-11] for WO and develop efficient and fast catalysts with rates comparable or even higher to that of PSII [12-14].
In the case of the reductive processes, the artificial photosynthetic scheme can be simplified to the water splitting scheme, in which water is oxidized to O2 and then the electron and protons are recombined to produce H2, which can be directly used as fuel. But it can also be generalized, and we can think about using the electron from WO to reduce other molecules such as CO2 (as in photosynthesis) or organic molecules to produce added value chemicals, which has less scaling and economic restrictions than the synthesis of energy carriers. In this line, it is important to understand how we can generate the reductive equivalents using light as driving force and access low valent metal species and stabilize them to promote reductive transformations. In this case, the combination of photo redox catalysts with well-defined molecular complexes or biocatalytic systems has been proven a powerful approach towards light-driven reduction of protons and organic molecules [15,16]. This strategy presents the following advantages:
- It facilitates the charge separation process in space.
- Reduces the energy barrier of reactants by multistep electron/proton-transfer processes (PCET processes in solution)
- Allows for better control of the selectivity through the modification of the photosensitizer (lifetime and redox potential) and the active sites of the (bio)catalysts by rational ligand design, and
- Let’s to rationally design the reactions according to the activity of the catalyst.
References
- Lewis NS (2016) Research opportunities to advance solar energy utilization. Science 351(6271): aad1920.
- Lewis NS, Nocera DG (2006) Powering the planet: Chemical challenges in solar energy utilization. Proc Natl Acad Sci USA 103(43): 15729-15735.
- Liu Q, Wu L, Jackstell R, Beller M (2015) Using carbon dioxide as a building block in organic synthesis. Nat Commun 6: 5933.
- Centi G, Perathoner S (2009) Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catalysis Today 148(3-4): 191-205.
- Qiao J, Liu Y, Hong F, Zhang J (2014) A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem Soc Rev 43(2): 631-675.
- Jessop PG, Ikariya T, Noyori R (1995) Homogeneous hydrogenation of carbon dioxide. Chem Rev 95(2): 259-272.
- Lee KJ, Elgrishi N, Kandemir B, Dempsey JL (2017) Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat Rev Chem 1: 0039.
- Zhang B, Sun L (2019) Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem Soc Rev 48: 2216-2264.
- Codolà Z, Gamba I, Parés FA, Casadevall C, Clémancey M, et al. (2019) Design of iron coordination complexes as highly active homogenous water oxidation catalysts by deuteration of oxidation-sensitive sites. J Am Chem Soc 141(1): 323-333.
- Casadevall C, Codolà Z, Costas M, Fillol JL (2016) Spectroscopic, electrochemical and computational characterisation of Ru species involved in catalytic water oxidation: Evidence for a [RuV(O)(Py2Metacn)] intermediate. Chem Eur J 22(29): 10111-10126.
- Casadevall C, Bucci A, Costas M, Fillol JL (2019) Water oxidation catalysis with well-defined molecular iron complexes. Adv Inorg Chem 74: 151-196.
- Duan L, Wang L, Li F, Li F, Sun L (2015) Highly efficient bioinspired molecular Ru water oxidation catalysts with negatively charged backbone ligands. Acc Chem Res 48(7): 2084-2096.
- Wang L, Duan L, Ambre RB, Daniel Q, Chen H, et al. (2016) A nickel (II) PY5 complex as an electrocatalyst for water oxidation. Journal of Catalysis 335: 72-78.
- Kärkäs MD, Åkermark B (2016) Water oxidation using earth-abundant transition metal catalysts: Opportunities and challenges. Dalton Trans 45(37): 14421-14461.
- Lang X, Zhao J, Chen X (2016) Cooperative photoredox catalysis. Chemical Society Reviews 45(11): 3026-3038.
- Larsen CB, Wenger OS (2018) Photoredox catalysis with metal complexes made from earth-abundant elements. Chemistry 24(9): 2039-2058.
© 2020 Carla Casadevall*. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






