- Submissions

Full Text
Environmental Analysis & Ecology Studies
Cassava Mill Effluents Recycling through Bioenergy Production: A Review
Sylvester CI*
Department of Biological Sciences, Nigeria
*Corresponding author: Sylvester CI, Department of Biological Sciences, Nigeria
Submission: June 5, 2018;Published: May 09, 2019

ISSN 2578-0336 Volume5 Issue4
Abstract
The processing of cassava tuber into finished products such as gari generates large wastes water. The effluents are known to contain cyanide and acidic pH in addition to other chemical characteristics such as heavy metals, chemical oxygen demand among others. The effluents have toxicological impacts on the receiving ecosystem (Soil and Surface Water) as well as some of its associated fauna and flora. During degradation processes by the indigenous microbes in the soil the effluents emits odour that are offensive to human. This study reviews recycling options of cassava mill effluents through bioenergy production. The study found that cassava mill effluents have demonstrated positive potentials for biogas, biohydrogen, bioethanol, bioelectricity using varying technologies. During bioenergy production, the characteristics of the raw effluents is improved upon in some of the parameters such as chemical oxygen demand, total solid, pH etc probably due to the activities of some microorganisms. As such there is the need for research to focus on other possible utilization of the sludge generated such as bio-fertilizer production as an option for reuse.
keywordsBioenergy; Biotechnology; Cassava wastewater; Environmental contamination; Reuse; Toxicology
Introduction
Environmental degradation associated with greenhouse gases mainly include methane and Carbon Dioxide and to lesser extent Hydrofluorocarbons, Perfluorocarbons and Sulphur Hexafluoride have been implicated to cause global warming [1]. Combustion of petroleum products also releases several diversities of emission depending on its physical constituents. Combustion of biomass also releases particulates and gaseous emissions [2,3]. Typically, some greenhouse gases can also be emitted from unsustainable discharge of wastes resulting from food processing under anaerobic conditions. To these effects, Ohimain Izah [1] reported the potential contribution of palm oil mill effluents to climate change in Nigeria. Studies have also shown most biomass have bioenergy potentials.
According to Jekanyinfa [4]; Ohimain [5], energy is a vital resource required by human for the survival of the economy. Energy is used in different forms including heat, electrical, mechanical and human energy [5]. Till date natural gas and petroleum are the most widely utilized energy sources accounting for over 50% of world energy requirements [6]. Ohimain [7] also reported that no other energy sources that is as flexible as petroleum products. As such most of the global energy is meant through petroleum products. According to Atadashi et al. [8], approximately 86% of global transportation fuel is provided by non- renewable energy sources such as petroleum. Furthermore, the demand of energy has been on the increasing trend probably due to an upsurge on global population [9,10].
Probably due to the challenges of fossil fuels including its environmental impacts, several countries including Nigeria have embarked on suitable alternative for the petroleum-based fuel Biomass has emerged as possible replacement [10,11]. Several biofuels have demonstrated good prospects for optimization and possibly commercialization. To this effects Bioethanol, Biogas, Biohydrogen etc have yielded possible results though research and development. In many parts of the world, bioethanol and biogas have been commercialized but they are also challenged by choice of feedstocks. For instance, in the production of bioethanol first generational feedstock which are basically food crops have food versus fuel crises. Hence research focused on the use of non-edible grasses and lignocellulosic wastes materials for production.
Several grasses such as elephant grass [12] wild Sorghum [13] etc have demonstrated possible potentials for bioethanol. Several wastes materials have been demonstrated for Biohydrogen, Bioethanol, Biogas, Bioelectricity production under varying conditions. Nigeria is the leading cassava producer accounting for over 20% of total output on global scale [14-25]. Typically, cassava is a staple food in Africa [23,26]. Cassava is among the widely consumed food in West African region in addition to maize and rice. Zhang et al. [14] reported that cassava is the 4th source of dietary carbohydrate energy crop with energy content of 720 x1012KJ/ day. The authors reported that cassava is 5th and 3rd among starch crop on global production and source of carbohydrate for human consumption respectively.
Approximately 60%, 33% and 7% of cassava produced on global scale is consumed as food (Gari, Fufu), animal feed and industrial purposes (viz: textile, paper, food, and fermentation industries, among others) respectively [14,26-28]. But in developing country like Nigeria, about 80% of cassava tubers produced are predominantly used for production of food especially gari (about 60%) and to lesser extent fufu, Lafon, etc (about 20%), while the reset 20% are used industrially. Some of the notable areas of utilization include Livestock Feeds, Confectionaries, Monosodium Glutamate, Sweeteners, Alcohol, Adhesive, Glues, Textiles, Soup Thickener, Laundry and Pharmaceuticals [29-31].
Cassava has also been utilized for the production of bread at certain percentages [32,33]. Cassava has also gained attention for bioethanol production [34-37]. Though the utilization has not yielded the desired result. During processing of cassava tuber into gari three major wastes streams including salivates, peels (Solid), gaseous emission (Air Pollutants) and cassava mill effluents (Liquid Wastes). The solid wastes such as cassava peels are a source of food to some domestic animals such as goat. Though Ubalua [38] considered cassava processing wastes especially the waste water to contribute to environmental pollution and aesthetic nuisance [38].
The cassava mill effluents are toxic to the environment and have the tendency to alter the receiving soil characteristics [16-18,23,24]. The impacts have been widely documented in literatures. Hence there is need for sustainable management practices of this waste stream. Therefore, this study focused on the bioenergy potentials of cassava mill effluents as sustainable options for avoiding the toxicological impacts associated with the effluents.
Environmental Impacts of Cassava Mill Effluents
Cassava mill effluents contain total solids, heady metals, total suspended solid, cyanide, acidic pH, high chemical oxygen demand, total dissolved solid and conductivity. Studies have indicated that the physiochemical characteristics of the effluents sometimes exceed the limits specified by Federal Environmental Protection Agency, Nigeria for effluents to be discharged into the environment [19,20,23]. Characteristics of the effluents are known to be injurious to the environment and some associated fauna. The cassava mill effluents are known to impacts of the three major components of the environment including air [39,40], soil [16-18,23,24,41,42] and water quality [43-46].
Ero [40], Derek [47] reported some of the challenges of odour pollution to include breathing and sleeping difficulty, coughing, stomach and loss of appetite, eye, nose and throat irritation, disturbance from external environment, annoyance etc. Furthermore, cassava mill effluents could cause an alteration in the quality of receiving soil and surface water about physicochemical and microbial characteristics. According to Uhegbu et al. [48], cassava mill effluents lead to alteration on some food crops (viz: Dioscorea dumetorum (Domestic Yam), Dioscorea dumetorum (Wild Yeam), Dioscorea rotundata (White Yam), Dioscorea alata (Water Yam), Xanthosoma sagittifolium (Red Cocoyam), Colocasiaesculenta (White Cocoyam), Ipomeabatatas (Red sweet potato), Ipomeabatatas (White sweet potato) with regard to the cyanide content.
Hence the consumption of food resources cultivated it cassava mill effluents contaminated soil may have some health implication because of the lethal nature of cyanide in soil. On aquatic ecosystem living organisms, cassava mill effluents are known to affects histopathological, haematological, enzymes, behavioural response, mortality etc in exposed fish [13,49,50]. The toxicity potentials of cassava mill effluents may be associated with the cyanide content [23], which have been described as respiratory poison that could kill fisheries [49]. In soil, raw cassava mill effluents have been reported to kill some vegetation cover (viz: Sida acuta, Mimosa pudica, Euphorbia hirata, Tridax procumbens, Chromelaena odorata) to some extent [51].
Authors have also reported that cassava mill effluents could plant germination and growth [52-54]. Probably due to nutrient potentials of cassava mill effluents on surface water could lead to eutrophication [23], thereby enhancing the growth of aquatic plants and marsh transformation [40]. The eutrophication could reduce oxygen content in water thereby causing behavioural response and enhancing mortality rate of aquatic organisms. Soil receiving cassava mill effluents have been reported to have lower microbial load [15,23,55].
This could be due to the cyanide content of the cassava which is lethal to some microbes. Environmental risk have been carried out in soil receiving cassava mill effluents in a rural community and result showed low to moderate contamination level in wet and dry seasons apart from lead that had considerable pollution in one of the locations for wet season for contamination factor and degree of contamination for iron, chromium, zinc, copper, cobalt, nickel, manganese, lead and cadmium [18]. The authors further reported that the pollution load index for these metals were within no pollution to moderate pollution, Nemerov integrated pollution index was within warning line of pollution to low level of pollution during the dry season and warning line of pollution to high pollution in wet season, while the metal pollution index and average pollution index showed slight pollution.
Enerijiofi et al. [55] reported that Potassium, Sodium, Copper and Calcium showed high contamination; Magnesium, Iron and lead showed considerable contamination, Zinc, Manganese, Nickel, Cadmium, Vanadium and Chromium showed moderate contamination for contamination factor; and Potassium, Sodium, Copper and Calcium showed high contamination, Zinc, Manganese, Nickel, Cadmium, Vanadium and Chromium showed moderation pollution and Magnesium, Iron and lead were low based on pollution status based on index of deaccumulation in cassava mill effluents contaminated soil.
Izah et al. [17] also reported iron, chromium, zinc, copper, cobalt, nickel, manganese, lead and cadmium in soil receiving cassava mill effluents to show un-contamination to moderately contamination for Index of geo accumulation, background rank to significant enrichment for enrichment factor and no enrichment to moderate enrichment for metal enrichment index, and pollution based on quantification of contamination. Izah et al. [24] showed low ecological risk in the soil for both wet and dry season.
Bioenergy Potentials of Cassava Mill Effluents
Several technologies have been demonstrated toward effective management of cassava mill effluents through biotechnological advancement. To this effect, cassava mill effluents have been shown to be used for bio surfactant production [56,57]. The effluents are me used as source of medium for the cultivation of Saccharomyces cerevisiae biomass [21,22,25,58,59], volatile fatty acids [60], enzymes, organic acids, bioenergy etc. Energy is typically one of the fundamental requirements for human existence. Biofuel from biomass is gaining prominence as many countries in including Nigeria seeking to substitute conventional fuel with biofuels such as biodiesel, bioethanol and biogas.
In recent time energy is produced from biomass including animal and plants resources. Food and agricultural processing activities lead to generation of several wastes stream, which have been reported to have bioenergy potentials [35]. For instance, cassava mill effluents which is one of the largest food processing wastes in Nigeria has bioenergy potentials. As such the organization of this section of the paper is mainly on different bioenergy products that could be generated from cassava mill effluents.
Bioethanol
Ethanol is a typically example of an oxygenated fuel that has tendency to replace fossil fuels [61,62]. This is probably due to its high-octane number [63] and low emission with regard to pollutant gases. Furthermore, due to its environmental benefits its used as blend for gasoline. Many nations have approved certain ethanol blend in conventional fuel including Nigeria (E10) [37]. Bioethanol is produced via different technology depending on the nature of the feedstock. According to Brooks [64], Dhabekar [65] bioethanol production from agricultural feedstock depends largely on the use of ideal microbial strain, suitable fermentation substrate and process technology.
Table 1:Bioethanol production from cassava mill effluents.
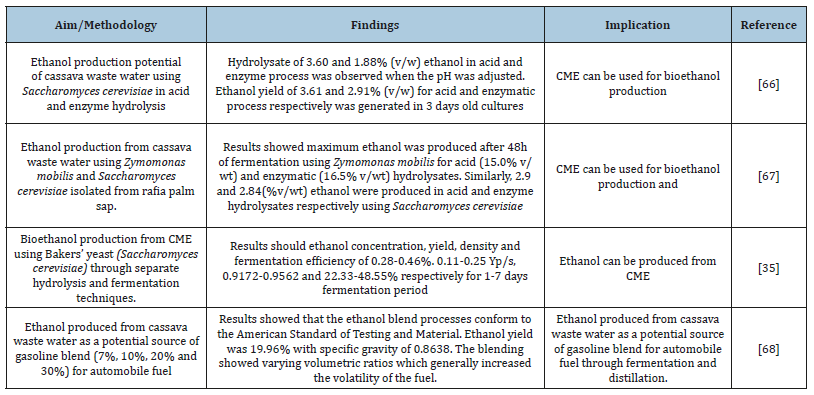
Recently, use of waste for ethanol production have been encouraged as a sustainable means of preventing the attendant environmental impacts associated with them. Bioethanol production offers a sustainable approach to manage cassava mill effluents [35,66-68]. Bioethanol production from cassava mill effluents could be from direct microbial conversion using acid resistant strain of yeast. Table 1 presents finding from ethanol production from cassava mill effluents. The conversion of cassava and its processing wastes have been established in literatures. Specifically, several technologies for conversion of carbohydrate crops such as cassava to ethanol have evolved.
But to this regard several attempt to produce ethanol with high yield following hydrolysis and fermentation of carbohydrate from cassava waste water have been made [14]. The production of ethanol from cassava effluent has been widely carried out using Saccharomyces cerevisiae [11]. In addition, studies have suggested that several internal conditions (constituents of the effluents, choice of microbes, sugar contents of the waste, configuration among others) and environmental factors (time, temperature, pH etc) affects yield of ethanol from wastes feedstock.
Biobutanol
Biobutanol have been reported to be a promising fuel for vehicles. This could be due to their high-octane number and energy content, low volatility, and improved air-to-fuel ratio similar to gasoline [14]. Like ethanol, butanol production through fermentation has a long historical origin. According to Zhang et al. [14], cassava tuber and its processing waste water is a promising lignocellulosic feedstock for acetone-butanol-ethanol production. The authors further reported that cassava bagasse hydrolysate is also a good substrate for n-butanol during acetone-butanol-ethanol fermentation processes.
Ouephanit et al. [69] reported that biobutanol and ethanol can be produced from tapioca starch wash wastewater using Clostridium spp through fermentation with. The authors also reported that the butanol (and combined butanol plus ethanol) production level was improved at pH 5.5 for both strain of bacteria (Clostridium butyricum TISTR 1032 and Clostridium acetobutylicum ATCC 824).
Biogas
Biogas is produced from several technologies that principally involve either aerobic or anaerobic digestion. Biogas production using anaerobic digester has gain prominence and has been widely studied. For instance, different configuration of digester used for biogas using palm oil mill effluents has been recently reviewed by Ohimain [70]. Biogas approximately consists of about 65% methane and 35% carbon dioxide under anaerobic system [9]. Biogas is a colourless, odourless, inflammable and combustible fuel [70-72] Biogas is produced by four major steps like hydrolysis, acidogenesis, acetogenesis and methanogenesis [1,9,70,73-75].
Table 2:Biogas production from cassava mill effluents.

Cassava mill effluents are also a good substrate for biogas production [76-79], with or without the use of bio stabilator. Table 2 present the findings of authors for biogas production using cassava mill effluents substrate. In addition, Paixao et al. [80] reported methane yield of 14.4l/d at temperature of 25 °C in hybrid reactor under certain conditions. Auphimai et al. [81] reported methane yield of 271.0-290.0ml CH4/g COD using batch reactor at temperature of 37 °C. During biogas production from cassava mill effluents, the resultant sludge is rich in potash, nitrogen, phosphorus and potassium. As such they can be used as biofertilizer [76].
According to Zhang et al. [14] co-digestion with manure can aid in maintaining an optimum pH for the methanogenic microbes, reduction in free ammonia/ammonium inhibition, enhancing optimal Carbon to Nitrogen ratio for effective digestion. To this regard, Ren et al. [82] reported that co-digestion of pig manure and cassava dregs enhance the population and diversity of methanogens during biogas production. Working conditions are affected by pH and temperature (environmental factors), organic loading rate, hydraulic retention time, microbial activity, pressure, nutrient level and composition, chemical equilibrium, mixing of effluent etc (internal factors) [1,9,70,73].
Studies have shown that biogas produced from wastewater have combustion characteristics. In addition, biogas is environmental, economic and socially sustainable. Biogas produced from cassava mill effluents could be used for both heat and power generation. The utilization of cassava mill effluents for biogas production could prevent the attendant environmental impacts associated with the wastewater.
Biohydrogen
Hydrogen has been regarding as fuel for the future. Biohydrogen can be diversely applied in different sectors including power generation and transportation fuel. Hydrogen from can be produced from biomass through several technologies including fermentation (with the aid of photosynthetic and chemosynthetic bacteria), electrolysis of water, steam reformation of methane, thermocatalytic reformation [83-85]. Through fermentation biohydrogen can be produced through light depended (photo) and light independent (dark) processes [83].
Microbes such as phototrophic indigenous purple nonsulphur bacteria (Rhodopseudomonas palustris PBUM001) [86], Thermoanaerobacterium species [87,88], Clostridum butyricum EB6 [89,90], Clostridium acetobutylicum [14] have been employed for biohydrogen production at varying fermentation processes. According to Lam & Lee [74], light independent fermentation processes are favorable due to the utilization of Bacillus, Enterobacter, Closridium species during acidogenesis. Typically, biohydrogen typically involves acidogenesis (conversion of monomers to intermediaries such as volatile fatty acids) and methanogenesis (conversion of volatile fatty acids to methane).
Carbohydrate-rich substrate have been widely reported as potential feedstock for biohydrogen production through fermentation [14,83]. The potentials of cassava feedstock for biohydrogen production have been reported in literature. In addition, waste rich in carbohydrate is ideal substrate for the fermentative hydrogen production. Thong et al. [91] reported hydrogen yield of 124.9-287mL H2/g starch at thermophilic temperature of 60 °C using continuous stirred tank reactor. Sreethawong et al. [92] reported hydrogen yield of 438mL H2/g COD removed at 37 °C under anaerobic sequencing batch reactor. Amorim et al. [93] reported hydrogen yield of 1.91mol H2/mol glucose at approximately 28 °C using anaerobic fluidized bed reactor.
Like biogas, several environmental parameters such as temperature, pH, and other operational conditions affects the yield [94-96], carbon to nitrogen and carbon to phosphorus ratios [14], in addition pre-treatment such as acidic and alkaline conditions enhance the yield of hydrogen [14,97]. Specifically, acidic condition enhanced the release of soluble carbohydrate while alkali condition stimulated soluble total organic carbon [14,97].
Bioelectricity using microbial fuel cells
Microbial fuel cell is a bio-electrochemical device that utilizes microorganisms to general electrical current from waste water i.e., effluents. Microbial fuel cell has the ability to transform chemical energy found in organic compounds into electricity via the catalysis of microorganisms [98,99]. As such it’s a promising alternative energy source and wastewater treatment [100-105]. Due to the architectural design of microbial fuel cells it has received attention in other field including engineering (mechanical, chemical, environmental, biotechnologist in addition to microbiologist [98]. Over the years, significant improvement has been made on the optimization of microbial fuel cell technology for the treatment of effluent while generating electrical current.
Several substrates can produce electricity using Microbial fuel cell technology, while reducing the pollution potential of such wastewater. Cassava mill effluents is a potential substrate for electricity generation using microbial fuel cells [106-108]. Table 3 present electricity generation from cassava waste. During electricity generation from the wastes chemical oxygen demand is reduced.
Table 3:Bioelectricity generation from cassava mill effluents using microbial fuel cells technology.

Conclusion and the Way Forward
Cassava mill effluents is a liquid waste generated during cassava processing into gari. In Nigeria, over 80% percent of cassava processing are handles by small-scale processors that typically use hydraulic presser to remove the water from the bagged mashed cassava. The effluents are discharged in the environment with little or no treatment. The toxicological impacts of cassava mill effluents have been reported to affects some domestic animals (sheep and goats), fisheries, plant growth and productivity. Furthermore, environmental risk assessment has shown that cassava mill effluents have some level of contamination in the receiving soil [108-110].
Hence there is the need for sustainable management practices, this study found that bioenergy is a potential means through which the attendant environmental impacts of the waste can be curtailed. As such there is the need for research to focus on means for collecting all the cassava mill effluents from the various processing plants for bioenergy production. In addition, the sludge generated during bioenergy production biohydrogen, bioelectricity, bioethanol, biogas should also be studied for other downstream applications such as bio-fertilizer.
References
- Ohimain EI, Izah SC (2014) Possible contributions of palm oil mill effluents to greenhouse gas emissions in Nigeria. British Journal of Applied Science and Technology 4(33): 4705-4720.
- Ohimain EI, Izah SC, Abah SO (2013) Air quality impacts of smallholder oil palm processing in Nigeria. Journal of Environmental Protection 4: 83-98
- Ohimain E I, Izah SC (2013) Gaseous emissions from a semi-mechanized oil palm processing mill in Bayelsa state, Nigeria. Continental Journal of Water, Air and Soil Pollution 4 (1): 15-25.
- Jekayinfa SO, Bamghoye AI (2007) Development of equation for estimating energy requirements in palm-kernel oil processing operations. Journal of Food Engineering 79: 322-329.
- Ohimain EI, Izah SC (2014) Contribution of manual energy to palm oil processing by smallholders in Nigeria. Sky Journal of Agricultural Research 3(7):137-141.
- Ohimain EI (2010) Petroleum geomicrobiology. In: Jain SK, Khan AA, Rain MK (Eds.), Geomicrobiology: Biodiversity and biotechnology, CRC Press/Taylor and Francis, Boca Raton, Florida, USA, pp. 349-374.
- Ohimain EI (2013) The challenges of liquid transportation fuels in Nigeria and the emergence of the Nigerian automotive biofuel programme. Research Journal of Applied Sciences, Engineering and Technology 5(16): 4058-4065.
- Atadashi IM, Aroua MK, Aziz AA (2011) Biodiesel separation and purification: A Review. Renewable Energy 36(2): 437-443.
- Ohimain EI, Izah SC (2014) Potential of biogas production from palm oil mills’ effluent in Nigeria. Sky Journal of Soil Science and Environmental Management 3(5): 50-58.
- Izah SC, Ohimain EI (2013) The challenge of biodiesel production from oil palm feedstock’s in Nigeria. Greener Journal of Biological Science 3(1): 1-12.
- Izah SC, Ohimain EI (2015) Bioethanol production from cassava mill effluents supplemented with solid agricultural residues using bakers’ yeast [Saccharomyces cerevisiae]. Journal of Environmental Treatment Techniques 3(1): 47-54.
- Ohimain EI, Kenabie P, Nwachukwu RES (2013) Bioenergy potentials of elephant grass, pennisetum purpureum schumach. Annual Research and Review in Biology 4(13): 2215-2227.
- Ohimain EI, Izah SC (2016) Productivity and Bioethanol potentials of wild sorghum (Sorghum arundinaceum). British Journal of Renewable Energy 1(2): 14-17.
- Zhang, M, Xie L, Yin Z, Khanal SK, Zhou Q (2016) Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresource Technology 215: 50-62.
- Izah SC, Aigberua AO (2017) Assessment of Microbial Quality of Cassava Mill Effluents Contaminated Soil in a Rural Community in the Niger Delta, Nigeria. EC Microbiology 13(4): 132-140.
- Izah SC, Bassey SE, Ohimain EI (2017) Assessment of heavy metal in cassava mill effluent contaminated soil in a rural community in the Niger Delta region of Nigeria. EC Pharmacology and Toxicology 4(5): 186-201.
- Izah SC, Bassey SE, Ohimain EI (2017) Geo-accumulation index, enrichment factor and quantification of contamination of heavy metals in soil receiving cassava mill effluents in a rural community in the Niger Delta region of Nigeria. Molecular Soil Biology 8(2): 7-20.
- Izah SC, Bassey SE, Ohimain EI (2017) Assessment of pollution load indices of heavy metals in cassava mill effluents contaminated soil: a case study of small-scale cassava processing mills in a rural community of the Niger Delta region of Nigeria. Bioscience Methods 8(1): 1-17.
- Izah SC, Bassey SE, Ohimain EI (2017) Changes in the treatment of some physico-chemical properties of cassava mill effluents using Saccharomyces cerevisiae. Toxic 5(4): E28.
- Izah SC, Bassey SE, Ohimain EI (2017) Removal of heavy metals in cassava mill effluents with Saccharomyces cerevisiae isolated from palm wine. MOJ Toxicology 3(4): 00057.
- Izah SC, Bassey SE, Ohimain EI (2017) Cyanide and macro-nutrients content of Saccharomyces cerevisiae biomass cultured in cassava mill effluents. International Journal of Microbiology and Biotechnology 2(4): 176-180.
- Izah SC, Bassey SE, Ohimain EI (2017) Amino acid and proximate composition of Saccharomyces cerevisiae biomass cultivated in Cassava mill effluents. Molecular Microbiology Research 7(3): 20-29.
- Izah SC, Bassey SE, Ohimain EI (2018) Impacts of cassava mill effluents in Nigeria. Journal of Plant and Animal Ecology 1(1): 14-42.
- Izah SC, Bassey SE, Ohimain EI (2018) Ecological risk assessment of heavy metals in cassava mill effluents contaminated soil in a rural community in the Niger Delta Region of Nigeria, Molecular Soil Biology 9(1): 1-11.
- Izah SC (2018) Estimation of Saccharomyces cerevisiae biomass cultured in cassava mill effluents. Environmental Analysis and Ecology studies 2(5): 1-3.
- Kemausuor F, Addo A, Darkwah L (2015) Technical and socioeconomic potential of biogas from cassava waste in Ghana. Biotechnology Research International 2015: 10.
- Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, et al. (2000) Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresource Technology 74(1): 81-87.
- Sanni LO, Onadipe OO, Ilona P, Mussagy MD, Abass A, et al. (2009) Successes and challenges of cassava enterprises in West Africa: A case study of Nigeria, Benin, and Sierra Leone. International Institute of Tropical Agriculture (IITA).
- Kigigha LT, Izah SC, Kpea TB (2015) Microbiological quality of fermented cassava flakes (Gari) sold in Yenagoa, Metropolis, Nigeria. Bull Adv Scientif Res 1(7): 157-160.
- Kigigha LT, Nyenke P, Izah SC (2018) Health risk assessment of selected heavy metals in Gari ( cassava flake) sold in some major markets in Yenagoa metropolis, Nigeria. MOJ Toxicol 4(2): 47-52.
- Adebayo AO, Oyewole OB, Obadina AO, Omemu MA (2013) Microbiological safety assessment of fermented cassava flour ‘‘Lafun’’ available in Ogun and Oyo states of Nigeria. Intern J Food Sci 2013: 5.
- Ohimain EI (2014) The prospects and challenges of cassava inclusion in wheat bread policy in Nigeria. International Journal of Science, Technology and Society 2(1): 6-17.
- Ohimain EI (2015) Recent advances in the production of partially substituted wheat and wheat less bread. Euro Food Res Technol 240(2): 257-271.
- Ukwuru MU, Egbonu SE (2013) Recent development in cassava-based products research. Academia J Food Res 1(1): 001-013.
- Izah SC (2016) Bioethanol production from cassava mill effluents supplemented with oil palm chaff, empty fruit bunch and cassava peels using Saccharomyces cerevisiae. M.Sc. thesis submitted to School of Post Graduate Studies, Niger Delta University, Wilberforce Island, Nigeria, p. 113.
- Ohimain EI (2010) Emerging bio-ethanol projects in Nigeria: Their opportunities and challenges. Energy Policy 38: 7161-7168.
- Ohimain EI (2012) The benefits and potential impacts of household cooking fuel substitution with bio-ethanol produced from cassava feedstock in Nigeria. Energy for Sustainable Development 16(3): 352- 362.
- Ubalua AO (2007) Cassava wastes: Treatment options and value addition alternatives. Afr J Biotechnol 6(18): 2065-2073.
- Ehiagbonare JE, Enabulele SA, Babatunde BB, Adjarhore R (2009) Effect of cassava effluents on Okada denizens. Scientific Research Essay 4(4): 310-313.
- Ero NR, Okponmwense M, Undated, The effect of cassava waste on the environment and its implication on the national economy. pp. 1-4.
- Eneje R, Ifenkwe I (2012) Effect of agricultural and industrial wastes on the physicochemical properties of a sandy clay loam soil. Intern J Appl Agric Res 7(3): 187-196.
- Eze VC, Onyilide DM (2015) Microbiological and physiochemical characteristics of soil receiving cassava effluents in Elele, Rivers state, Nigeria. J Appl Environ Microbiol 3(1): 20-24.
- Oghenejoboh KM (2015) Effects of cassava wastewater on the quality of receiving water body intended for fish farming. Bri J Appl Sci Technol 6(2): 164-171.
- Omotioma M, Mbah GO, Akpan IJ, Ibezim OB (2013) Impact assessment of cassava effluents on barika stream in Ibadan, Nigeria. Intern J Environ Sci Manage Eng Res 2(2): 50-56.
- Okunade DA, Adekalu KO (2013) Physico-chemical analysis of contaminated water resources due to cassava wastewater effluent disposal. Euro Intern J Sci Technol 2(6): 75-84.
- Afuye GG, Mogaji KO (2015) Effect of cassava effluents on domestic consumption of ‘shallow well’ water in Owo Local Government Area, Ondo State, Nigeria. Physical Sci Res Intern 3(3): 37-43.
- Derek MB (1992) Atmospheric pollution - A global problem (2nd edn.), Blackwells Publishers, Oxford, UK.
- Uhegbu FO, Akubugwo EI, Iweala EEJ (2012) Effect of Garri processing effluents [waste water] on the cyanide level of some root tubers commonly consumed in the South East of Nigeria. Afr J Food Agric Nutri Develop 12(5): 6748-6758.
- Adeyemo OK (2005) Haematological and histopathological effects of cassava mill effluent in Clarias gariepinus. African Journal of Biomedical Research 8(3): 179-183.
- Asogwa CN, Ezenwajiaku FO, Okolo CA, Ekeh FN, Nwibo DD, et al. (2015) Behavioural and biochemical responses of juvenile catfish (Clarias gariepinus) exposed to graded concentrations of cassava waste water. Animal Research International 12(1): 2136-2142.
- Otunne RN, Kinako PDS (Undated) Effects of cassava effluent on Egbema denizens: a case of Mmahu community in Egbema. Intern J Res Develop, pp. 1-5.
- Olorunfemi DI, Lolodi O (2011) Effect of cassava processing effluents on antioxidant enzyme activities in Allium cepa L. Biokemistri 23(2): 49-61.
- Nwakaudu MS, Kamen FL, Afube G, Nwakaudu AA, Ike IS (2012) Impact of cassava processing effluent on agricultural soil: A Case Study of Maize Growth. J Emerging Trends in Eng Appl Sci 3(5): 881-885.
- Orhue ER, Imasuen EE, Okunima DE (2014) Effect of cassava mill effluent on some soil chemical properties and the growth of fluted pumpkin (Telfairia occidentalis Hook F.). Journal of Applied and Natural Science 6(2): 320-325.
- Enerijiofi KE, Bassey ES, Fagbohun GJ (2017) Assessment of the impact of cassava mill effluent (CME) on the Microbial diversity, physicochemical parameters and heavy Metal concentrations in the receiving soil. Ife Journal of Science 19(2): 399-407.
- Izah SC, Aseibai ER (2018) Potentials of biosurfactants production from cassava mill effluents. In: Microbiological Applications for National Economic Development. 5th Annual Nigerian society for microbiology south east zonal symposium held in Federal polytechnic Nekede, Nigeria.
- Nitschke M, Pastore GM (2006) Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour Technol 97(2): 336-341.
- Izah SC, Bassey SE, Ohimain EI (2017) Assessment of some selected heavy metals in Saccharomyces cerevisiae biomass produced from cassava mill effluents. EC Microbiology 12(5): 213-223.
- Izah SC (2017) Potentials of yeast biomass production from food processing wastes effluents. EC Nutrition 8(3): 72-74.
- Mahmud Hasan SD, Giongo C, Fiorese ML, Gomes SD, Ferrari TC, et al. (2015) Volatile fatty acids production from anaerobic treatment of cassava waste water: Effect of temperature and alkalinity. Environ Technol 36: 2637-2646.
- Chandel AK, Chan ES, Rudravaram R, Narasu ML, Rao LV, et al. (2007) Economics and environmental impact of bioethanol production technologies: An appraisal. Biotechnol Molecular Biol Rev 2(1): 14-32.
- Iqbal HMN, Kamal S (2012) Economical bioconversion of lignocellulosic materials to value-added products. J Biotechnol Biomaterial 2(5): e112.
- Joshi B, Bhatt MR, Sharma D, Joshi J, Malla R, et al. (2011) Lignocellulosic ethanol production: Current practices and recent developments. Biotechnol Molecular Biol Rev 6(8): 172-182.
- Brooks AA (2008) Ethanol production potential of local yeast strains isolated from ripe banana peels. Afr J Biotechnol 7(20): 3749-3752.
- Dhabekar A, Chandak A (2010) Utilization of banana peels and beet waste for alcohol production. Asiatic J Biotechnol Resources 1: 8-13.
- Akponah E, Akpomie OO (2012) Optimization of bio-ethanol production from cassava effluent using Saccharomyces cerevisiae. African Journal of Biotechnology 11(32): 8110-8116.
- Akponah E, Akpomie OO, Ubogu M (2013) Bio-ethanol production from cassava effluent using Zymomonas mobilis and Saccharomyces cerevisiae isolated from rafia palm (Elaesis guineesis) SAP. European Journal of Experimental Biology 3(4): 247-253.
- Aisien F, Aguye MD (2010) Blending of ethanol produced from cassava waste water with gasoline as source of automobile fuel. Electronic Journal of Environment, Agriculture and Food Chemistry 9(5): 946-950.
- Ouephanit C, Virunanon C, Burapatana V, Chulalaksananukul W (2011) Butanol and ethanol production from tapioca starch wastewater by clostridium spp. Water Science & Technology 64(9): 1774-1780.
- Ohimain EI, Izah SC (2017) A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renewable & Sustainable Energy Reviews 70: 242-253.
- Igoni AH, Abowei MFN, Ayotamuno MJ, Eze CI (2007) Effect of total solids concentration of municipal solid waste in anaerobic batch digestion on the biogas produced. J Food Agric Environ 5(2): 333-337.
- Igoni AH, Abowei MFN, Ayotamuno MJ, Eze CI (2008) Comparative evaluation of batch and continuous anaerobic digesters in biogas production from municipal solid waste using mathematical models. Agric Eng Inter: the CIGR E journal 10: 1-12.
- Nayono SE (2010) Anaerobic digestion of organic wastes for energy. Hyderabad, Telangana, India.
- Lam MK, Lee KT (2011) Renewable and sustainable bio-energies production from palm oil mill effluent (POME): Win-win strategies toward better environmental protection. Biotechnology Advances 29(1): 124-141.
- Trisakti B, Manalu V, Taslim I, Turmuzi M (2015) Acidogenesis of palm oil mill effluent to produce biogas: Effect of hydraulic retention time and ph. Procedia-Social Behavioral Sci 195: 2466-2474.
- Eze JI (2010) Converting cassava (Manihot spp) waste from Gari processing industry to energy and bio-fertilizer. Global Journal Researches Eng 10(4): 113-117.
- Budiyono, Kusworo TD (2011) Biogas production from cassava starch effluent using microalgae as bio-stabilisation. Internat. J. of Sci. and Eng 2(1): 4-8.
- Kullavanijaya P, Thongduang P (2012) Enhanced biogas production in anaerobic digestion of cassava wastewater though supplementation of biodiesel waste as co-substrate. International Journal of Renewable Energy Research 2(3): 510-515.
- Jijai S, Srisuwana G, Thong OS, Ismail N, Siripatana C (2014) Specific Methanogenic Activities (SMA) and biogas production of different granules size and substrates. The 1st Environment and Natural Resources International Conference 6th-7th November 2014, The Sukosol hotel, Bangkok, Thailand.
- Paixao MA, Tavares CRG, Bergamasco R, Bonifacio ALE, Costa RT (2000) Anaerobic digestion from residue of industrial cassava industrialization with acidogenic and methanogenic physical separation phases. Appl Biochem Biotechnol 84(6): 809-819.
- Auphimai C, Rukruam W, Chaiprasert P (2014) Efficacies of various inoculum sources on methane production from agro-industrial wastewaters. In: Thong OS, Waewsak J (Eds.), 2013 International Conference on Alternative Energy in Developing Countries and Emerging Economies (2013 Aedcee). Elsevier Science Bv, Amsterdam, Netherland, pp. 167-172.
- Ren J, Yuan X, Li J, Ma X, Zhao Y, et al. (2014) Performance and microbial community dynamics in a two-phase anaerobic co-digestion system using cassava dregs and pig manure. Bioresour Technol 155: 342-351.
- Ohimain EI, Izah SC (2015) Estimation of potential biohydrogen from palm oil mills’ effluent in Nigeria using different microorganisms under light independent fermentation. Journal of Environmental Treatment Techniques 3(2): 97-104.
- Atif AY, Fakhrul RA, Ngan MA, Morimoto M, Iyuke SE, et al. (2005) Fed batch production of hydrogen from palm oil mill effluent using anaerobic microflora. International Journal of Hydrogen Energy 30(13-14): 1393- 1397.
- Foo KY, Hameed BH (2010) Insight into the applications of palm oil mill effluent: A renewable utilization of the industrial agricultural waste. Renewable and Sustainable Energy Reviews 14(5): 1445-1452.
- Jamil Z, Annuar MSM, Ibrahim S, Vikineswary S (2009) Optimization of phototrophic hydrogen production by Rhodopseudomonas palustris PBUM001 via statistical experimental design. International Journal of Hydrogen Energy 34(17): 7502-7512.
- Thong OS, Prasertsan P, Intrasungkha N, Dhamwichukorn S, Birkeland NK (2008) Optimization of simultaneous thermophilic fermentative hydrogen production and COD reduction from palm oil mill effluent by Thermoanaerobacterium-rich sludge. International Journal of Hydrogen Energy 33(4): 1221-1231.
- Thong OS, Prasertsan P, Intrasungkha N, Dhamwichukorn S, Birkeland NK (2007) Improvement of biohydrogen production and treatment efficiency on palm oil mill effluent with nutrient supplementation at thermophilic condition using an anaerobic sequencing batch reactor. Enzyme and Microbial Technology 41(5): 583-590.
- Chong ML, Abdul RN, Rahim RA, Aziz SA, Shirai Y, et al. (2009) Optimization of biohydrogen production by clostridium butyricum EB6 from palm oil mill effluent using response surface methodology. International Journal of Hydrogen Energy 34(17): 7475-7482.
- Chong ML, Rahim RA, Shirai Y, Hassan MA (2009) Biohydrogen production by clostridium butyricum EB6 from palm oil mill effluent. International Journal of Hydrogen Energy 34(2): 764-771.
- Thong OS, Hniman A, Prasertsan P, Imai T (2011) Biohydrogen production from cassava starch processing wastewater by thermophilic mixed cultures. Int J Hydrog Energy 36(5): 3409-3416.
- Sreethawong T, Chatsiriwatana S, Rangsunvigit P, Chavadej S (2010) Hydrogen production from cassava wastewater using an anaerobic sequencing batch reactor: Effects of operational parameters, COD: N ratio, and organic acid composition. Int J Hydrog Energy 35(9): 4092- 4102.
- Amorim NCS, Alves I, Martins JS, Amorim ELC (2014) Biohydrogen production from cassava wastewater in an anaerobic fluidized bed reactor. Braz J Chem Eng 31: 603-612.
- Luo G, Xie L, Zou Z, Wang W, Zhou Q (2010) Evaluation of pre-treatment methods on mixed inoculum for both batch and continuous thermophilic biohydrogen production from cassava stillage. Bioresour Technol 101(3): 959-964.
- Luo G, Xie L, Zou Z, Wang W, Zhou Q (2010) Exploring optimal conditions for thermophilic fermentative hydrogen production from cassava stillage. Int J Hydrog Energy 35(12): 6161-6169.
- Luo G, Xie L, Zou Z, Zhou Q, Wang JY (2010) Fermentative hydrogen production from cassava stillage by mixed anaerobic microflora: Effects of temperature and pH. Appl Energy 87(12): 3710-3717.
- Wang W, Luo G, Xie L, Zhou Q (2013) Enhanced thermophilic fermentative hydrogen production from cassava stillage by chemical pre-treatments. Water Sci Technol 68(1): 59-67.
- Mathuriya AS, Yakhmi JV (2016) Microbial fuel cells-applications for generation of electrical power and beyond. Crit Rev Microbio 42(1): 127-143.
- Chaturvedi V, Verma P (2016) Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresources Bioprocessing 3: 38.
- Mathuriya AS (2014) Eco-Affectionate face of microbial fuel cells. Crit Rev Environ Sci Technol 44(2): 97-153.
- Mathuriya AS (2016) Novel microbial fuel cell design to operate with different wastewaters simultaneously. Journal of Environmental Sciences 42: 105-111.
- Rozendal RA, Hamelers HVM, Rabaey K, Keller J, Buisman CJN (2008) Towards practical implementation of bio-electrochemical wastewater treatment. Trends Biotechnol 26(8): 450-459.
- Logan BE (2009) Exo-electrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7(5): 375-381.
- Li WW, Sheng GP, Yu HQ (2013) Electricity generation from food industry wastewater using microbial fuel cell technology. In: Food Industry Wastes. Elsevier, Amsterdam, Netherlands, pp. 249-260.
- Edem DE, Opara CC, Evbuomwan BO, Oforkansi BC (2015) Effects of novel substrates in electricity generation in a mediator-less microbial fuel cell. Greener J Sci Eng Technol Res 5(1): 11-19.
- Chiwes W, Kaewkannetra P (Undated) Production of bioelectricity via microbial fuel cell using sediment batteries technique.
- Quan X, Tao K, Mei Y, Jiang X (2014) Power generation from cassava alcohol wastewater: Effects of pre-treatment and anode aeration. Bioprocess Biosystems Eng 37(11): 2325-2332.
- Leano EP, Babel S (2011) Electricity generation from anaerobic sludge and cassava wastewater subjected to pre-treatment methods using microbial fuel cell. Clean Energy and Technology (CET), 2011 IEEE First Conference on Sirindhorn International Institute of Technology (SIIT), Khlong Nueng, Thailand.
- Kaewkannetra P, Chiwes W, Chiu TY (2011) Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel 90(8): 2746-2750.
- Prasertsung N, Reungsang A, Ratanatamskul C (2012) Alkalinity of cassava wastewater feed in anodic enhance electricity generation by a single chamber microbial fuel cell. Engineering Journal 16(5): 17-28.
© 2019 Sylvester CI. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






