- Submissions

Full Text
Environmental Analysis & Ecology Studies
Effect of Three Cultivars Succulent Plants Leaves Coloring Under Continuous Stress
Hui Xu, Zhang Yan, Tianlai Li and Rui Wang*
Ministry of Education, Key Laboratory of Ministry of Education, China
*Corresponding author: Rui Wang, Ministry of Education, Key Laboratory of Ministry of Education, China
Submission: March 08, 2019;Published: April 01, 2019

ISSN 2578-0336 Volume5 Issue3
Abstract
The succulent color of the succulents is often loved, and the economic value is high. However, due to seasonal factors, cultivation techniques and other factors, the colorful succulents in the market are rare, cause the viewing effect is not good, and the economic value is low. Therefore, this experiment studied the effects of three stresses of acid, alkali, water and salt on the leaf coloration of three succulents, and it has important theoretical guidance and practical significance for the cultivation and management of succulents. The results showed that under the stress, the a* value, anthocyanin content, antioxidant enzymes (SOD, POD, CAT) activities of the three succulents increased with stress and the prolongation of stress time, while the chlorophyll content is reduced. When three succulents were subjected to stress, antioxidant enzymes acted as free radicals, and their activity increased sharply on the 5th day of treatment. Anthocyanins were synthesized in large quantities on the 10th day as antioxidants to scavenge free radicals in the body. On the 15th day, it is expressed in the coloration of the leaves, the leaf hue a* rises rapidly, and the leaves red deepens. In general, in a certain range, strong acid and alkali can promote the leaf coloration of sedum succulents.
Keywords:Stress coloring; Physicochemical properties
Introduction
Adversity can increase the anthocyanin content of plants and inhibit the synthesis of chlorophyll. The content of anthocyanins makes the plants brighter. The anthocyanins of plant leaves can not only reflect the color of plants, but also have protective functions on plants. Therefore, under stress, the content of anthocyanins in plants tends to increase [1-3]. Studies have found that anthocyanin content increases under a range of water stress [4]. Therefore, in succulents, anthocyanins are the main cause of redness of leaves, and the amount of anthocyanin can reflect the depth of the red leaves of succulents. The ratio of anthocyanin to chlorophyll content increased, the a* value of leaf hue increased, and the plant leaves became red. An increase in anthocyanin content and a decrease in chlorophyll content increase the color of the plant.
The color of the colorful leaf plants will change with the change of pH value. The pH value can affect the enzymatic activity related to anthocyanins, and then change the synthesis mode of anthocyanins. Suitable acidification can make plants leaf color is more vivid. However, some studies suggest that the leaf color is greatly affected by the pH value, and the leaf color changes from red to green as the pH increases, because the pH value not only affects the synthesis of anthocyanins, but also affects its stability. As the pH increased, anthocyanins continued to degrade [5], and alkaline soils inhibited leaf coloration [6].
Under stress conditions, the higher the activity of antioxidant enzymes (SOD, POD, CAT) of three succulents, the higher the content of anthocyanins in leaves, indicating that anthocyanins act as antioxidants when a large amount of free radicals accumulate in plants. It is synthesized in large quantities to scavenge free radicals. In addition, when the antioxidant enzyme activity is increased, the chlorophyll content in the plant is decreased, indicating that the antioxidant enzyme destroys the synthesis of chlorophyll. chlorophyll destruction in plant leaves is related to free radical action [7]. Stress can lead to the accumulation of a large amount of active oxygen in plants, which hinders the synthesis of chlorophyll and causes a decrease in chlorophyll content in plants [8-10].
At present, the colorful and succulent plants in the market are rare, at same time viewing effect is not good, and the economic value is low. In addition, research on the staining of succulents has rarely been reported. Adversity can increase the anthocyanin content of plants and inhibit the synthesis of chlorophyll. The anthocyanin content will make the plants brighter. Therefore, this experiment studied the effects of three stresses of acid, alkali, water and salt on the leaf coloration of three succulent succulents, and it has important theoretical guidance and practical significance for the cultivation and management of succulents.
Materials and Methods
Plant material
The experiment was conducted in the Facility Environmental Laboratory of the Horticultural College of Shenyang Agricultural University from 9-11h 2015. The treatment materials for three kinds of succulent plants: Monroe, Pretty in Pink, Olivia. All the same size, growth of three kinds of succulent plants.
Experiment design
We desiged Acid and alkali stress set 13 treatments: P1~P13 (hydroponic solution pH value respectively 1~13), with HCL and NAOH solution pH value adjustment. Water stress with 5 treatments: W1, W2, W3, W4, W5 (the osmotic pressure of the solutions were 110mOsm/L, 220mOsm/L, 330mOsm/L, 440mOsm, /L, mOsm/L 550), a control group CK (Normal Hydroponics, no water stress), and the pH of each treatment were maintained at 6.5 the solution osmotic pressure with mannitol, regulation. There are 5 treatments for salt stress: N1, N2, N3, N4 and N5 (The concentration of solution salt is 100mmol/L, 200mmol/L, 300mmol/L, 400mmol/L, 500mmol/L), and the pH values of each treatment are all 6.5, and the salt concentration of solution is adjusted by 400mmol/L. A control group (CK solution pH=6.5, EC=80mmol/L, is the most suitable for the growth of the concentration of succulent plants).
Determination of leaf hue a* value
Determination of leaf chromaticity: Leaf chromaticity was measured using a Minolta CR-400 automatic color difference meter (Lister C E, 1996; Voss D H, 1992). The measurement is first performed using a standard white board calibration. The a* value is recorded to represent the leaf color, where the a* value represents red/green, the a* value is large red, and the a* value is green deep.
Determination of anthocyanin content
We adopted Solvent extraction method (Li 2000). 0.2g of liquid nitrogen frozen leaves were cut into pieces, placed in a test tube, added with 10mL of 1% hydrochloric acid-methanol solution, and immersed in the dark for 24h at room temperature. The supernatant was extracted, and 1% hydrochloric acid-methanol solution was used as a blank. For the control, the optical density value was measured at 530nm, and the anthocyanin content was calculated according to the following formula: The relative content of anthocyanins=OD530/0.1 W (Pigment unit g-1).
Chlorophyll content determination
We used Ethanol-acetone extraction method (Li 2000). Take fresh leaves and wipe off the dirt. Take 0.2g of liquid nitrogen frozen leaves and cut into pieces. Add 10mL of 80% acetone: 95% ethanol to 1:1 mixture, so that the whole tissue is immersed in the solution. The mixture was immersed in the dark at room temperature for 24h, and the supernatant was taken. The optical density was measured at 664nm and 645 nm using an ethanol-acetone solution as a blank control. The chlorophyll content was calculated according to the following formula:
Chlorophyll Content (mg/g)=(20.29OD645 + 8.04OD665)×V/1000/W
Determination of enzyme activity (SOD,POD,CAT)
Calculate the enzyme activity using the following formula:
Total SOD activity (U/g▪FW) =(ACK-AE) ×V/ (0.5 ACK×W×) Vt
ACK-control tube light absorption value AE-sample tube light absorption value
V-total liquid volume (mL)
W - sample fresh weight (g)
Vt-measure sample amount (mL)
POD activity (△A470/min·gFW) =(△A470×Vt)/( W×VS×0.01×t)
△A470-Change in absorbance during reaction time
Vt-Total amount of extract (mL)
S-The amount of extract used in the measurement (mL)
t-reaction time W-fresh weight of sample (g)
CAT activity (△A240/min·gFW) =△A240×V/Va/W
△A240-The change in absorbance during the reaction time V-the amount of enzyme solution (mL)
Va-total volume of crude enzyme extract (mL) W-fresh weight of sample (g)
Statistical analysis
Excel and DPS data analysis software are used to make a significant analysis of the data.
Result
Effect of three kinds of stress on plant morphology
After the previous three kinds of Echeveria fleshy form screening, found that the solution pH value is 3-11, three species of succulent plants can be normal growth, for the further study of three kinds of succulent plants under extreme conditions of coloring, P3, P4, P10, P11, W2, W3, W4, N2, N3 N4, and CK analysis.
Effect of three kinds of stress on the a* value
The treatments of acid, alkali, water and salt, the a* values of the leaves of Monroe and Pink Lady rose gently increase and began to rise sharply on the 15th day, reaching the highest value in 25 days. The treatments of P3, P4, P10 and P11 were 52.23%, 48.86%, 46.37% and 55.75% higher than the control, respectively. Monroe’s W2, W3, and W4 treatments were 91.63%, 97.06%, and 106.95% higher than the control, respectively; the hue a* values of Olivia’s treatments were not significantly increased, reaching the highest at 25th, P3, P4, and P10. The P11 treatment was 24.62%, 25.9%, 12.78%, and 28.14% higher than CK, respectively. The W2, W3, and W4 treatments in Olivia were 26.2%, 29.07%, and 29.29% higher than the control, respectively (Figure 1-3).
Figure 1:Three succulents pictures
A: Echeveria Monroe;
B: Echeveria Pretty in Pink;
C: Echeveria Olivia.

Figure 2:Effect of acid stressand alkali on three kinds of succulent plants leaves the hue a* value.
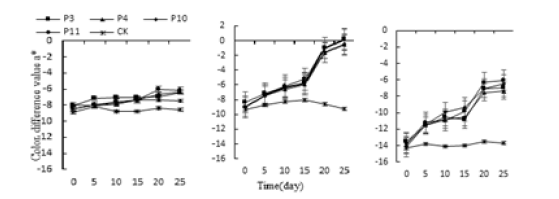
Figure 3:Effect of water stress on three kinds of succulent plants leaves the hue a* value.
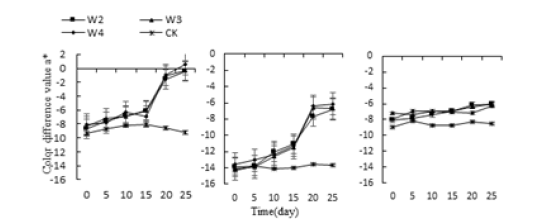
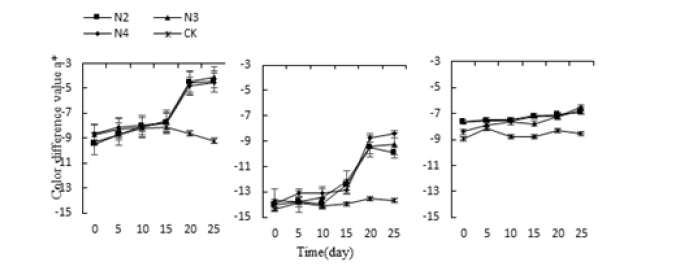
The stress of salt, the a* value of the leaf color of Monroe and Pink Lady did not change significantly from 0 to 5 days and rose sharply on the 15th day and reached the highest value in 25 days (Figure 4). Monroe was in N2. The highest values under N3 and N4 treatments were 51.9%, 55.59%, and 57.81% higher than the control, respectively. Olivia had no significant change compared with the control, only a small increase, and the highest increase on the 25th day, each treatment was 19.11%, 21.11%, 23.56% higher than the control. The results of the above experiments showed that under the acid-base stress, the a* value of leaf hues of three succulents increased with the extension of stress time. When reaching the highest peak, the a* value of Monroe’s leaves was the largest, and the leaves changed from green to red. Pink Beauty and Olivia are both green; under the stress of water, the a* of all three increase with the increase of water stress, but only the leaf a* value of Monroe rises above 0, the leaves are green turned to red. Under the stress of salt, the hue a* value of the leaves increased with the increase of salt stress.
Figure 4:Effect of salt stress on three kinds of succulent plants leaves the hue a* value.
Effect of three kinds of stress on succulent plants anthocyanin
When the conditions of acid-base stress and water stress, the anthocyanin content of the leaves of the three succulent plants began to increase on the 10th day, and the three succulents reached the highest value on the 25th day (Figure 5&6). The highest values of Monroe under P3, P4, P10 and P11 treatments were 1098.91%, 1042.39%, 1051.08% and 1114.13% higher than the control, respectively. The highest values under W2, W3 and W4 treatment were 1010.86% and 1081.52% higher than the control, respectively., 1193.47%; Under the stress of salt, the anthocyanin content of the three succulents continued to rise under the salt treatment, and rose sharply on the 15th day, and reached the highest value on the 25th day (Figure 7).
Figure 5:Effects of acid and alkali stress on three kinds of Anthocyanins of succulent plants.
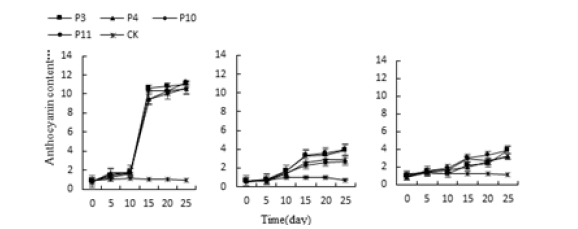
Figure 6:Effect of water stress on three kinds of meat of anthocyanin.
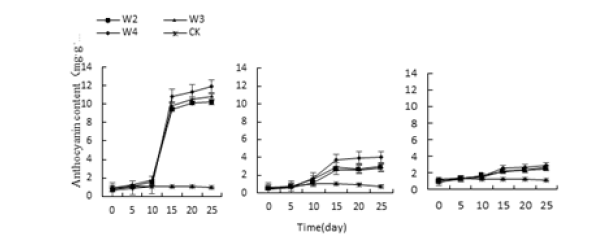
Figure 7:Effect of water stress on three kinds of meat of anthocyanin.
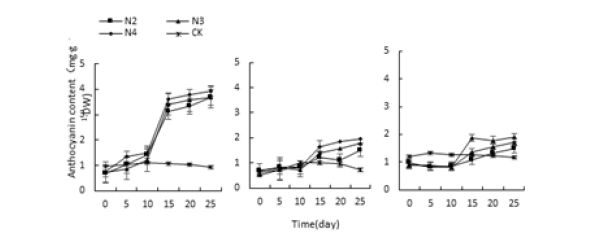
Monroe Pink Beauty was 382.19%, 416.44%, and 447.95% higher than the control, respectively; Olivia was 213.44%, 313.54%, and 337.50% higher than the control, respectively. It can be seen that under the stress of acid, alkali, water and salt, the anthocyanin content of the three increased with the increase of stress degree and time, and the content of anthocyanin increased the most. The difference between Livia and the control is not obvious.
Effect of three kinds of stress on chlorophyll
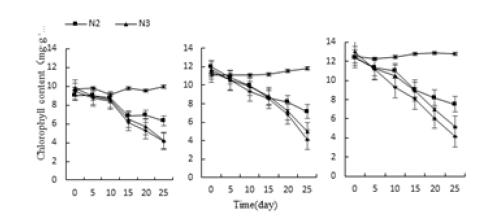
Under the treatments of acid, alkali, water and salt stress, the chlorophyll content of the three vegetative plants continued to decrease and was the lowest at the 25th day of culture (Figure 8-10). The lowest values of Monroe P3, P4, P10 and P11 treatment were 39.09%, 33.17%, 32.16% and 39.50% lower than the control, respectively. The chlorophyll content under W2, W3 and W3 treatment was 27.14% and 29.95% lower than that of the control, respectively. 32.36%; Monroe’s N2, N3, and N4 treatments were 36.48%, 57.38%, and 58.59% lower than the control, respectively; It can be seen that the chlorophyll content of the three decreases with the increase of the degree of stress. Compared with the control, Olivia’s chlorophyll content decreased the most, Monroe’s decline was the smallest, followed by pink beauty, indicating that Olivia’s acid and alkali resistance is the weakest.
Figure 8:Effects of acid and alkali stress on three kinds of meat plant chlorophyll.

Figure 9:Effect of water stress on three kinds of meat plant chlorophyll.
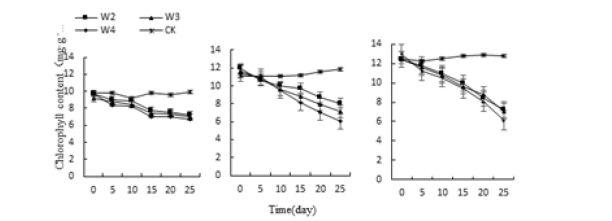
Figure 10:Effect of water stress on three kinds of meat plant chlorophyll.
Effect of three kinds of stress on the SOD activity
Figure 11:Effects of acid and alkali stress on three kinds of succulents SOD activity.

Figure 12:Effect of water stress on three kinds of meat plant SOD activity.
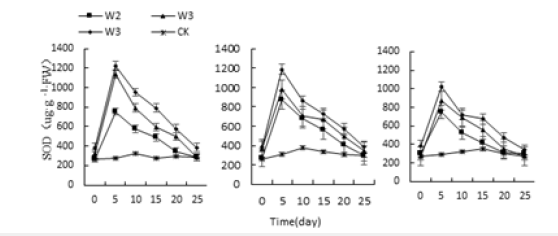
Figure 13:Effects of salt stress on three kinds of meat plant SOD activity.

The stress of acid, alkali and water, the SOD activity of Monroe, Pink Lady and Olivia increased first and then decreased under each treatment, and the highest value appeared on the 5th day (Figure 11-13). Monroe’s the highest values of P3, P4, P10 and P11 treatment were 181.68%, 128.37%, 150.16% and 170.51% higher than the control, respectively. The highest values under W2, W3 and W4 treatment were 175.41%, 315.15% and 348.73% higher than the control, respectively. During the whole salt stress cycle, the SOD activity of the three succulents increased sharply from 0 to 5 days, then began to decline. On the 25th day, there was no significant difference from the control, the 5th day, Monroe increased 251.06%, 358.22%, and 462.15%, respectively, under N2, N3, and N4 treatments. It can be seen that the SOD activity of the three increased with the severity of stress and compared with the control. Monroe has the highest activity of SOD enzymes, followed by Pink Lady and Olivia.
Effect of three kinds of stress on POD activity
Figure 14:Effects of acid and alkali stress on three kinds of Succulents POD activity.
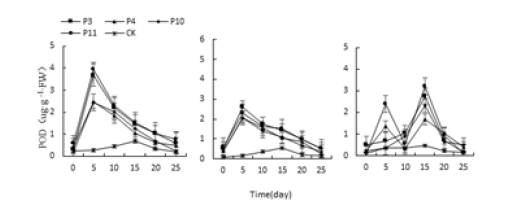
Figure 15:Effect of water stress on three kinds of meat plant POD activity.
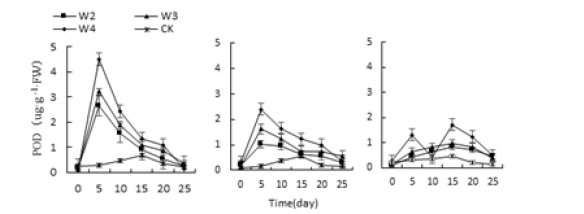
Figure 16:Effects of salt stress on three kinds of meat plant POD activity.
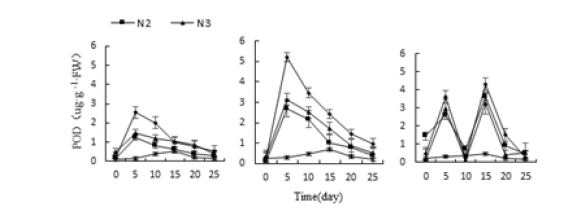
Three different adverse conditions, Monroe, Pink Lady and Olivia’s POD activity increased first and then decreased under each treatment (Figure 14-16), and Monroe and Pink Lady appeared on the 5th day. The highest value, Olivia peaked on the 15th day. The highest values of Monroe’s P3, P4, P10 and P11 treatments were 121.11%, 78.92%, 77.50% and 130.35% higher than the control, respectively; the highest values of Monroe under W2, W3 and W4 treatments were 846.42%, 1046.42%, 1507.14%higher than the control, respectively; When the treatment of N2, N3 and N4, the highest value of POD activity of leaves was 867.85%, 102.51% and 174.71% higher than the control. Under the treatment of P4 and P10, Olyvia’s POD activity increased then decreased. P4 and P10 treatments were 74.18% and 79.01% higher than the control, respectively. Under P3 and P11 treatment, the activity of POD was high-low-high-low.
The trend was the peak on the 5th and 15th days, the highest on the 15th day, and the P3 and P11 treatments increased by 72.78% and 81.67%, respectively. Which showed that the changes in POD activity of the three polyps were the same as those of SOD and increased with the increase of the degree of stress.
Effect of three kinds of stress on CAT activity
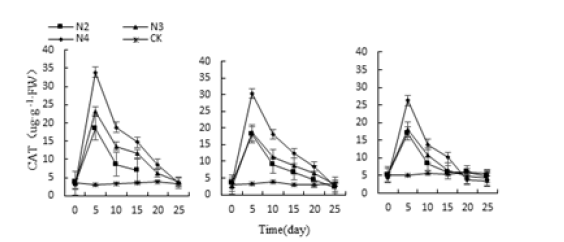
Under the stress of three different adversities, the CAT activities of Monroe, Pink Lady and Olivia increased first and then decreased under each treatment, and the highest value appeared on the 5th day (Figure 17-19). Monroe’s the highest values of P3, P4, P10 and P11 treatment were 850.34%, 374.82%, 287.07% and 904.76% higher than the control, respectively; The highest value of the meat plant Monroe under W2, W3 and W4 treatment was 346.27%, 569.38% and 1203.41% higher than the control, Respectively; At same time succulent Monroe under N2, N3 and N4 treatment was 534.29%, 688.43%, 1053.41% higher than the control, respectively. Thus, the CAT activity of the three increased with the severity of stress, and compared with the control, Monroe had the highest CAT activity, followed by Pink Lady and Olivia.
Figure 17:Effects of acid and alkali stress on three kinds of Succulents CAT activity.
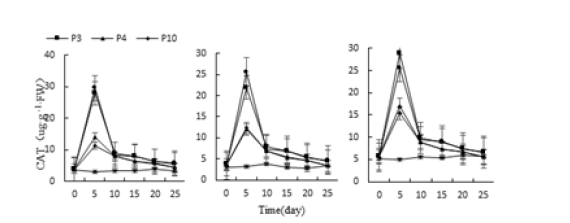
Figure 18:Effect of water stress on three kinds of meat plant CAT activity.

Figure 19:Effects of salt stress on three kinds of meat plant CAT activity.
Discussion
The anthocyanins of plant leaves can not only reflect the color of plants, but also have protective functions on plants. Therefore, under stress, the content of anthocyanins in plants tends to increase [1-3]. The soil is neutral or slightly acidic, which is favorable for the leaf color of the colored leaves. The color of the colorful leaf plants will change with the change of pH value. The pH value can affect the enzymatic activity related to anthocyanins, and then change the synthesis mode of anthocyanins. Suitable acidification can make plant leaves color more vivid. The study found that five-year-old red maple, acidic soils accelerate the synthesis of anthocyanins [11].
Under a certain range of water stress, anthocyanin content will increase [4]. This is consistent with our treatment results. However, some studies suggest that the leaf color is greatly affected by the pH value, and the leaf color changes from red to green as the pH increases, because the pH value not only affects the synthesis of anthocyanins, but also affects its stability. As the pH increased, anthocyanins continued to degrade [5], and alkaline soils inhibited leaf coloration [6]. This is different from the results of this treatment. Our experiment showed that within a certain range, strong acid and alkali can promote the coloration of succulent leaves of sedum.
The anthocyanin content of the three succulents was positively correlated with the hue a* value, and the chlorophyll content was negatively correlated with the hue a* value. Therefore, in three succulents’ plants, anthocyanins are the main reason for the leaves to be red, and the amount of anthocyanins can reflect the red shade of succulent leaves. The ratio of anthocyanin to chlorophyll content increased, the a* value of leaf hue increased, and the plant leaves became red. Previous studies have found that an increase in anthocyanin content and a decrease in chlorophyll content increase the color of plants. The color changes with the increase of anthocyanin content, and the chlorophyll decreases and becomes brighter. Under the condition of water stress, the anthocyanin content of Photinia frondosa decreased, and the chlorophyll content decreased. The ratio of the two increased, and the leaf a* increased. This is the same as the results of our study [12-16].
In the stress, the higher the activity of antioxidant enzymes (SOD, POD, CAT) of three succulents, the higher the content of anthocyanins in leaves, indicating that anthocyanins act as antioxidants when a large amount of free radicals accumulate in plants. It is synthesized in large quantities to scavenge free radicals. The study found that sorghum red pigment has a scavenging effect on free radicals. In a certain concentration range, the concentration of sorghum red pigment is consistent with its ability to scavenge hydroxyl radicals mulberry red pigment versus hydroxyl radical,superoxide Anionic radicalsa, lkyl radicals and hydrogen peroxide radicals all have scavenging effects. And the scavenging ability is better for hydroxyl radicals, superoxide anion radicals and hydrogen peroxide radicals [13-18].
In our experiments, we found that the antioxidant enzyme activity of plants increased on the 5th day, and the synthesis of anthocyanins increased significantly on the 10th day [18-20]. The red color of the leaves strengthened at 15 days, and the h* a value increased. In addition, when the antioxidant enzyme activity is increased, the chlorophyll content in the plant is decreased, indicating that the antioxidant enzyme destroys the synthesis of chlorophyll. Stress can lead to the accumulation of a large amount of active oxygen in plants, which hinders the synthesis of chlorophyll and causes a decrease in chlorophyll content in plants [8-10].
Conclusion
In adversity stress 10-25th days, the anthocyanin content of three kinds of succulent plants leaves will be decreased with the time and degree of water stress and enhance increased, while the chlorophyll content decreased, resulting in the increase of the ratio of anthocyanin and chlorophyll, the leaf red deepened; Three kinds of succulent plants suffered from stress, antioxidant enzymes as radical material, its activity increased sharply in the 5th day of treatment.
The anthocyanin in 10th days are in large quantities, as antioxidants and free radical scavenging substances in the body. The 15th day, the leaves of hue a* rapid rise, deepen the red leaf; Three species of succulent plants had different sensitivity to stress, there are differences between varieties. Olivia had the strongest resistance to stress, followed by Pretty in Pink and Monroe [21,22]. Therefore, under the stress of adversity, Monroe had the most significant leaf coloring, followed by Pretty in Pink and Olivia. In the production practice can enhance succulent plants coloring using appropriate acid and alkali, obtain more brightly coloured fleshy plants, improve the business value of succulent plants.
References
- Zhang YY, Qi DM, Liu H (2008) Flower color diversity of ornamental sunflower and its relationship with anthocyanins. Journal of Horticulture 35(6): 863-868.
- Tholakalabavi A, Zwiabek J, Thorpe RA (1994) Effect of mannitol and glucose-induced osmotic stress. On growth, water relations, and solute composition of cell suspension cultures of poplar in relation to anthocyanin accumulation. In Vitro Cellular Development Biology 30(3): 164-170.
- Brandt K, Giannini A, Lercari B (1995) Photomorphogenic responses to UV radiation III: a comparative study of UVB effects on anthocyanin and flavonoid accumulation in wild type and Aurea mutant of tomato. Photochemistry and Photobiology 62(4): 1081-1087.
- Kong YJ, Kong MG, Hu XJ (2006) Effects of drought stress on several physiological indexes of astragalus membranaceus seedlings. Journal of Central South Forestry University 26(4): 42-46.
- Tang QR, Chen YY, Zhou PH (2003) Study on the stability of anthocyanin and the change of pH value of leaf cell liquid in red deadlock wood. Hunan Forestry Science and Technology 30(4): 24-25.
- Yuan T, Su XH (2004) Introduction and cultivation of colored leafy woody flowers and golden leaves. Journal of Horticulture 31(1): 112-114.
- Wu ZT (1991) Relationship between superoxide radicals and chlorophyll destruction during leaf senescence. Journal of Plant Physiology 7(4): 277-279.
- Xu XN (1995) Effects of water stress on physiological characteristics. Erliu Journal of Anhui Agricultural University 45(1): 42-47.
- Nie HT (1993) Preliminary study on drought resistance identification of mango leaves. Tropical crop research 25(3): 55-59.
- Jia LQ, Li JY (2003) Effects of water stress on seedlings of pistacia chinensis and qingxiangmu. Journal of Beijing Forestry University 25(3): 55-59.
- Steven A (1990) Messenger hruby bett a. response of interveinally chlorotic red maple trees treated with medicaps or by soil acidification. Environ Hort 8(1): 5-9.
- Cao J, Jiang WB, Weng ML (2007) Effects of drought and flood stress on photosynthetic characteristics of Photinia frondosa in summer and autumn. Journal of Horticulture 34(1): 163-172.
- Xu JG, Tian CR, Hu PQ (2005) Study on the scavenging free radical activity of natural mulberry red pigment in vitro. Food Science 26(12): 77-79.
- Li CH, Wang LS, Shu QY (2008) Changes in flower pigment composition and flower color of red azalea in the flowering process. Journal of Horticulture 35(7): 1023-1033.
- Li HS (2000) Principles and techniques of plant physiological and biochemical experiments. Higher Education Press, Beijing, China.
- Mc Pheeters K, Skivrin RM (1983) Histogenic layer maniputation in chimera thornless evergreen trailing blackberry. Euphycioa 32(2): 351- 360.
- Sun B, Liu XD, Zheng DC (2008) Response of leaf color change of false color maple to acid treatment of FeSO4 in soil. Journal of Northeast Forestry University 36(9): 51-52.
- Yu XN, Zhang QX (2007) Research progress on the colorful formation of colorful leaf plants. Journal of Horticulture 27(5): 533-538.
- Zhang HR, Wang WY (2007) Study on scavenging hydroxyl radical activity of sorghum red by fluorescence method. Spectroscopy and Spectral Analysis 27(3): 547-551.
- Zhang JF (2003) Research progress on salt tolerance mechanism and salt-tolerant plant selection in plants. World Forestry Research 21(2): 14-17.
- Zhang ZL, Zhai WW (2003) Plant Physiology Experiment Guide (3rd edn), Higher Education Press, Beijing, China 17(5): 75-125.
- Zhang ZS, Hu DY, Guang YG (1977) Study on Introduction and Reproduction of Colorful Leaf Garden Plants in Beijing. Beijing Garden 43(2): 5-10.
© 2019 Rui Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






